Abstract
Objectives
Neiyi Prescription of QIU (NYPQ) is a traditional Chinese medicine prescription for the treatment of endometriosis (EMS). Here, we aimed to examine the effects and mechanisms of NYPQ on angiogenic ability in EMS.
Study design
EMS rats were established with estradiol valerate and autologous transplantation. EMS rats were intraperitoneally injected with chloroquine (CQ, 40 mg/kg), rapamycin (RAPA, 1 mg/kg), and monoclonal antibody VEGF (anti-VEGF, 3 mg/g/d) or administered 5, 10, 20 mg/g/d NYPQ decoction through oral gavage for 4 weeks, respectively. By the before and end of the treatment period, the volume of the endometriotic lesions was measured. The pathological morphology, angiogenesis, and the number of autophagosomes of the endometriotic lesion were observed by hematoxylin and eosin staining, immunohistochemistry, and transmission electron microscope, respectively. The cell viability, apoptosis, and angiogenesis of HUVECs were detected by MTT, flow cytometry, and lumen formation experiment, respectively. The expression levels of VEGF, autophagy-/apoptosis-/PPARγ/NF-κB- pathway-related proteins in endometrium tissues or HUVECs were detected by western blot assays.
Results
The autophagy agonist rapamycin reduced the lesion size, the microvessel density, and VEGF expression, and promoted the production of autophagosomes and the expression of autophagy-related proteins, while the autophagy inhibitor chloroquine had the opposite effects. In vivo, NYPQ could dose-dependently reduce lesion volume and microvessel density, ameliorate histopathological features and promote autophagosome production of ectopic endometrium. Moreover, serum-containing NYPQ could significantly inhibit the cell viability and tube formation of HUVECs and elevate HUVECs apoptosis. Besides, NYPQ significantly reduced VEGF and promoted autophagy-/apoptosis-related protein expressions. Also, NYPQ might promote autophagy and inhibit angiogenesis by activating the PPARγ/NF-κB pathway.
Conclusions
Collectively, these findings indicate that NYPQ has therapeutic potential in experimentally induced peritoneal endometriosis, and its mechanism may be related to the activation of the PPARγ/NF-κB signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis (EMS), a common gynecological disease, is defined by the presence of endometrial-like tissue outside the uterus [1]. The main symptoms of EMS are dysmenorrhea, chronic pelvic pain, abnormal menstruation, and infertility [2, 3]. According to statistics, the incidence of EMS accounts for about 10–15% of all women of childbearing age [4]. Although EMS is a benign hormone-dependent disease in histology, it has biological behaviors such as invasion, infiltration, metastasis, and recurrence, which seriously affect women's health and family life [5].
In recent years, autophagy and angiogenesis are generally considered to be related to the pathogenesis of EMS. Autophagy is a kind of programmed cell death, which plays an important role in cell growth, differentiation, immunity, death, and other significant processes [6]. It was found that the autophagy level of endometrial tissue in EMS significantly decreased [7], and the levels of autophagy markers LC3 I/II and beclin-1 in EMS mice were significantly lower than that in control mice [8]. The growth of ectopic endometrium depends on the formation of new blood vessels. It is found that the epithelial and stromal cells of endometriotic implants express high levels of VEGF, which can promote the proliferation and migration of endothelial cells, enhance vascular permeability and participate in angiogenesis cascade reaction [9, 10]. In addition, a part of anti-angiogenic drugs has proved the significance of inhibiting angiogenesis for improving EMS [11].
In China, Traditional Chinese medicine (TCM) has become an adjunct to routine clinical treatment of EMS due to its low toxicity and few side effects [12]. Neiyi Prescription of QIU (NYPQ) is a prescription for treating EMS, which was founded by the late Professor Qiu Xiaomei, a Chinese national TCM doctor, and has been used for EMS therapy for decades in China [13]. The prescription is composed of Scutellaria barbata, Caulis Lonicerae, Caulis Sargentodoxae, Spica Prunellae, Hedyotis diffusa, Corydalis yanhusuo, Clematis chinensis, Salvia miltiorrhiza, barley malt, fried hawthorn, rabdosia herb, and Concha Concha. It is a heat-clearing agent with the effect of clearing heat and detoxifying, relieving swelling and pain, promoting blood circulation, and removing blood stasis. Many scholars have conducted research on the efficacy of NYPQ in the treatment of EMS and adenomyosis [14, 15].
However, the molecular mechanism of NYPQ in treating EMS is still unclear. To better understand Professor Qiu Xiaomei’s therapeutic prescription, a model of EMS was established in rats, then the effect and underlying mechanism of NYPQ on autophagy and angiogenesis were evaluated on the development of EMS. Results suggested that NYPQ may have a therapeutic effect against EMS because it can regulate autophagy and angiogenesis via the PPARγ/NF-κB signaling pathway. This study highlights novel insights for researchers to explore the mechanism of NYPQ systematically, and provides a guideline in the further investigation of this prescription.
Materials and methods
Animal and experiments
Hundred and ten Sprague Dawley rats (female, 190 ± 10 g,) were purchased from Shanghai Slack Laboratory Animal Co., Ltd (Shanghai, China). The rats were raised in the Animal Center of Hangzhou Yingyang Biotechnology Co., Ltd (Zhejiang, China), and kept in separate cages in a clean animal breeding room with a temperature of 25 ± 2 °C and a humidity of 50–60%. After three days of adaptive feeding, rats (n = 80) were given estradiol valerate (0.2 mg/kg body mass, YZ-100063, Solarbio, China) once a day for 3 consecutive days. A large number of denucleated keratinocytes were observed on vaginal smears of rats, indicating that the estrous period of rats tends to be consistent. After the last administration of estradiol valerate, the rats fasted for 12 h and were anesthetized by intraperitoneal injection of pentobarbital sodium (45 mg/kg, Sigma). The abdominal hair was shaved and the abdomen of the rats was cut open. Subsequently, endometrial tissue was harvested from the myometrium under a dissecting microscope and trimmed into 0.5 cm × 0.5 cm pieces. And then, the autologous fragments were transplanted into the inner surface of the abdominal wall. Finally, the abdominal cavity was closed with absorbable surgical silk thread. After the operation, rats were injected with cefuroxime sodium (C7340, Solarbio, China) intraperitoneally for anti-infection, once a day for 5 days. After 4 weeks, the rats were anesthetized and exposed the abdominal cavity again to observe the transplanted site of the endometrium. If the endometrium grew into transparent vesicles with liquid accumulation, the EMS model was successfully constructed. Rats (n = 10) in the sham group were only cut off the adipose tissue around the uterus after laparotomy. Four weeks after successful modeling, the length, width, and height of ectopic endometrium in each group were measured and recorded by electronic vernier caliper, and its volume (V) = 0.52 × length × width × height was calculated. Animal care and experimental protocols were approved by the ethics committee of Hangzhou Eyong Biotechnological Co., Ltd. Animal Experiment Center [No. SYXK (Zhe)2020-0024].
To study the effect of autophagy on endometriosis, the experiment was divided into four groups: control group (rats undergoing a sham operation, n = 10), model group (EMS rats, n = 10), CQ (IC4440, Solarbio, China) group (EMS rats received intraperitoneal injection of 40 mg/kg CQ three per week, n = 10) and RAPA (R8140, Solarbio, China) group (EMS rats received intraperitoneal injection of 1 mg/kg RAPA three times a week, n = 10). Rats in the control group and model group were intraperitoneally injected with the same amount of normal saline. All rats were treated for 4 weeks.
To study the effect of NYPQ on rat endometrium, the experiment was divided into five groups: model group, low-dose NYPQ (L-NYPQ) group, medium-dose NYPQ (M-NYPQ) group, high-dose NYPQ (H-NYPQ) group and anti-VEGF group (positive control), ten in each group. NYPQ was diluted to 0.4 ml with distilled water, and then rats in L-NYPQ, M-NYPQ, and H-NYPQ groups were gavaged with 5, 10, and 20 mg/g/d NYPQ, respectively. Rats in the anti-VEGF group were intraperitoneally injected with 3 mg/g/d VEGF monoclonal antibody (# MA1-21465, Thermo Fisher, USA), and rats in the model group were gavaged with the same amount of distilled water. All rats were treated for 4 consecutive weeks. NYPQ was processed into Chinese medicine fluid extract by good manufacturing practices (GMP) certified preparation center of the First Affiliated Hospital of Zhejiang Chinese Medical University, containing 2 g/ml of the crude drug. Before and after treatment, the endometriotic lesion volume was calculated.
According to the keratinization of the vaginal epithelium, all samples were taken at the later stage of hyperplasia, while the control group took the eutopic endometrium and the other groups took the ectopic endometrium. Part of the collected tissues was frozen in a liquid nitrogen tank, and the other part was put in a 10% fixed solution (E672001, Sangon, China).
Hematoxylin and eosin (H&E) staining
The fixed tissue was dehydrated and transparent, and then embedded in wax, and then the wax block was cut into 5 μm sections. After the sections were deparaffinized and hydrated, they were stained with hematoxylin (G1142, Solarbio, China) and eosin (G1100, Solarbio, China). Afterward, the sections were dehydrated, transparent, and sealed with a neutral balsam mounting medium (E675007, Sangon Biotech, China). The pathological morphology of ectopic endometrium can be observed by taking pictures under a microscope (BX53M, Olympus, Japan, × 200 or × 400).
Immunohistochemistry
Paraffin sections were dewaxed conventionally and then put into citric acid repair solution to be repaired by microwave heating. Subsequently, paraffin sections were incubated in 3% hydrogen peroxide solution (10011208, Sinopharm Chemical Reagent, China) for 10 min and 3% BSA (E661003, Sangon, China) for 30 min. After blocking, the sections were reacted with anti-CD31 antibody (AF6191, Affinity, USA) and the secondary antibody (ab205718, Abcam, China). After that, SABC was added to the sections and incubated for 30 min. Sections were required for color development in DAB kits (G1211, servicebio, China) and counterstained with hematoxylin. After sealing with neutral gum, the results were observed by an inverted phase-contrast microscope (magnification: ×200 and ×400), and the number of capillaries were counted by Image Pro-Plus 6.0 software (Media Cybernetics, Inc., MD, USA).
Measurement of autophagosomes
The freshly isolated tissue was fixed with 3% glutaraldehyde (111-30-8, Alfa Aesar, USA), and then dehydrated and embedded to make ultra-thin sections. The sections were observed under a transmission electron microscope (H7650, Hitachi, Japan), and 30 cells were randomly selected from each group to record the number of autophagosomes, and then the number of autophagosomes in each cell was calculated.
Western blotting
The total protein from ectopic endometria was extracted by RIPA buffer (E-BC-R327, Elabscience, China) and quantified by BCA kit (E-BC-K318-M, Elabscience, China). Identical amounts of total protein were denatured and then subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Proteins were transferred to nitrocellulose membranes (B500, ABM, USA), and blocked by 3% BSA. Afterward, the membranes were incubated with primary antibodies and secondary antibodies followed by visualization using ECL Substrates (E-BC-R347, Elabscience, China) on the iBright Imaging System (610020-9q, QINXIANG, China). The antibody used in this experiment include: VEGF (AF5131, Affinity, USA), LC3 I/II (AF5402, Affinity, USA), Beclin-1 (ab62557, Abcam, USA), PPARγ (ab272718, Abcam, USA), NF-κBp65 (ab16502, Abcam, USA), Bax (ab182733, Abcam, USA), Bcl-2 (ab194583, Abcam, USA), cleaved-caspase-3 (AF7022, Affinity, USA), GAPDH (ab8245, Abcam, UK), goat anti-rabbit (1:10000, ab205718, Abcam, UK) and goat anti-mouse (1:10000, ab6789, Abcam, UK). GAPDH was used as an internal control.
Acquisition of serum-containing NYPQ
Rats were randomly divided into two groups: the control group and the NYPQ group, with 10 rats in each group. The rats in the NYPQ group were given 20 g/kg/d NYPQ, and the rats in the control group were given the same amount of distilled water twice a day for 5 days. In the last 2 h after gavage, blood samples were collected from the abdominal aorta, and serum was isolated, inactivated at 56 ℃ for 30 min, sterilized by microporous filtration. All serum was stored at 4 °C for later use.
Cell culture and NYPQ containing serum treatment
Human Umbilical Vein Endothelial Cells (HUVECs) were purchased from the Shanghai Institute of Cell Biology (Shanghai, China) and cultured in 1640 medium (Hyclone, Beijing, China) containing 10% fetal bovine serum (FBS, Hyclone, Beijing, China) and 1 × streptomycin in a humidity incubator with 5% CO2 at 37 °C and passaged when they reached 90% confluence. Next, HUVECs were planted in six-well plates at a density of 1 × 106 per well and were treated with RPMI-1640 medium (with free FBS and 1% penicillin–streptomycin solution) containing 10% control rat serum, 5%, 10%, and 20% serum-containing NYPQ for the appointed time at 37 °C, respectively. 10% control rat serum-treated HUVECS served as the control group.
Cell viability
MTT kit (M1020, Servicebio, China) was employed to examine the viability of HUVECs. HUVEC was inoculated in a 96-well culture plate and cultured in an incubator. After the cells adhered to the wall, they were intervened with serum for 24 h and 48 h, respectively. MTT solution was added to the culture plate to react with the cells for 4 h, and then formazan solution was used to dissolve the crystals. A multiple detection reader (C3-6550-01, AS ONE, Japan) was employed to count the absorbance at 570 nm.
Cell apoptosis assay
An apoptosis detection kit (CW2574S, CWBIO, China) was employed to detect apoptosis of HUVECs. HUVECs (5 × 105) were seeded in six-well plates for cell culture and adherent overnight. The cells were cultured with serum-containing NYPQ. After incubating for 48 h, adherent cells were washed with PBS and digested with 0.25% trypsin; Cell suspension containing 105 cells was reacted with 5 μL Annexin V-FITC and 5 μL propidium iodide (PI) for 15 min at room temperature. After adding 400 μL binding buffer, the cells were analyzed by flow cytometry (C6, BD, USA).
Lumen formation assay
HUVECs (2 × 105/mL) were inoculated into the 96-well plate coated with Matrix (354480, Coring, USA) at a density of 100 μL per well. In addition, 10% control rat serum, 5%, 10%, and 20% serum-containing NYPQ were added to the 96-well plate to incubate with HUVECs for 24 h, respectively. The number of tubes was observed by optical microscope and calculated by Image-Pro Plus 6.0 software.
Statistical analysis
The results were represented as mean ± standard deviation. Graph Prism v8.0 (Graphpad Software, California, USA) and SPSS 20.0 (SPSS, Chicago, USA) were employed to analyze the data. In pairwise comparison between groups, the independent sample T-test was used for those with homogeneous variance, and the Kruskal–Wallis H test was used for those with uneven variance. Differences between multiple groups were analyzed by a one-way analysis of variance. P < 0.05 was accepted to be statistically significant.
Results
Effects of autophagy on lesion volume and endometrial pathology in EMS rats
As shown in Fig. 1A, the lesion volume of the model group increased significantly after treatment, and so did the autophagy inhibitor CQ group, but the lesion volume was decreased significantly in the autophagy agonist RAPA group and was lower than that of the model group (P < 0.01). In addition, we observed the effect of autophagy on the pathological morphology of rat endometrium by H&E staining (Fig. 1B). In the control group, the epithelial structure of the endometrium is columnar, uniform in size, and closely arranged, with dense microvilli, orderly arrangement of interstitial cells, abundant glands, and oval-like nucleus shape. In the endometrium of the model group and CQ group, interstitial cells increased and thickened, microvilli fell off, glands almost disappeared, the endometrium was extremely thin, there were a large number of eosinophils and neutrophils, and the surrounding tissues were rich in blood vessels. In the RAPA group, the epithelial cells were closely arranged with uniform cell size, but some microvilli fell off and glands were rich, and their pathological morphology was better than that in the model group and CQ group.
Effects of chloroquine and rapamycin on the endometriotic lesion in a rat model of EMS. A The volume of the lesion in chloroquine and rapamycin groups after 4 weeks of treatment. B Representative images of endometriotic lesion by H&E staining at magnification × 200 (Scale bars = 100 μm) and × 400 (Scale bars = 50 μm) after chloroquine and rapamycin treatment. The data are presented as the means ± SD. **P < 0.01 vs. model group
Effects of autophagy on microvessel density (MVD) and autophagosomes of endometrial tissue in EMS rats
In this study, CD31 antibody was used to evaluate the MVD of endometrial tissue in EMS rats. Immunohistochemical results showed that the MVD of the model group was significantly higher than that of the control group. While on the basis of the model group, MVD of the CQ group increased, while MVD of the RAPA group decreased (Fig. 2A–B, P < 0.05).
Effects of chloroquine and rapamycin on the microvessel density in a rat model of EMS. A Representative immunohistochemical images of ectopic endometrium from chloroquine and rapamycin treated EMS rats. Magnification: × 20, × 200, and × 400. B microvessel density (mm−2) in endometriotic lesions after chloroquine and rapamycin treatment, analyzed with quantitative analysis of immunohistochemical sections. The data are presented as the means ± SD. **P < 0.01 vs. control group. ▲P < 0.05, ▲▲P < 0.01 vs. model group
Under a transmission electron microscope, the structure of endometrial epithelial cells in the control group was clear, the structure of mitochondria was complete and mitochondria were abundant (Fig. 3A). In the model group, the endometrial epithelial cells were seriously damaged, the mitochondrial membrane was incomplete, the crista structure disappeared, there were more vacuoles in the cytoplasm and the organelles decreased significantly. Moreover, the number of autophagosomes in the model group was less than that in the control group. However, CQ further reduced the number of autophagosomes in the endometrial tissue of model rats, but RAPA facilitated the formation of autophagosomes.
Effects of chloroquine and rapamycin on autophagy of endometriotic lesion in a rat model of EMS. A observation of autophagosome of endometriotic lesion in a rat model of EMS by transmission electron microscope (scale bar = 2 µm). B Representative western blots of VEGF, LC3, and Beclin-1 protein from ectopic endometrium. And, the protein expression levels were quantified using Image J software and normalized to GAPDH protein levels. The data are presented as the means ± SD. **P < 0.01 vs. control group. ▲P < 0.05, ▲▲P < 0.01 vs. model group
Furthermore, we detected the expression levels of VEGF and autophagy-related proteins. As expected, the VEGF levels of EMS model rats were higher than that of the control group, while the expressions of autophagy-related proteins LC3II/I and beclin-1 were inhibited. Similarly, in comparison with the model group, CQ promoted the expression of VEGF but inhibited the expression of LC3-II/I, which were markedly reversed by RAPA treatment (Fig. 3B, P < 0.05).
Effects of NYPQ on lesion volume and endometrial pathology in EMS rats
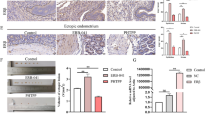
The low-dose, medium-dose, and high-dose of NYPQ and anti-VEGF treatment effectively reduced the lesion volume of model rats (Fig. 4A, P < 0.05). As shown in Fig. 4B, compared with the control group, the model group and L-NYPQ group have abundant blood vessels and many interstitial cells, but microvilli and glands were almost invisible. From M-NYPQ, H-NYPQ, and anti-VEGF groups, we found that epithelial cells were closely arranged, microvilli and glands were increased, and the overall pathological morphology was better than that of the model group; these characteristics were identified by H&E staining analyses.
Effects of NYPQ on the endometriotic lesion in a rat model of EMS. A The volume of the lesion in NYPQ-treated groups after 4 weeks of treatment. B Representative images of endometriotic lesion by H&E staining at magnification × 200 (Scale bars = 100 μm) and × 400 (Scale bars = 50 μm) after NYPQ and VEGF antibody treatment. The data are presented as the means ± SD. **P < 0.01 vs. model group
Effects of NYPQ on MVD, autophagy, and PPARγ/NF-κB signaling pathway of endometrial tissue in EMS rats
Immunohistochemical results showed that M-NYPQ and H-NYPQ can significantly reduce MVD of model rats, although L-NYPQ has a tendency to reduce MVD, the effect is not significant (Fig. 5). Furthermore, NYPQ can also restore the number of autophagosomes lost in the model group to a normal level in a concentration-dependent manner (Fig. 6A–F). Additionally, NYPQ inhibited the protein expression levels of VEGF, NF-κB p65 and promoted the expression levels of LC3 II/I, beclin-1 and PPARγ in a concentration-dependent manner, suggesting that NYPQ may promote autophagy and inhibit angiogenesis by regulating the PPARγ/NF-κB signaling pathway (Fig. 6G).
Effects of NYPQ on the microvessel density in a rat model of EMS. microvessel density (mm−2) in endometriotic lesions after NYPQ treatment, analyzed with quantitative analysis of immunohistochemical sections. The data are presented as the means ± SD. **P < 0.01 vs. control group. ▲P < 0.05, ▲▲P < 0.01 vs. model group
Effects of NYPQ on the autophagy of endometriotic lesion in a rat model of EMS. A observation of autophagosome of endometriotic lesion in a rat model of EMS by transmission electron microscope after NYPQ treatment (scale bar = 2 µm). B Representative western blots of VEGF, LC3, Beclin-1, PPARγ, NF-κB p65 protein from ectopic endometrium after NYPQ treatment. And, the protein expression levels were quantified using Image J software and normalized to GAPDH protein levels. The data are presented as the means ± SD. **P < 0.01 vs. control group. ▲P < 0.05, ▲▲P < 0.01 vs. model group
Effects of serum containing NYPQ on apoptosis and angiogenesis of HUVECs
We collected the serum of rats by gavaged with NYPQ and used it to culture HUVECs. After 24 h of culture, 10% and 20% serum-containing NYPQ could significantly inhibit the viability of HUVECs except that 5% serum-containing NYPQ had no significant effect (Fig. 7A, P < 0.05). After 48 h of culture, serum-containing NYPQ inhibited the activity of HUVECs in a concentration-dependent manner (P < 0.05). Moreover, serum-containing NYPQ dose-dependently elevated the apoptosis rate and suppressed the number of tubes in HUVECs (Fig. 7B–C, P < 0.05). Consistent with the results of in vivo experiments, serum-containing NYPQ inhibited the protein expression levels of VEGF, NF-κB p65 and promoted the expression levels of LC3 II/I, beclin-1, and PPARγ. Besides, serum-containing NYPQ also inhibited the expression of Bcl-2 and promoted the expression of Bax and cleaved caspase-3 in HUVECs (Fig. 7D).
Effects of serum containing NYPQ on the vitality, apoptosis, and lumen formation capacity of human umbilical vein endothelial cells. A Cell counting kit‐8 assay was utilized to measure the cell vitality of HUVECs treated with serum-containing NYPQ. B Cell apoptosis of HUVECs treated with serum-containing NYPQ as determined by flow cytometry. C Vascular formations of human umbilical vein endothelial cells treated with serum-containing NYPQ. D Representative western blots of VEGF, LC3, Beclin-1, PPARγ, NF-κB p65, Bcl-2, Bax, Caspase-3 protein from HUVECs after serum-containing NYPQ treatment. *P < 0.05, **P < 0.01 vs. control group
Discussion
Autophagy is closely related to angiogenesis. Angiogenesis plays a pivotal role in the occurrence and development of EMS, and its essence is the process of proliferation, migration, differentiation and recombination of vascular endothelial cells [16]. Studies have shown that autophagy can regulate the steady-state of endothelial cells by regulating oxidative stress, lipid metabolism, and VEGF receptor [17, 18]. Siracusa et al. found that VEGF expression and MVD in diseased tissues were significantly reduced by injecting autophagy inducer RAPA into EMS rats, indicating that autophagy reduced angiogenesis in EMS [19]. Consistent with the previous study, we also found that RAPA has the effect of reducing lesion volume, MVD, and VEGF expression, while autophagy inhibitor CQ did the opposite effects in this study.
Adopting TCM theories into clinical practice would contribute to the development of a more effective strategy for treating diseases, including endometriosis. NYPQ as a complex prescription was designed based on the syndrome typing of TCM and followed the rules for drug synergism and compatibility [13]. It is widely used to treat adenomyosis currently [13, 15, 20, 21]. It is interesting to explore the effects of NYPQ on other diseases, such as EMS. More importantly, many drugs in NYPQ have the effects of anti-angiogenesis and inhibiting VEGF secretion [22,23,24,25]. Therefore, we speculated that the mechanism of NYPQ in the treatment of EMS may be anti-angiogenesis. VEGF is the key mediator of angiogenesis, and blocking the level of VEGF has been proved to reduce MVD and hinder the growth of lesions [26]. In the current study, NYPQ showed an inhibitory effect on VEGF in vivo and in vitro and decreased MVD and lesion volume, which indicated that NYPQ improved EMS through anti-angiogenesis. A study by Domigan et al. found that VEGF can regulate the autophagy of endothelial cells [27]. Therefore, we focus on the relationship between NYPQ and autophagy in EMS. Both beclin-1 and LC 3 are the hallmark proteins of autophagy. When autophagy is formed, LC3-I will enzymatically decompose a peptide and convert it into LC3-II. Therefore, the level of autophagy can be assessed by comparing LC3-II/LC3-I [28]. Borahay et al. found that one of the mechanisms of Mullerian inhibiting substance treatment of EMS is to induce an increase in LC3-II to promote autophagy in EMS cells [29]. Similarly, in this study, NYPQ promoted the expression of beclin-1 and LC3-II/LC3-I in a concentration-dependent manner, indicating that NYPQ promoted autophagy in EMS.
The PPAR family has three types (α, β/δ, γ), among which PPARγ is found to be involved in the process of anti-angiogenesis. Studies have shown that PPARγ agonists can inhibit endothelial cell proliferation, migration, tube formation and promote cell apoptosis [30, 31]. Cumulative evidence indicated that PPARγ can inhibit pathological angiogenesis by inhibiting NF-κB activation [32, 33]. Angiogenesis in EMS is NF-κB-dependent, which may be due to the fact that a variety of angiogenic factors such as VEGF are affected by NF-κB regulation. A recent study showed that inhibiting the NF-κB pathway in EMS rats can significantly reduce MVD [5]. In addition, the use of NF-κB inhibitors has also proved to be a new strategy for the treatment of EMS [34]. Consistent with the previous studies, we found that NYPQ can promote the expression levels of PPARγ and inhibit the expression levels of NF-κB p65, suggesting that NYPQ may exert a therapeutic effect through the PPARγ/NF-κB pathway.
Autophagy and apoptosis are not independent of each other but affect each other [35]. It has been proved that autophagy in human endometrial cells can promote apoptosis, and its specific mechanism is that autophagy accumulation can increase the ratio of Bax/Bcl-2 and activate caspase-3 to promote apoptosis [36]. In addition, autophagy-induced apoptosis may counteract angiogenesis [37]. In line with these facts, NYPQ promoted the apoptosis of HUVECs but inhibited its tube formation ability. Significantly, we found that NYPQ can regulate autophagy and angiogenesis simultaneously in EMS in this study. However, our research also has some shortcomings. Firstly, the causal relationship between autophagy and angiogenesis has not been clarified. Moreover, NYPQ contains a complex mixture of compounds, requiring further study to identify the active ingredients and molecular mechanisms leading to anti-angiogenesis in vitro and in vivo. In addition, the relationship between serum-containing NYPQ and NYPQ observed in human serum after oral ingestion and metabolism needs to be further verified.
Conclusions
In conclusion, this study found that NYPQ inhibited angiogenesis, promoted apoptosis and autophagy in rats with endometriosis and HUVECs. And, this effect of NYPQ on endometriosis was associated with the activation of the PPARγ/NF-κB pathway. However, the protective effect of NYPQ for endometriosis remains not thoroughly studied. Therefore, further clinical trials are required to confirm its efficacy.
References
Saunders PTK, Horne AW (2021) Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell 184(11):2807–2824. https://doi.org/10.1016/j.cell.2021.04.041
Chiaffarino F, Cipriani S, Ricci E et al (2021) Endometriosis and irritable bowel syndrome: a systematic review and meta-analysis. Arch Gynecol Obstet 303(1):17–25. https://doi.org/10.1007/s00404-020-05797-8
Peng C, Huang Y, Zhou Y (2021) Dydrogesterone in the treatment of endometriosis: evidence mapping and meta-analysis. Arch Gynecol Obstet 304(1):231–252. https://doi.org/10.1007/s00404-020-05900-z
Garcia-Fernandez J, García-Velasco JA (2020) Endometriosis and reproduction: what we have learned. Yale J Biol Med 93(4):571–577
Wang Y, Nicholes K, Shih IM (2020) The origin and pathogenesis of endometriosis. Annu Rev Pathol 15:71–95. https://doi.org/10.1146/annurev-pathmechdis-012419-032654
Yang HL, Mei J, Chang KK, Zhou WJ, Huang LQ, Li MQ (2017) Autophagy in endometriosis. Am J Transl Res 9(11):4707–4725
Zhan L, Yao S, Sun S, Su Q, Li J, Wei B (2018) NLRC5 and autophagy combined as possible predictors in patients with endometriosis. Fertil Steril 110(5):949–956. https://doi.org/10.1016/j.fertnstert.2018.06.028
Ruiz A, Rockfield S, Taran N et al (2016) Effect of hydroxychloroquine and characterization of autophagy in a mouse model of endometriosis. Cell Death Dis 7(1):e2059. https://doi.org/10.1038/cddis.2015.361
Shifren JL, Tseng JF, Zaloudek CJ et al (1996) Ovarian steroid regulation of vascular endothelial growth factor in the human endometrium: implications for angiogenesis during the menstrual cycle and in the pathogenesis of endometriosis. J Clin Endocrinol Metab 81(8):3112–3118. https://doi.org/10.1210/jcem.81.8.8768883
Li G, Lin Y, Zhang Y et al (2022) Endometrial stromal cell ferroptosis promotes angiogenesis in endometriosis. Cell Death Discov 8(1):29. https://doi.org/10.1038/s41420-022-00821-z
Kiani K, Rudzitis-Auth J, Scheuer C et al (2019) Calligonum comosum (Escanbil) extract exerts anti-angiogenic, anti-proliferative and anti-inflammatory effects on endometriotic lesions. J Ethnopharmacol 239:111918. https://doi.org/10.1016/j.jep.2019.111918
Flower A, Liu JP, Lewith G, Little P, Li Q (2012) Chinese herbal medicine for endometriosis. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858
Zhu YP, Wu YP (2013) Effect of Neiyi Prescription of QIU on expressions of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in rats with endometriosis. Chin Arch Tradit Chin Med 31(03):644–646. https://doi.org/10.13193/j.archtcm.2013.03.198.zhuyp.069 (716-717)
Yang HD, Xia MT, Wu YP, Wang XE (2016) Effect of medicated serum of Qiu’s Neiyi Recipe on angiogenic ability of human umbilical vein endothelial cells. Chin Arch Tradit Chin Med 34(10):2545–2547. https://doi.org/10.13193/j.issn.1673-7717.2016.10.068
Ying P, Li H, Jiang Y et al (2021) Qiu’s Neiyi recipe regulates the inflammatory action of adenomyosis in mice via the MAPK signaling pathway. Evid Based Complement Alternat Med 2021:9791498. https://doi.org/10.1155/2021/9791498
Pellicer N, Galliano D, Herraiz S, Bagger YZ, Arce JC, Pellicer A (2021) Use of dopamine agonists to target angiogenesis in women with endometriosis. Hum Reprod 36(4):850–858. https://doi.org/10.1093/humrep/deaa337
Kardideh B, Samimi Z, Norooznezhad F, Kiani S, Mansouri K (2019) Autophagy, cancer and angiogenesis: where is the link? Cell Biosci 9:65. https://doi.org/10.1186/s13578-019-0327-6
Yalaza C, Canacankatan N, Gürses İ, Aytan H, Taşdelen B (2020) Altered VEGF, Bcl-2 and IDH1 expression in patients with adenomyosis. Arch Gynecol Obstet 302(5):1221–1227. https://doi.org/10.1007/s00404-020-05742-9
Siracusa R, D’Amico R, Impellizzeri D et al (2021) Autophagy and mitophagy promotion in a rat model of endometriosis. Int J Mol Sci 22(10):5074. https://doi.org/10.3390/ijms22105074
Wang Q, Chen J (2020) Clinical observation on 48 cases of uterine adenomyosis treated by hongteng sanjie decoction and goserelin. Chin J Tradit Med Sci Technol 27(6):992–993
Ying P, Yang HD, Zhang Y, Lu SY (2020) Clinical observation of qiu’s Neiyi formula combined with GnRH-a after laparoscopic adenomyectomy. Chin J Integr Tradit West Med 40(04):401–405
Dai ZJ, Lu WF, Gao J et al (2013) Anti-angiogenic effect of the total flavonoids in Scutellaria barbata D. Don BMC Complement Altern Med 13:150. https://doi.org/10.1186/1472-6882-13-150
Cao Y, Zhang TT, Xie SW et al (2009) Effects of caulis sargentodoxae granule on expressions of vascular endothelial growth factor and its receptor-2 in rats with endometriosis. Zhong Xi Yi Jie He Xue Bao 7(4):360–365. https://doi.org/10.3736/jcim20090411
Lin J, Wei L, Shen A et al (2013) Hedyotis diffusa Willd extract suppresses Sonic hedgehog signaling leading to the inhibition of colorectal cancer angiogenesis. Int J Oncol 42(2):651–656. https://doi.org/10.3892/ijo.2012.1753
Wan L, Zhao Y, Zhang Q et al (2019) Alkaloid extract of Corydalis yanhusuo inhibits angiogenesis via targeting vascular endothelial growth factor receptor signaling. BMC Complement Altern Med 19(1):359. https://doi.org/10.1186/s12906-019-2739-6
Laschke MW, Menger MD (2012) Anti-angiogenic treatment strategies for the therapy of endometriosis. Hum Reprod Update 18(6):682–702. https://doi.org/10.1093/humupd/dms026
Domigan CK, Warren CM, Antanesian V et al (2015) Autocrine VEGF maintains endothelial survival through regulation of metabolism and autophagy. J Cell Sci 128(12):2236–2248. https://doi.org/10.1242/jcs.163774
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):542–545. https://doi.org/10.4161/auto.4600
Borahay MA, Lu F, Ozpolat B et al (2013) Mullerian inhibiting substance suppresses proliferation and induces apoptosis and autophagy in endometriosis cells in vitro. ISRN Obstet Gynecol 2013:361489. https://doi.org/10.1155/2013/361489
Wagner N, Wagner KD (2020) PPARs and angiogenesis-implications in pathology. Int J Mol Sci 21(16):5723. https://doi.org/10.3390/ijms21165723
Zhang S, Zhuang L, Liu Q et al (2021) Rosiglitazone affects the progression of surgically-induced endometriosis in a rat model. Mol Med Rep 23(1):35. https://doi.org/10.3892/mmr.2020.11673
Kim YW, West XZ, Byzova TV (2013) Inflammation and oxidative stress in angiogenesis and vascular disease. J Mol Med 91(3):323–328. https://doi.org/10.1007/s00109-013-1007-3
Keshamouni VG, Arenberg DA, Reddy RC, Newstead MJ, Anthwal S, Standiford TJ (2005) PPAR-gamma activation inhibits angiogenesis by blocking ELR+CXC chemokine production in non-small cell lung cancer. Neoplasia 7(3):294–301. https://doi.org/10.1593/neo.04601
Celik O, Hascalik S, Elter K, Tagluk ME, Gurates B, Aydin NE (2008) Combating endometriosis by blocking proteasome and nuclear factor-kappaB pathways. Hum Reprod 23(11):2458–2465. https://doi.org/10.1093/humrep/den246
D’Arcy MS (2019) Cell death: a review of the major forms of apoptosis, necrosis and autophagy. Cell Biol Int 43(6):582–592. https://doi.org/10.1002/cbin.11137
Devis-Jauregui L, Eritja N, Davis ML, Matias-Guiu X, Llobet-Navàs D (2021) Autophagy in the physiological endometrium and cancer. Autophagy 17(5):1077–1095. https://doi.org/10.1080/15548627.2020.1752548
Mehrzadi S, Pourhanifeh MH, Mirzaei A, Moradian F, Hosseinzadeh A (2021) An updated review of mechanistic potentials of melatonin against cancer: pivotal roles in angiogenesis, apoptosis, autophagy, endoplasmic reticulum stress and oxidative stress. Cancer Cell Int 21(1):188. https://doi.org/10.1186/s12935-021-01892-1
Funding
This study was supported by the Natural Science Foundation of Zhejiang Province, China (no. LQ20H270003); National Natural Science Foundation of China (no. 82104909); Traditional Chinese Medicine Administration Project of Zhejiang Province, China (no. 2022ZB128); Health Commission Project of Zhejiang Province, China (no. 2020ZY664) and Science and Technology Project of Jinhua, Zhejiang province, China (2019-4-159).
Author information
Authors and Affiliations
Contributions
YG and HDY: initiated this study and designed the experiments. HDY, QFZ, HL, XQX: performed experiments and XLJ analyzed the data. HDY and HL: wrote the manuscript. YG reviewed the manuscript. All authors read the manuscript and approved it.
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, HD., Zhu, QF., Li, H. et al. Effect of Neiyi Prescription of QIU on autophagy and angiogenic ability of endometriosis via the PPARγ/NF-κB signaling pathway. Arch Gynecol Obstet 306, 533–545 (2022). https://doi.org/10.1007/s00404-022-06537-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-022-06537-w