Abstract
The endoscopic resection of juvenile nasopharyngeal angiofibroma (JNA) emerges as an alternative approach to open procedures due to reduced morbidity and comparable recurrence rates. The purpose of this study was to present our experience with the endoscopic management of JNA using retrospective chart review of ten male patients (mean age 15.7 years) with JNA who were treated endoscopically at our institution between the years 2003 and 2010. According to the Radkowski’s system, one patient was at stage Ia, two at stage Ib, one at stage IIa, two at stage IIb, two at stage IIc (infratemporal fossa invasion) and two at stage IIIa (clivus erosion). Six patients underwent preoperative embolization. The endoscopic treatment involved total ethmoidectomy, middle meatal antrostomy, sphenoidotomy, clipping of the sphenopalatine artery and its branches and drilling of the pterygoid basis. All patients underwent magnetic resonance imaging 3 months postoperatively and then if indicated clinically. Mean follow-up was 23.7 months (range 3–70). All but one patient were free of macroscopic disease. A patient with stage IIb JNA developed a recurrence after 9 months. The residual tumor was resected endoscopically and the sphenopalatine foramen widened by drilling. The patient is free of disease 25 months postoperatively. The intra-operative blood loss was not excessive (200–800 ml, mean: 444 ml) and no patient required a blood transfusion. Patients were discharged after 4–8 days (mean 5 days). One patient developed postoperative infraorbital nerve hypoesthesia. Results showed that endoscopic treatment of stage I and IIa/b JNA is a valid alternative to external approaches. For select tumors with limited infratemporal fossa invasion and skull base erosion, the endoscopic approach may also be indicated. It is a safe and effective treatment modality due to the lack of external scars, minimal bone resection and blood loss and low recurrence rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Juvenile nasopharyngeal angiofibroma (JNA) is a benign vascular tumor typically affecting young adolescents. The reported incidence is 3.7 new cases per million males per year [1]. JNA has a propensity for local destruction, intracranial or intraorbital extension and profuse bleeding during resection. Despite complete macroscopic removal, a postoperative recurrence may still occur.
Macroscopically, JNA is a soft multilobular tumor with a well-defined capsule [2]. It consists of fibrotic and vascular elements, the latter being more prominent at the periphery. The larger the tumor, the greater the fibrotic element [3]. Many intratumoral vessels have an incomplete muscular wall and lack the ability to contract; thus any attempt to remove the tumor in a piecemeal fashion results in severe bleeding. The tumor originates from the superior edge of the sphenopalatine foramen and advances submucosally, through natural ostia, along canals and nerves and by bone erosion to the infratemporal fossa, pterygoid canal, parasellar region, sphenoid basis and the orbit [3].
The natural history of JNA is that of progression despite some reported cases of spontaneous involution [4]. Facial deformity, reduced vision, exopthalmos and opthalmoplegia may develop. Therefore, despite its benign nature, JNA should be treated as radically as possible to prevent recurrence which is reported to be between 23 and 27.5% [1, 5]. A number of conservative modalities such as external beam radiotherapy and hormone therapy have been used but the treatment of choice is surgical resection after preoperative embolization. Lateral rhinotomy provides ample operative access to most JNAs but in advanced stage disease a craniotomy may be needed. The disadvantages of these approaches are the external scar and the increased morbidity.
Minimally invasive surgery of select benign [6] and malignant [7] nasal tumors has emerged as an alternative to the open radical procedures of the past. The endoscopic resection of JNA was introduced in the early 90 s [8] and nowadays, several authors advocate its use [2, 9–12].
The aim of this study was to present the results of the endoscopic resection of JNA at our institution and review the limitations and possibilities of the endoscopic technique compared with open approaches in the light of current evidence.
Materials and methods
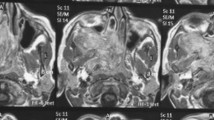
The medical charts of ten male patients with a mean age of 15.7 years (range 10–21) who underwent endoscopic resection of JNA at our department between the years 2003 and 2010 were reviewed. All patients but two, presented with nasal obstruction and epistaxis (one patient required multiple transfusions at presentation). The two patients complained solely of unilateral nasal blockage. The mean duration of symptoms was 7.6 months (range 3–18 months). Diagnosis and staging were based on nasal endoscopy, computed tomography (CT) and magnetic resonance imaging (MRI). We adopted the Radkowski’s classification scheme (Table 1). Patient characteristics and tumor localization are shown in Table 2. No cases with intracranial or intraorbital extension were treated endoscopically. Six patients, all at Radkowski’s stage IIa/b/c and IIIa, underwent embolization of the external carotid artery branches within 24 h before operation. A patient with stage IIIa JNA is shown in Fig. 1a, b. The operation was carried out by the same surgeon (JC) and in one occasion a computer-assisted navigation system was employed. The nasal cavity was prepared with cotton pledgets soaked in local anesthetic and vasoconstrictor. The surgical procedure consisted of anterior and posterior ethmoidectomy, middle meatal antrostomy, removal of the posterior wall of the maxillary sinus when necessary and sphenoidotomy. The sphenopalatine artery and its large branches were identified and clipped. Small bleeders were cauterized by bipolar diathermy. The basis of the pterygoid plates and the Vidian canal were consistently drilled. The tumor was delivered through the nasopharynx and the oral cavity in one piece. The new cavity was filled with bioresorbable nasal dressing (Nasopore®) and Merocel® nasal packs in order to ensure adequate hemostasis. The nasal packs were removed on postoperative day 3 and the patients were instructed to start nasal douching with normal saline on day 6. Follow-up examination with nasal endoscopy was arranged at 3, 6 and 12 months postoperatively for the first year and then twice per year. Patients with large postoperative cavities attended for follow-up regularly in the first 3 months to have nasal crusts endoscopically cleared. A postoperative MRI scan was requested at 3 months and thereafter when indicated by the endoscopic findings.
a Axial CT scan of a 21-year-old patient with JNA stage IIIa. The lesion occupies the nasopharynx and penetrated the left pterygopalatine fossa (open arrows) through an enlarged sphenopalatine foramen. Black arrow demarcated the erosion of the clivus. b Coronal T2-weighted MRI scan of the same patient. The enhancing lesion invades into the left infratemporal fossa (arrows)
Results
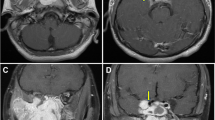
Visible tumor was completely resected in all patients. Intra-operative blood loss ranged from 200 to 800 ml (mean 444 ml); no blood transfusion was required (Table 2). Postoperative hospital stay ranged from 4 to 8 days (mean 5 days). Duration of follow-up was between 3 and 70 months (mean 23.7 months). One patient with JNA extending to the infratemporal fossa developed post-operative infraorbital nerve hypesthesia (Fig. 2). Nasal crusting persisted for a few months in patients with extensive JNAs and large postoperative cavities (two patients with IIIa, two patients with IIc). Nasal douching and endoscopic removal of crusts sufficed in all cases. Nine patients had no endoscopic or radiological evidence of residual or recurrent disease. One patient (stage IIb) developed a recurrence 9 months postoperatively. He underwent repeat endoscopic resection and drilling of the sphenopalatine foramen. The patient is free of disease 25 months after revision.
Discussion
In our patient cohort there was no need for blood transfusion; postoperative morbidity was minimal and hospital stay was short. The recurrence rate was low (1 patient) but this should be viewed with caution since four out of ten patients had a follow-up of less than a year. In three patients, the fibrotic capsule of the tumor facilitated traction and en bloc endoscopic removal of the infratemporal segment of the lesion. We, therefore, favor the endoscopic resection of JNA entering the pterygopalatine fossa and for select tumors with infratemporal fossa invasion or clivus erosion. The maximum extent of JNA which can be safely resected by the endonasal route continues to be a topic of debate.
The radical external approaches are the mainstay of treatment for JNAs of any extent. They provide ample access to the tumor and space for surgical manipulations. Profuse bleeding, a hallmark of JNA resection, can be controlled easily by the surgeon who has both hands free to use suction and diathermy. The external scar of a lateral rhinotomy can be inconspicuous if the incision is closed meticulously or avoided at all if a midfacial degloving approach is adopted. The transoral Le Fort I approach provides wide exposure of the postanasal space and pterygopalatine and infratemporal fossae bilaterally. If there is large intracranial extension of JNA or high risk of severing the internal carotid artery or the cavernous sinus intraoperatively, a craniofacial approach is employed. Infratemporal or parapharyngeal extensions of JNA are best managed by the Fisch type C infratemporal fossa approach [13].
The morbidity of the external approaches in the treatment of JNA is well documented. Blood loss due to the extensive osteotomies for access [14] may necessitate blood transfusions with all the associated risks of disease transmission. Additionally, osteotomies increase the operative time and may interfere with the normal facial growth of the adolescent patient [15]. Cerebrospinal fluid leak, facial and infraorbital nerve damage, lacrimal dysfunction, facial deformities and dental malocclusion have been reported with transfacial approaches [5, 16]. The lateral approach to the infratemporal fossa may result in trismus, hypesthesia and conductive hearing loss [13].
The buildup of experience with the endoscopic treatment of inflammatory sinonasal disease has paved the way to the endoscopic resection of nasal tumors with many advantages: bone resection is minimized, blood loss is reduced and the normal growth of the adolescent facial skeleton is not altered significantly [17]. By the endonasal access, the scar and the manipulation of the soft tissues of the anterior face are avoided. The operative time and hospital stay are therefore reduced. Another advantage of the endoscopic resection of JNA is the improved, multiangled and magnified view of the tumor limits which facilitates tumor resection [18].
Limited JNA in the nasal cavity and close surroundings
The endoscopic resection of tumors in the nasal cavity, paranasal sinuses and pterygopalatine fossa is well established compared with the external approaches [9, 12, 19–22]. Intraoperative visualization of the tumor limits in the complex area of the pterygopalatine fossa, with endoscopes of various angles, is superior to that of the unaided eye. Enepekides reports the recurrence rate for tumors up to Radkowski’s stage II, between 0 and 7% [15].
JNA with infratemporal or orbital invasion
Tumors with infratemporal fossa or orbital involvement are not an absolute contraindication for endoscopic surgery. Hofmann et al. [10] operated six patients with Fisch IIIa JNAs after preoperative embolization. The mean intraoperative blood loss was 825 ml and two recurrences were reported after a mean follow-up of 54.5 months. One was irradiated with gamma knife and the other remained asymptomatic. Other authors also advocate endoscopic resection for tumors with limited infratemporal fossa extension but not for tumors with orbital invasion [2, 11, 14, 18]. The infratemporal fossa is a distant area for access through the nose, contains the internal carotid artery and jugular vein and lies close to the orbital apex and the cavernous sinus. Complete removal of a tumor in this region and control of bleeding during endoscopic surgery may be difficult to achieve. A proposed solution is the four-hand technique [18] which requires removal of the posterior bony septum so that two surgeons can work at the field. Endoscopic access to the infratemporal fossa may be augmented by limited external approaches such as the Caldwell-Luc in an attempt to avoid the morbidity of the lateral external approaches [23].
JNA with intracranial extension
The rate of intracranial extension of JNA is reported up to 11% but the true dural infiltration is rare [5, 24].The risks of manipulating a tumor at the parasellar region, irrespective of surgical approach, include damage to the optic and oculomotor nerves and the cavernous sinus. Injury to the internal carotid artery may result in uncontrollable bleeding. In such extensive tumors, the likelihood that the tumor receives feeding vessels from the internal carotid artery is high. This has two important implications: first, embolization of the external carotid system is unlikely to produce a bloodless field and second, emboli may pass through anastomosing vessels into internal carotid branches with deleterious effects.
Despite these problems, experienced surgeons attempted to resect advanced JNAs with limited intracranial extension or skull base erosion endoscopically. Large, fibrotic JNAs have a strong, well-defined capsule which may allow complete en bloc resection with traction from the non-infiltrated cavernous sinus. Nicolai et al. [11] reported a patient with extension of JNA lateral to the cavernous sinus who was operated endoscopically without recurrence 4 years postoperatively. Despite this, the authors recommend an open approach as more appropriate. Roger et al. [25] operated on nine patients with skull base erosion; three patients had also tumor extension into the foramen lacerum. Removal was complete in all but one patient who had asymptomatic residual tumor not requiring additional treatment. Onerci et al. [26] reported four patients with skull base erosion and minimal intracranial extension who underwent endoscopic resection. Two patients were free of disease and two patients had stable residual tumor around the cavernous sinus 2 years postoperatively. Hackman et al. [23] presented a series of 17 patients with advanced JNA, the largest to date, who were treated either by an exclusively endoscopic or an endoscopic plus a limited external approach. Recurrences were noted in four patients (mean follow up 4 years); all of them were treated successfully by a second endoscopic procedure. The authors favored the Caldwell-Luc approach for all combined cases and proposed that the intranasal component of the tumor is resected first and the skull base component at a later stage when the patient has stabilized. El-Banhawy et al. [27] have used the endoscope as adjunct to the midfacial degloving approach for JNA extending intracranially. They have operated 15 patients (4 patients with orbital involvement), two of whom presented with a recurrence. Endoscopes of different angles improved visualization in areas not directly exposed by the midfacial degloving approach, i.e., the base of the pterygoids, the vaginal process and the greater wing of the sphenoid, the medial and inferior aspect of the orbit and the olfactory cleft.
JNAs with an extensive intracranial component require multimodality treatment with preoperative embolization, transfacial or transcranial approach and gamma knife radiotherapy if large residual tumor remains around the cavernous sinus [28].
Adjunctive procedures
Resection of JNA is facilitated by a bloodless surgical field. Intraoperative measures to decrease the vascularity of the tumor include local mucosal application of vasoconstrictors, infiltration of the pterygopalatine fossa with lignocaine-adrenaline through the major palatine foramen and clipping of the sphenopalatine artery and its branches. Early intraoperative cauterization of the vidian artery (branch of the internal carotid artery), at the pterygoid plates, may reduce significantly the vascularity of the skull base component of a large JNA [23]. Budzynowska et al. [29] have used intratumoral injections of tissue adhesive glue to minimize vascularity. Preoperative embolization of external carotid branches 24–48 h preoperatively provides a bloodless field, reduces the blood loss and the need for transfusion [1, 9, 12, 21, 30–32]. If the tumor extends intracranially or into the skull base, the likelihood of internal carotid artery contribution to its vascularity is high. Embolization may lead to occlusion of the opthalmic or cerebral arteries [23]. There is also concern of incomplete resection due to blunting of tumor borders after embolization, particularly if deep invasion of the sphenoid is present [30, 33]. Intratumoral embolization, whenever applicable, avoids occlusion of intracranial or intraorbital vessels and should be the first choice for reducing the tumor vascularity [10, 34]. JNAs may be operated endoscopically despite the lack of preoperative embolization without significant hemorrhage or increased rate of recurrence [19, 20].
Recurrence of JNA after endoscopic resection
The recurrence rate after surgical removal of JNA irrespective of tumor extent or surgical approach ranges between 23 and 27.5% [1, 5]. Recurrence of JNA usually reflects persistent disease due to incomplete resection [15]. Predisposing factors for incomplete resection is the involvement of the infratemporal fossa, the sphenoid sinus, the basis of the pterygoid plates including the Vidian canal and the intracranial invasion [5, 35]. Some authors have also linked the risk of recurrence with the vascularity of the tumor [36]. Management options for residual or recurrent tumors are (a) a wait-and-see policy with follow-up endoscopy and imaging, (b) reoperation (endoscopic or open approach) and (c) radiotherapy. Several authors reported that asymptomatic residual nodules remain unchanged or regress without any additional treatment [5, 9, 10]. It has been postulated that JNAs undergo a natural cycle of aggressive growth followed by regression in some cases [37]; therefore it is not known if undetected residual disease during surgery will gain clinical significance postoperatively. The timeframe for anticipating progression or involution of JNA is 3 years [38]; thus, long-term monitoring is justified. Small accessible recurrences may be resected endoscopically [9, 10] or monitored with endoscopy and imaging every 3 months. Monitoring is also justified for small residual disease in sensitive areas (e.g., around the cavernous sinus) [24]. Symptomatic or progressing recurrences call for additional treatment: intranasally accessible tumors may be treated endoscopically [23, 39], and tumors with lateral or intracranial extension require an open anterior or lateral approach [24]. Gamma knife has been used successfully to control residual disease in the orbit or brain after surgery in select cases [40]. A promising solution for JNA remnants in inaccessible or dangerous areas may be the use of adjuvant antiangiogenic medications or inhibitors of the vascular endothelial growth factor. Antiangiogenic treatment with etoposide, thalidomide and selecoxib has been used successfully on two patients with unresectable and highly vascular residual JNA [36].
Conclusion
Experience with the endonasal approach in the management of JNA is growing steadily. The advantages of endoscopic resection of JNAs with limited extension into the pterygopalatine fossa over the open approaches are clear. The infratemporal fossa or clival extension of JNA may be managed with endoscopic surgery alone or in combination with limited external approaches. Critical factors for successful endoscopic resection of JNA are preoperatively, proper selection of candidates after careful evaluation of tumor extent and availability of measures to decrease tumor vascularity. Intraoperatively, the Vidian canal and pterygoid basis should be drilled to avoid persistence of tumor foci and the sphenopalatine artery with its branches clipped. Postoperatively, endoscopy and imaging will detect early, significant recurrent disease. High expertise of the surgical team with endoscopic surgery is required for successful management of JNA.
References
Glad H, Vainer B, Buchwald C, Petersen BL, Theilgaard SA, Bonvin P, Lajer C, Jakobsen J (2007) Juvenile nasopharyngeal angiofibromas in Denmark 1981–2003: diagnosis, incidence, and treatment. Acta Otolaryngol 127:292–299
Wormald PJ, Van Hasselt A (2003) Endoscopic removal of juvenile angiofibromas. Otolaryngol Head Neck Surg 129:684–691
Sennes LU, Butugan O, Sanchez TG, Bento RF, Tsuji DH (2003) Juvenile nasopharyngeal angiofibroma: the routes of invasion. Rhinology 41:235–240
Spielmann PM, Adamson R, Cheng K, Sanderson RJ (2008) Juvenile nasopharyngeal angiofibroma: spontaneous resolution. Ear Nose Throat J 87:521–523
Herman P, Lot G, Chapot R, Salvan D, Huy PT (1999) Long-term follow-up of juvenile nasopharyngeal angiofibromas: analysis of recurrences. Laryngoscope 109:140–147
Karkos PD, Fyrmpas G, Carrie SC, Swift AC (2006) Endoscopic versus open surgical interventions for inverted nasal papilloma: a systematic review. Clin Otolaryngol 31:499–503
Banhiran W, Casiano RR (2005) Endoscopic sinus surgery for benign and malignant nasal and sinus neoplasm. Curr Opin Otolaryngol Head Neck Surg 13:50–54
Bernal-Sprekelsen M, Vazquez AA, Pueyo J, Carbonell Casasus J (1998) Endoscopic resection of juvenile nasopharyngeal fibromas. HNO 46:172–174
Eloy P, Watelet JB, Hatert AS, de Wispelaere J, Bertrand B (2007) Endonasal endoscopic resection of juvenile nasopharyngeal angiofibroma. Rhinology 45:24–30
Hofmann T, Bernal-Sprekelsen M, Koele W, Reittner P, Klein E, Stammberger H (2005) Endoscopic resection of juvenile angiofibromas—long term results. Rhinology 43:282–289
Nicolai P, Berlucchi M, Tomenzoli D, Cappiello J, Trimarchi M, Maroldi R, Battaglia G, Antonelli AR (2003) Endoscopic surgery for juvenile angiofibroma: when and how. Laryngoscope 113:775–782
Schick B, el Rahman el Tahan A, Brors D, Kahle G, Draf W (1999) Experiences with endonasal surgery in angiofibroma. Rhinology 37:80–85
Andrews JC, Fisch U, Valavanis A, Aeppli U, Makek MS (1989) The surgical management of extensive nasopharyngeal angiofibromas with the infratemporal fossa approach. Laryngoscope 99:429–437
Pryor SG, Moore EJ, Kasperbauer JL (2005) Endoscopic versus traditional approaches for excision of juvenile nasopharyngeal angiofibroma. Laryngoscope 115:1201–1207
Enepekides DJ (2004) Recent advances in the treatment of juvenile angiofibroma. Curr Opin Otolaryngol Head Neck Surg 12:495–499
Radkowski D, McGill T, Healy GB, Ohlms L, Jones DT (1996) Angiofibroma. Changes in staging and treatment. Arch Otolaryngol Head Neck Surg 122:122–129
Bothwell MR, Piccirillo JF, Lusk RP, Ridenour BD (2002) Long-term outcome of facial growth after functional endoscopic sinus surgery. Otolaryngol Head Neck Surg 126:628–634
Robinson S, Patel N, Wormald PJ (2005) Endoscopic management of benign tumors extending into the infratemporal fossa: a two-surgeon transnasal approach. Laryngoscope 115:1818–1822
Andrade NA, Pinto JA, Nobrega Mde O, Aguiar JE, Aguiar TF, Vinhaes ES (2007) Exclusively endoscopic surgery for juvenile nasopharyngeal angiofibroma. Otolaryngol Head Neck Surg 137:492–496
Borghei P, Baradaranfar MH, Borghei SH, Sokhandon F (2006) Transnasal endoscopic resection of juvenile nasopharyngeal angiofibroma without preoperative embolization. Ear Nose Throat J 85:740–743, 746
Jorissen M, Eloy P, Rombaux P, Bachert C, Daele J (2000) Endoscopic sinus surgery for juvenile nasopharyngeal angiofibroma. Acta Otorhinolaryngol Belg 54:201–219
Scholtz AW, Appenroth E, Kammen-Jolly K, Scholtz LU, Thumfart WF (2001) Juvenile nasopharyngeal angiofibroma: management and therapy. Laryngoscope 111:681–687
Hackman T, Snyderman CH, Carrau R, Vescan A, Kassam A (2009) Juvenile nasopharyngeal angiofibroma: the expanded endonasal approach. Am J Rhinol Allergy 23:95–99
Danesi G, Panciera DT, Harvey RJ, Agostinis C (2008) Juvenile nasopharyngeal angiofibroma: evaluation and surgical management of advanced disease. Otolaryngol Head Neck Surg 138:581–586
Roger G, Tran Ba Huy P, Froehlich P, Van Den Abbeele T, Klossek JM, Serrano E, Garabedian EN, Herman P (2002) Exclusively endoscopic removal of juvenile nasopharyngeal angiofibroma: trends and limits. Arch Otolaryngol Head Neck Surg 128:928–935
Onerci TM, Yucel OT, Ogretmenoglu O (2003) Endoscopic surgery in treatment of juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol 67:1219–1225
El-Banhawy OA, Shehab El-Dien A, Amer T (2004) Endoscopic-assisted midfacial degloving approach for type III juvenile angiofibroma. Int J Pediatr Otorhinolaryngol 68:21–28
Roche PH, Paris J, Regis J, Moulin G, Zanaret M, Thomassin JM, Pellet W (2007) Management of invasive juvenile nasopharyngeal angiofibromas: the role of a multimodality approach. Neurosurgery 61:768–777 (discussion 777)
Budzynowska K, Pietniczka M, Dowzenko A, Borowska K, Czepiel W (2008) Safe extirpating of AFJ after preoperative tumor obliteration with tissue adhesive glue. Otolaryngol Pol 62:408–411
Mann WJ, Jecker P, Amedee RG (2004) Juvenile angiofibromas: changing surgical concept over the last 20 years. Laryngoscope 114:291–293
Naraghi M, Kashfi A (2003) Endoscopic resection of nasopharyngeal angiofibromas by combined transnasal and transoral routes. Am J Otolaryngol 24:149–154
Giavroglou C, Constantinidis J, Triaridis S, Daniilidis J, Dimitriadis A (2007) Angiographic evaluation and embolization of juvenile nasopharyngeal angiofibroma. HNO 55:36–41
Lloyd G, Howard D, Phelps P, Cheesman A (1999) Juvenile angiofibroma: the lessons of 20 years of modern imaging. J Laryngol Otol 113:127–134
Liang Y, Wang D, Huang W, Ling F, Liu Y, Lu F (2003) Direct intratumoral embolization of hypervascular tumors of the head and neck. Chin Med J (Engl) 116:616–619
Howard DJ, Lloyd G, Lund V (2001) Recurrence and its avoidance in juvenile angiofibroma. Laryngoscope 111:1509–1511
Renkonen S, Hagstrom J, Vuola J, Niemela M, Porras M, Kivivuori SM, Leivo I, Makitie AA (2011) The changing surgical management of juvenile nasopharyngeal angiofibroma. Eur Arch Otorhinolaryngol 268:599–607
Lund VJ, Lloyd GA, Howard DJ (1989) Juvenile angiofibroma—imaging techniques in diagnosis. Rhinology 27:179–185
Marshall AH, Bradley PJ (2006) Management dilemmas in the treatment and follow-up of advanced juvenile nasopharyngeal angiofibroma. ORL J Otorhinolaryngol Relat Spec 68:273–278
El Sharkawy AA, Elmorsy SM (2011) Transnasal endoscopic management of recurrent juvenile nasopharyngeal angiofibroma. Int J Pediatr Otorhinolaryngol [Epub ahead of print]
Park CK, Kim DG, Paek SH, Chung HT, Jung HW (2006) Recurrent juvenile nasopharyngeal angiofibroma treated with gamma knife surgery. J Korean Med Sci 21:773–777
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fyrmpas, G., Konstantinidis, I. & Constantinidis, J. Endoscopic treatment of juvenile nasopharyngeal angiofibromas: our experience and review of the literature. Eur Arch Otorhinolaryngol 269, 523–529 (2012). https://doi.org/10.1007/s00405-011-1708-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-011-1708-6