Abstract
Reliable staging of the neck is an important factor for the estimation of prognosis of head and neck cancer patients. A total of 608 patients with oral squamous cell carcinomas treated from March 1975 to December 2000 were enrolled. In radical neck dissection (RND) group, the number of lymph nodes ranged from 6 to 116; while in selective neck dissection (SND) group, from 1 to 87 (P < 0.001). In SND group, the number of metastatic nodes ranged from 0 to 8 nodes, while in RND group, from 0 to 47 (P < 0.001). The number of dissected lymph nodes correlates with the presence of positive nodes (P = 0.001). In RND group, this correlation is described by the equation Y = −0.0117X 2 + 1.7262X. Factors affecting neck metastasis were number of dissected nodes (P < 0.001), lymphatic embolization (P = 0.044) and neural invasion (P = 0.030). In SND group, this equation is Y = −0.012X 2 + 1.5102X; the number of dissected nodes (P = 0.002) and lymphatic embolization (P = 0.001) were significant for metastasis finding. For patients with tumors at stages I and II, a significant impact on survival and neck recurrence rates were observed. In conclusion, we report the importance of the number of retrieved nodes in likelihood of positive cervical node finding. Node yield is an important factor in oral cancer staging, and, more important, in early stage carcinomas, it is associated with survival and recurrence rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reliable staging of the neck is an important factor for accurate prognostic definition and treatment planning of patients with head and neck cancer, since the presence of neck metastasis is regarded as the most important prognostic factor in patients with head and neck squamous cell carcinoma [1, 2].
The gold-standard diagnostic testing for the presence of metastatic lymph nodes has been considered the pathological examination of the neck dissection specimen [3, 4]. Despite this, to stage a neck properly, the AJCC states that only six nodes will suffice for patients submitted to a selective neck dissection and ten in cases of a radical neck dissection [5]. A neck dissection removes the regional lymph nodes with the fatty tissue that harbors them and meticulous dissection may lead to higher yield rates. Anatomic studies estimate the number of neck lymph nodes to be as high as 105 [6]. Previous studies showed that the number of retrieved nodes may be as high as 155 nodes [7]. The number of nodes yielded in neck dissection is highly variable, even within the same institution [8].
The purpose of this report is to assess the number of nodes, in our series of patients, that should be retrieved to ensure maximum precision in neck staging and which factors influence the probability of positive lymph nodes yielded.
Patients and methods
Patients with primary tumors of the oral cavity and oropharynx were, retrospectively, enrolled in this study. The data regarding all patients treated from March 1975 to December 2000 were recovered from the medical charts. The following inclusion criteria were considered: histological diagnosis of squamous cell carcinoma, primary tumor restricted to the oral cavity and oropharynx, no previous treatment, treated with curative intent, surgery as primary form of treatment, neck dissection performed at the time of first treatment and no distant metastasis at diagnosis.
The patients were restaged, based on the record description and pathological report, according to the 2002 AJCC Classification [5]. For comparison purposes and statistical analysis, only the homolateral neck dissection was considered. All specimens were dissected immediately after the removal by a surgical pathologist. Three histological slides were prepared from each node.
The statistical analysis was performed using the Stata 10.1 for Macintosh and the FreeMath Software for MacOs 10. Associations between the presence of positive nodes and gender, primary site and tumor characteristics were analyzed by the χ2 test. The relation between the age and positive nodes was analyzed by the t test. The Spearman test was used for non-parametric correlations, while the Pearson test was used in parametric correlations. The Kaplan–Meier and Cox regression models were used for survival analysis. A random sample analysis was used. It created an array for each or each patient with the number of cells equal to that of dissected nodes and randomly valued each cell as zero (0), if the node was negative, one (1) if the node was positive and smaller than 3 cm and (2) if larger than 3 cm. Up to 200 random samples were drawn and a second array was created were the cells received values zero (0) if the staging was incorrect when compared with the patient’s pathological staging and one (1) if it was correct. The percentage of correct staging is equal to the sum of the array cells divided by two. The number of lymph nodes included in the random sample was increased by five each time the algorithm was executed until the total number of dissected nodes was achieved. Statistical significance was considered when P < 0.05.
Results
A total of 608 patients that conformed to the inclusion criteria were identified for this report. There were 525 males (86.3%) and 83 females (13.7%). The age at diagnosis ranged from 22 to 87 years (mean 55.8 years and median 56 years). Tobacco consumption was reported by 491 patients (80.8%), and 405 (66.6%) reported alcohol consumption.
The most common primary sites were the oral tongue accounting for 242 patients (39.8%), floor of the mouth with 139 patients (22.9%) and retromolar region with 76 patients (12.5%). The distribution of patients according to primary tumor site is shown in Table 1. According to clinical T stage, 35 tumors (5.8%) were classified as T1, 265 (43.6%) as T2, 176 (28.9%) as T3 and 132 (21.7%) as T4a. The most frequent surgery performed for the primary tumor was pelveglossectomy (removal of oral tongue and floor of the mouth) in 224 patients (36.8%), pelveglossectomy with segmental mandibulectomy in 133 patients (21.9%) and the commando resection in 104 patients (17.1%). The distribution of the surgeries is shown in Table 2. Bucopharyngectomies involved the resection form structures belonging to both the oral cavity and oropharynx, usually involving the tonsil, tonsilar pillar and pterygoid muscles) while retromolar surgery involves the removal of the retromolar trigone and ascending ramus of the mandible.
The neck was considered clinically negative in 293 patients (48.2%). The clinical stage (N) of the neck is shown in Table 3. Neck dissection ipsilateral to the tumor was performed in all patients. A radical neck dissection accounted for 336 patients (55.3%), a modified radical neck dissection for 144 patients (23.9%) and a selective neck dissection (levels I–III) for 128 patients (20.9%). In 19 patients (3.1%), an extended radical neck dissection including at least one non-lymphatic structure (nerve, external carotid artery) involved by the tumor was performed. A contralateral neck dissection was performed in 154 patients (27.3%). In 549 patients (90.3%), the neck dissection was removed en bloc with the primary tumor.
All pathological specimens were analyzed at the institution. Separation of the nodes from the fat tissue surrounding them was performed through careful manual dissection after the fixation of the specimen. Peritumoral vascular infiltration was found in 25 patients (4.1%) and lymphatic embolization in 319 patients (52.5%). Neural infiltration was observed in 228 patients (37.5%). The number of lymph nodes recovered in the neck dissection specimen ranged from 1 to 116 in the homolateral neck (mean 43.4 nodes, median 41 nodes and SD 18.042). In patients submitted to a radical neck dissection, the number of lymph nodes ranged from 6 to 116 (mean 46.0 nodes, median 44 nodes and SD 17.495); while in patients submitted to selective neck dissection the number of nodes ranged from 6 to 87 (mean 33.6 nodes, median 32 nodes and SD 16.687). There was a significant difference between the radical and selective dissections groups regarding the number of retrieved nodes (P < 0.001). The number of metastatic nodes in the homolateral side ranged from 0 to 47 (mean 1.6, median 1 node and SD 3.399). In patients submitted to the selective neck dissection, the number of involved nodes ranged from 0 to 8 nodes (mean 0.64 nodes, median 0 nodes and SD 1.219). For the radical dissection group, the number of positive nodes ranged from 0 to 47 (mean 1.84 nodes, median 1 node and SD 3.730). There was a significant difference in the number of metastatic nodes in the two groups (P < 0.001). In the contralateral side of the neck, the number of dissected nodes ranged from 1 to 89 (mean 16.3 nodes, median 10 nodes and SD 14.686), with the number of involved nodes ranging from 0 to 9 nodes (mean 0.4 node, median 0 nodes and SD 0.806).
For patients submitted to neck dissection, a significant correlation was found between the number of dissected lymph nodes and the presence of at least one positive node in the neck (P = 0.001). In patients submitted to a radical neck dissection, this correlation can be plotted and described by a second-degree polynomial equation in which the Y axis represents the probability of finding positive nodes and the X axis, the number of dissected nodes. In our series, this equation is Y = −0.0117X 2 + 1.7262X (Fig. 1). The threshold value of the number of nodes that needed to be resected to achieve the maximum probability of positive nodes may be defined as the value which annulated the derivative of the equation. In this series, this value is 68.04. For values of dissected nodes, below or above this value, the probability of positive node finding is lower than at this specific point. For the diagnosis of two or more positive nodes, a significant correlation with the number of dissected nodes was also demonstrated (r = 0.759, P < 0.001) and the equation that describes this relation is Y = −0.006X 2 + 1.022X. The threshold value for maximum probability of diagnosis is 85.16 nodes. Using the random sample algorithm, the 80% rate of correct staging is achieved with a lymph node count of at least 35 nodes (Table 4).
The number of dissected nodes influences the probability of positive lymph nodes, but other factors may also contribute to this finding. No significant correlation was found between the presence of positive nodes and gender (P = 0.188), age group (P = 0.360) and vascular invasion (P = 0.714). The probability of finding positive nodes in the neck was associated with primary tumor site in the oropharynx (P < 0.001), lymphatic invasion (P < 0.001), perineural invasion (P < 0.001), T stage (P < 0.001) and invasion’s depth (P < 0.001).
In a second stage, a multinomial logistic regression model was used to identify the effect of previously identified factors on the probability of node metastasis. In this model, the number of dissected nodes (P < 0.001), lymphatic embolization (P = 0.044) and neural invasion (P = 0.030) remained significant. The depth of invasion (P = 0.525), primary site (P = 0.392) and T stage (P = 0.305) was non-significant.
The process was repeated for patients submitted to selective neck dissection. In these patients, a significant correlation is also demonstrated (P < 0.001, r = 0.540) and the relation is described by the equation Y = −0.012X 2 + 1.5102X (Fig. 2). The threshold will be achieved with a lymph node count of 62.9 nodes. For the diagnosis of two or more nodes, the threshold value is 57.2 nodes. Again, the random sample algorithm showed that if at least 35 nodes were dissected and analyzed, the rate of correct staging would be more than 80% (Table 4).
In this group of patients, the following factors were found to affect the probability of positive nodes: number of dissected nodes (P < 0.001), lymphatic embolization (P < 0.001) and gender (P = 0.028). The tumor depth of invasion (P = 0.108), primary site (P = 0.782), T stage (P = 0.078), vascular infiltration (P = 0.824) and neural infiltration (P = 0.177) were non-significant in this group. The multinomial logistic regression showed that the number of dissected nodes (P = 0.002) and lymphatic embolization (P = 0.001) remained significant. Gender was shown to be non-significant (P = 0.440).
In our series, there is a significant difference between the mean number of dissected nodes in necks staged as negative or positive for metastasis. In N0 patients, 41.0 nodes were found in the homolateral neck, while in N+ patients, 45.7 nodes were found (P = 0.001).
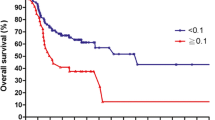
The impact of the number of dissected nodes on survival was also analyzed. In univariate analysis, it was statistically significant (P = 0.048), but not in multivariate analysis. Patients were then divided into groups according to clinical stage. In this analysis, a significant impact on survival regarding the number of dissected nodes was found in patients with early stage carcinomas (stages I and II). For this group of patients, the following factors were found to be significant in a univariate analysis: T stage (P = 0.000, HR 1.672, CI 1.430–1.955), number of dissected nodes (P = 0.018, HR 1,022, 95% CI 1.004–1.040), presence of lymphatic embolus (P = 0.000, HR 2.141, CI 1.578–2.904), neural infiltration (P = 0.001, HR 1.664, 95% CI 1.251–2.214) and involved surgical margins (P = 0.000, HR 1.642, 95% CI 1.245–1.789). A multivariate analysis was performed with stepwise comparison of these parameters. Those found to be non-significant were removed from the model and at the final step, the number of dissected nodes, neural infiltration and surgical margins remained significant (Table 5).
The role of the number of dissected necks in neck recurrence was also assessed. Again, its importance could only be demonstrated in stages I and II oral cancer patients. In fact, this was the only identifiable factor in this series that significantly increases the risk of neck recurrence in this group of patients in multivariate analysis (P = 0.028, HR 1.039, 95% CI 1.004–1.074). Other tested factors were T stage (P = 0.158), vascular invasion (P = 0.448), lymphatic embolization (P = 0.808), perineural infiltration (P = 0.356), type of neck dissection (P = 0.623), status of surgical margins (P = 0.859) and postoperative radiotherapy (P = 0.442).
We also found a significant correlation between T stage and the number of dissected nodes (P = 0.023). Patients with T1 and T2 cancers had a mean of 41.8 dissected nodes (SD 17.25), while T3 and T4 cancers had a mean of 45.02 dissected nodes (SD 18.66) and this difference was statistically significant (P = 0.028). No significant correlation was found between the time of chronological time of surgery (P = 0.502) or the executing surgeon (P = 0.909) and the number of retrieved nodes.
We also calculated the lymph node ratio. It is obtained by dividing the number of metastatic nodes by the total number of dissected nodes. In our series, the lymph node ratio ranged from 0 to 97.92% (mean 3.85% and median 1.19%). The lymph node ratio has a significant impact on survival in univariate analysis, considering the whole series (P = 0.000, HR 1.031, 95% CI 1.021–1.040). Other identifiable prognostic factors were gender (P = 0.008), T stage (P = 0.000), N stage (P = 0.000), TNM stage (P = 0.000), skin invasion (P = 0.000), lymphatic embolization (P = 0.000), perineural infiltration (P = 0.000), number of dissected nodes (P = 0.048) and postoperative radiotherapy (P = 0.001). Multivariate analysis showed the following factors to be significant: gender, T stage, N stage, lymphatic embolization, perineural infiltration and lymph node ratio (Table 6).
Discussion
This study reports a single institution experience with consecutive patients submitted to neck dissections. It demonstrates that the number of retrieved nodes is an important factor in the staging of neck metastasis. The sheer number of lymph nodes yielded may influence the accuracy of the pathological examination and the probability of discovering positive nodes. It also shows other factors that may influence this probability. The use of a mathematical estimation model for the description of the probability of positive nodes versus the number of dissected nodes allow us to establish the number of dissected nodes that presents the higher probability of positive node finding. A low nodal yield may understage a neck, influencing both treatment planning and prognosis assessment.
The role of the number of dissected nodes in staging of malignant neoplasms has also been shown in other sites. It has been shown that the proportion of lymph node metastasis increases as a function of the number of retrieved nodes. In a previous article, a survival benefit was reported with higher nodal yield in colorectal adenocarcinoma [9]. Another report showed a survival benefit in node negative colorectal adenocarcinoma with a number of dissected lymph nodes greater than 13 when compared with those with <6 retrieved nodes [10]. In patients with Dukes B colorectal carcinoma, the number of examined lymph nodes was the only significant factor related to survival in a multivariate analysis [11].
For gastric carcinoma, the same role of the number of dissected nodes has been shown to be a significant prognostic factor for survival. In a multivariate analysis of patients with stages I and II disease, T stage, age and number of dissected nodes were shown to be relevant. The authors also suggest that staging is unreliable when a critical number of nodes are not achieved [12].
In a previous study addressing the node yield of the neck dissection, the likelihood of finding cervical metastasis increased with more than 20 dissected nodes when compared with <13 nodes. It suggests that the finding of pathologically positive nodes is dependent on the extension of the neck dissection. In this article, the neck dissections were not separated according to type (radical or selective) or laterality (unilateral vs. bilateral), but it clearly shows the importance of nodal yield in staging of the neck [13].
Another point that should be addressed when evaluating the role of the nodal yield is quality control. The number of dissected nodes should warrant optimal stage accuracy. For pancreatic adenocarcinoma, this number has been shown to be 15, allowing for discrimination of approximately 90% of the pN1a cohort in a series [14].
In our paper, we report the importance of the number of retrieved nodes in likelihood of positive cervical node finding. We also demonstrate the number of retrieved nodes that correspond to the maximum probability of finding neck metastasis. These findings suggest that the node yield of the neck is per se an important prognostic factor in determining the probability of correctly staging oral squamous cell carcinoma, and for patients with early stage carcinomas a risk factor for overall and disease-free survival expectancies.
Another variable evaluated was the lymph node ratio. In breast cancer, the number of dissected nodes was considered a significant prognostic factor in patients with stages I and II carcinomas, with a longer survival for patients above the threshold of 14 examined nodes. In node-positive patients, the lymph node ratio was an excellent predictor of metastasis development and survival [15].
For other solid neoplasms, lymph node ratio has also been shown to be a significant prognostic factor. In cases of pancreatic adenocarcinoma, the increase in the lymph node ratio is associated with a decrease in survival that is statistically significant in multivariate analysis [16]. For colon cancer, the lymph node ratio is also statistically significant, with different breakpoints based on the clinical staging and that influence both disease-free and overall survival [17, 18]. The same relationship between lymph node ratio and survival was observed for esophageal cancer, with a higher rate corresponding to lower disease-specific survival [19].
The limitations of the size as a prognostic marker for lymph node metastasis were initially shown in melanoma. The melanoma staging committee uses the number of involved nodes, without reference to size to stage the regional nodes (AJCC). The limitations of neck staging based on the size of metastatic lymph nodes as opposed to the number of involved were already shown with regard to oral cancer. In a previous study, no significant difference could be shown between N1 and N2a patients [20].
In our report, we state the role of lymph node ratio as a prognostic marker for oral cancer. This ratio may be useful in adjusting the number of involved nodes by the number of dissected nodes and it is easy to calculate.
References
Myers EN, Fagan JJ (1998) Treatment of the N-neck in squamous cell carcinoma of the upper aerodigestive tract. Otolaryngol Clin North Am 31:671–686
Kowalski LP, Magrin J, Waksman G et al (1993) Supraomohyoid neck dissection in the treatment of head and neck tumors: survival results in 212 cases. Arch Otolaryngol Head Neck Surg 119(9):958–963
Grandi C, Alloisio M, Moglis D et al (1985) Prognostic significance of lymphatic spread in head and neck carcinomas: therapeutic implications. Head Neck Surg 8:67–73
Snow GB, Annyas AA, van Slooten EA, Bartelink H, Hart AA (1982) Prognostic factors of neck node metastasis. Clin Otolaryngol 7:185–192
Greene FL, Compton CC, Fritz AG, Shah JP, Winchester DP (2006) AJJC Cancer staging atlas. Springer Science+Media Inc, New York
Rouviere H (1932) Anatomie des lymphatiques de l’homme. Editora Masson et Cie, libraries de lÁcademie de medicine. Paris
Haagensen CD, Feind CR, Herter FP, Slanetz CA, Weinberg JA (1972) The lymphatics in cancer. WB Saunders, Philadelphia
Agrama MT, Reiter D, Topham AK et al (2001) Node counts in neck dissection: are they useful in outcomes research? Otolaryngol Head Neck Surg 124:433–435
Goldstein NS (2002) Lymph node recoveries from 2427 pT3 colorectal resection specimens spanning 45 years. Am J Surg Pathol 26:179–189
Tepper JE, O’Connell MJ, Niedzwiecki D et al (2001) Impact of number of nodes on outcome in patients with rectal cancer. J Clin Oncol 19:157–163
Caplin S, Cerottini JP, Bosman FT et al (1998) For patients with Duke’s B (TNM stage II) colorectal carcinoma, examination of six of fewer lymph nodes is related to poor prognosis. Cancer 83:666–672
Bouvier A-M, Haas O, Piard F, Roignot P, Bonithon-Kopp C, Faivre J (2002) How many nodes must be examined to accurately stage gastric carcinoma? Cancer 94:2862–2866
Agrama MT, Reiter D, Cunnane MF, Topham A, Keane WM (2003) Nodal yield in neck dissection and the likelihood of metastases. Otolaryngol Head Neck Surg 128:185–190
Tomlinson JS, Jain S, Bentrem DJ, Sekeris EG, Maggard MA, Hines OJ, Rber HA, Ko CY (2007) Accuracy of staging node-negative pancreas cancer: a potential quality measure. Arch Surg 142(8):767–773
van der Wal BC, Butzelaar RM, van der Meij S, Boermeester MA (2002) Axillary lymph node ratio and total number of removed lymph nodes: predictors of survival in stage I and II breast cancer. Eur J Surg Oncol 28(5):481–489
Pawlik TM, Gleisner AL, Cameron JL, Winter JM, Assumpcao L, Lillemoe KD, Wolfgang C, Hruban RH, Schulick RD, Yeo CJ, Choti MA (2007) Prognostic relevance of lymph node ratio following pancreaticoduodenectomy for pancreatic cancer. Surgery 141(5):610–618
Berger AC, Sigurdson ER, LeVoyer T, Hanlon A, Mayer RJ, Macdonald JS, Catalano PJ, Haller DG (2005) Colon cancer survival is associated with decreasing ratio of metastatic to examined lymph nodes. J Clin Oncol 23(24):8706–8712
Schumacher P, Dineen S, Barnett C Jr, Fleming J, Anthony T (2007) The metastatic lymph node ratio predicts survival in colon cancer. Am J Surg 194(6):827–831
Greenstein AJ, Litle VR, Swanson SJ, Divino CM, Packer S, Wisnivesky JP (2006) Prognostic significance of the number of lymph node metastases in esophageal cancer. J Am Coll Surg 206(2):239–246
Hall SF, Groome PA, Rothwell D, Dixon PF (1999) Using TNM staging to predict survival in patients with squamous cell carcinoma of the head and neck. Head Neck 21:30–38
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Köhler, H.F., Kowalski, L.P. How many nodes are needed to stage a neck? A critical appraisal. Eur Arch Otorhinolaryngol 267, 785–791 (2010). https://doi.org/10.1007/s00405-009-1144-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00405-009-1144-z