Abstract
Purpose
This retrospective cohort study determined the relative efficacy of blastocyst and cleavage-stage transfers in patients with differing numbers of zygotes.
Methods
A total of 1116 women whose embryo transfers were planned independently of patient characteristics were included. Cleavage-stage (D3) and blastocyst-stage (D5) transfer outcomes were analyzed per number of zygotes. The D5 group included transfer cancellations as the intention-to-treat population. The effect of the embryo transfer date on the clinical outcomes (clinical pregnancy and implantation rates) was analyzed using multivariate logistic regression.
Results
Among the patients, 584 and 532 underwent D3 and D5 embryo transfers, respectively. The clinical pregnancy rates were significantly higher in D5 patients with ≥ 6 zygotes (25.7% vs 48.3%). The multivariate logistic regression analysis for clinical pregnancy did not show significant differences between the blastocyst and cleavage-stage transfers in patients with ≤ 5 zygotes (0.874 [0.635-1.204]). Compared to the cleavage-stage, blastocyst-stage transfers for patients with ≥ 6 zygotes resulted in a three-fold increase in clinical pregnancy rates (3.122 [1.797-5.425]).
Conclusion
Blastocyst transfers were not inferior to cleavage-stage embryo transfers among patients with few zygotes and were preferable for patients with several zygotes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Extending embryo culture to the blastocyst stage has been proposed to improve uterine and embryonic synchrony, select the most competent embryos, and allow elective single embryo transfers to avoid multiple pregnancies that create health risks for both the mother and offspring [1]. Despite concerns about the increased risk of adverse perinatal outcomes [2], blastocyst transfers are as likely to yield healthy babies as cleavage transfers [3,4,5,6], and they also have better obstetric outcomes [7].
The number of cycles needed and time to conception is significantly lower for blastocyst transfers [8]. However, most clinics offer extended culture only when abundant embryos are available because it remains unclear whether in vitro extended culture supports embryo development as effectively as in vivo. According to the European Society for Human Reproduction and Embryology Vienna Consensus, competence and benchmark values of the blastocyst development rate are ≥ 40% and ≥ 60%, respectively [9]. Not every embryo will reach the blastocyst stage, even under optimal culture conditions. Consequently, extended culture transfers are cancelled nearly three times more often than cleavage transfers (odds ratio 2.85; 95% confidence interval [CI] 1.97–4.11) across all age groups [10].
Although no previous studies have elucidated whether cleavage-stage embryo transfers help patients with poor prognosis by eliminating the risk of arrested embryo development in extended culture, cleavage transfers remain the primary preference for patients with few embryos. This study was conducted to answer two questions. First, do cleavage transfers provide a benefit by avoiding transfer cancellations during extended culture? Second, what is the relative efficacy of blastocyst and cleavage transfers for patients with differing numbers of zygotes?
Materials and methods
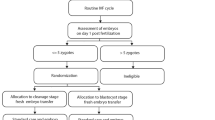
This retrospective cohort study included all infertile couples who attended the Reproductive Endocrinology and Assisted Reproduction Unit at Akdeniz University Hospital in Antalya, Turkey, from January 2018 to December 2019. Our clinic’s digital database was screened for all fresh cycles with at least one mature egg, excluding fertility preservation, preimplantation genetic testing, and natural in vitro fertilization (IVF) cycle treatments. Only the first treatment was included for patients with multiple treatments (n = 1710). Embryo transfers were planned to avoid the weekend shift during the study period. Patients whose oocytes were collected on Monday and Tuesdays routinely received a cleavage-stage embryo transfer (D3), while Wednesday and Thursday pick-up patients routinely received a blastocyst-stage embryo transfer (D5). As Friday pick-up patients were eligible to be assigned to either cleavage (D3) or blastocyst (D5) transfers according to cycle characteristics, they were excluded (n = 217), and only patients whose embryo transfers were planned independently of patient and cycle characteristics were included. Total fertilization failures and cases with all embryos arrested before D3 (n = 39) were also excluded. The embryos from the remaining 1454 patients were assigned to either freeze-all (n = 338) or fresh transfer cycles (n = 1116), with the decision being made by the chief physician at the time of the oocyte pick-up based on common medical issues. Subsequently, the freeze-all cycles were excluded (n = 338) in the comparison of clinical outcomes (n = 1116) between the D3 (n = 584) and D5 (n = 532) groups (Fig. 1).
An intention-to-treat population was defined for the D5 group. Rather than being excluded, patients who did not have any embryos for transfer after extended culture were counted as negative. The clinical pregnancy rate was defined as the number of cycles with a viable heartbeat per number of fresh transfer cycles, with or without embryo transfer. The implantation rate was defined as the number of viable gestational sacs per number of embryos transferred; however, embryos lost in extended culture were calculated as transferred but not implanted. The primary outcome measures were clinical pregnancy and implantation rates. The secondary outcome measures were cryopreservation (CRs) and embryo utilization rates (EURs), which are defined as the sum of the transferred and cryopreserved embryos per total number of zygotes [9].
The patients underwent IVF according to standard stimulation protocols, which involved pituitary downregulation with a gonadotropin-releasing hormone (GnRH) agonist administered in the mid-luteal phase of the prior cycle (long protocol) or a GnRH antagonist starting on the 6th day of stimulation (short protocol). Controlled ovarian stimulation was achieved with a human menopausal gonadotropin and/or recombinant follicle-stimulating hormone. In both groups, human chorionic gonadotropin was administered when the leading follicle(s) reached 17 mm.
In all cases, oocyte retrieval was performed 34–36 h after human chorionic gonadotropin injection. All inseminations were performed by intracytoplasmic sperm injection, and all embryos were cultured in a single-step culture medium (G-TL, Vitrolife, Sweden) under oil in the same benchtop incubator (Miri Multi-Room Incubator, ESCO, Singapore) under 6% CO2 and 5% O2 until the day of transfer. Embryo assessments were performed according to the ALPHA Istanbul Consensus guidelines [11]. For luteal support, all fresh transfer patients received 8% progesterone gel daily (Crinone, Merck, Switzerland) beginning the evening after the oocyte retrieval; this was continued until a negative pregnancy test, or a viable fetus was documented by transvaginal ultrasonography.
The distribution of continuous variables is presented as mean ± standard deviation, and categorical variables are given in percentages. Differences in continuous variables among groups were compared using the Mann–Whitney U test. The Chi-square test was used to analyze implantation rates, CRs, and EURs. The clinical pregnancy outcomes per number of zygotes produced (1 to ≥ 9) were analyzed in total and separately. Multiple logistic regression analysis was performed to calculate the effects of common confounders on clinical outcomes. The relationships between age, number of zygotes, embryo transfer, and utilization rates were analyzed using Spearman’s rank-order correlation coefficients. A p value < 0.05 was considered statistically significant. SPSS for Windows version 23.0 (IBM, USA) was used to perform the statistical analyses.
This study was conducted following ethical standards stipulated in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards and was approved by the Institutional Review Board of Akdeniz University, Faculty of Medicine (approval number: 27012021/71). The requirement of informed consent was waived owing to the retrospective nature of the study.
Results
Women in the D3 group were significantly older than those in the D5 group. The mean number of mature eggs (MIIs) was lower in the D3 group than in the D5 group. Seventy patients in the D5 group did not produce at least one viable embryo that could be transferred, and the transfer cancellation rate for the D5 group was 13.16%. The D3 group had a significantly higher number of embryos transferred per embryo transfer than that of the D5 group. Despite the lower number of mature eggs, the CRs and EURs were significantly higher in the D3 group compared with that of the D5 group (Table 1).
Results of the clinical pregnancy rates per number of zygotes and implantation rates are provided in Table 2. A post hoc subgroup analysis was also conducted on two different numbers of zygotes (≤ 5 and ≥ 6). The clinical pregnancy rates in the D3 group were significantly lower than those in the D5 group for patients with ≥ 6 zygotes (25.7% [29/113] vs 48.3% [72/149]), respectively (Table 2). In total, the D5 group resulted in a 26.04% implantation rate, which was significantly higher than the 20.52% of the D3 group (Table 2). For patients with ≤ 5 zygotes, the transfer cancellation rate was significantly higher than that for patients with ≥ 6 zygotes (16.7% [64/383] vs 4% [6/149], p < 0.001, respectively) in the D5 group. There was a weak positive correlation between age and EUR (rho = 0.095, p < 0.001), and no correlation between age and embryo transfer rate (rho = − 0.035, p = 0.178). However, regarding the number of zygotes, strong (rho = 0.513, p < 0.001) and weak (rho = 0.188, p < 0.001) correlations were found in embryo utilization and transfer rates, respectively.
A logistic regression analysis investigated the effect of the transfer date on clinical pregnancy rates. Age, previous IVF failures, follicle-stimulating hormone, antral follicle counts, cumulus–oocyte complexes, and MIIs were added into the same model as potential confounding factors. There was no significant difference in the clinical pregnancy rates between the blastocyst and cleavage transfers (aOR 0.874 (95% CI) [0.635–1.204]) for patients with ≤ 5 zygotes. Conversely, for patients with ≥ 6 zygotes, blastocyst transfers resulted in a three-fold increase in clinical pregnancy rates (aOR 3.122 (95% CI) [1.797–5.425]) when compared to that of cleavage transfers (Table 3).
Discussion
This study, investigating whether cleavage transfers provide a benefit by avoiding transfer cancellations during extended culture and the relative efficacy of blastocyst and cleavage transfers in relation to the available number of zygotes, found that blastocyst transfers were not inferior to cleavage-stage embryo transfers among patients with few zygotes. Our findings are supported by Haas et al. [12], who reported comparable cumulative pregnancy rate per patient for patients with one or two cleavage-stage embryos, regardless of embryo quality; however, when the pregnancy rates were analyzed per embryo transfer, blastocyst transfers resulted in higher pregnancy rates. Levi-Setti et al. [13] found that the cycle outcomes of patients (female age < 39, ≥ 4 zygotes) were comparable between blastocyst and cleavage transfers. Yang et al. [14], also demonstrated that the implementation of a time-lapse algorithm for cleavage transfers remains inferior to blastocyst transfers. Their findings were attributed to either embryo self-selection during extended culture [15] or better synchronization of the embryo and uterus [16]. In contrast, clinical outcomes of the blastocyst and cleavage transfers for patients with only one viable embryo on day 3 favored cleavage transfers [17], but the poorer outcomes from blastocyst transfers did not appear related to embryo loss in extended culture.
Most recently, De Croo et al. [18] retrospectively analyzed the live birth rates of four different embryo transfer strategies: cleavage transfers for all, blastocyst transfer for patients with > 9 zygotes, blastocyst transfer for patients with > 4 zygotes, and blastocyst transfer for all per oocyte collection cycle. They found that blastocyst transfers resulted in comparable live birth rates per retrieval among all the groups; however, the clinical outcomes regarding the number of zygotes available were not reported. In the present study, the effect of the zygote cohort size on the efficacy of embryo self-selection in extended culture was investigated. The clinical pregnancy rates for the patients with ≥ 6 zygotes after extended culture to the blastocyst stage were higher than those at the cleavage stage. One possible explanation for the poor cleavage transfer outcomes is that the conventional morphological criteria for cleavage embryos might be insufficient for choosing the most competent embryos [19]. In addition to the conventional morphological evaluation, the time-lapse selection of cleavage embryos resulted in lower implantation rates than in blastocyst transfers for patients with > 10 mature oocytes [14]. This result is understandable, given that more potentially competent embryos will be produced when the ovarian reserve and response are increased, thereby increasing the effectiveness of embryo self-selection. Additionally, a larger cohort size might also help reduce oocyte-borne deficiencies, which is the major determinant of embryo developmental competence [20].
Although there is a paucity of data in the literature regarding the impact of ovarian reserve and ovarian response on the chromosomal status of the embryos produced, euploidy rates per embryo are reportedly not affected by the patient’s ovarian reserve and response [21]. However, the probability of finding at least one euploid embryo increases with the number of oocytes retrieved [22] and embryos biopsied [23]. The blastocyst cohort size is also associated with at least one euploid embryo being found [24]. Recent studies support this conclusion, as clinical pregnancy and live birth rates have been shown to increase according to the number of eggs retrieved [25] and oocytes fertilized [26]. Here, the improved implantation and pregnancy rates of blastocyst transfers for patients with a larger zygote cohort could be attributed to the production of more embryos with implantation potential and improved embryo selection properties resulting from extended culture.
The live birth [27] and clinical pregnancy [28] rates of first fresh embryo transfers are higher for blastocyst transfers, consistent with our study’s findings. However, for patients with a poor prognosis who produce a limited number of embryos, the current literature is insufficient to assist practitioners in developing embryo transfer date strategies. Despite the benefits of blastocyst transfers, it is speculated that an in vitro environment is inferior to an in vivo environment. Higher transfer cancellation rates of blastocyst transfers are mainly attributed to suboptimal conditions of the extended in vitro culture [29]. Per the UK National Institute of Health guidelines (NICE Guidelines), cleavage transfers are recommended to avoid transfer cancellations when few embryos are available [30]. The American Society for Reproductive Medicine also recommends avoiding transfer cancellations [10]. Performing a cleavage transfer eliminates the risk of transfer cancellation for those patients. To date, it remains unclear whether these patients benefit from cleavage transfers.
A major challenge of assisted reproduction is the treatment’s cost-effectiveness. Reducing the number of failed embryo transfers, thereby decreasing the time to conception, is critical [31]. To date, clinical pregnancy and live birth rates for blastocyst transfers are higher than those of cleavage transfers for first fresh embryo transfers [32]. Further studies have shown that blastocyst transfers reduce the mean number of cycles and days needed per live birth compared to those of cleavage transfers [8]. According to current knowledge, most aneuploid embryos show arrested development during extended culture [15, 16]. Aneuploidy rates of slow-developing embryos in extended culture are significantly elevated, regardless of patient age [33]. The clinical pregnancy and live birth rates of embryos with delayed blastulation and poor expansion patterns are also lower than those of fully expanded blastocysts [34]. Therefore, performing an extended culture for all patients may decrease the transfer of incompetent embryos, which will reduce costs and shorten the time to conception [35].
Our study demonstrated that cleavage-stage transfers yield higher CRs and EURs when compared to blastocyst transfers. However, the clinical outcome does not seem to be improved at the cleavage stage at first fresh embryo transfers. Therefore, extended culture may also help to reduce the workload and space needed for cryopreservation by reducing the number of embryos cryopreserved when compared to the cleavage stage. However, cumulative pregnancy rates should be evaluated before drawing any firm conclusions on the effects of CRs and EURs.
Increasing evidence demonstrates that the number of oocytes retrieved after controlled ovarian stimulation influences the clinical outcome [36]. In our study, blastocyst transfers were more effective for patients with many zygotes. However, some patients with few zygotes still do not seem to benefit from blastocyst transfers according to our data. Several strategies have been proposed to improve ovarian response, especially for poor responders, but currently no standard management, in terms of protocol and drugs, has been defined [37]. New options to improve the effectiveness and efficacy of IVF treatment, by increasing the oocyte cohort are needed [38] so that more patients can benefit from extended culture. Strategies like duostim (double stimulation in the same ovarian cycle) [39] or embryo pooling might be useful for such patients.
To our knowledge, the following methods used here were a novel approach to the dilemma of cleavage or blastocyst transfers for patients with few embryos. First, transfer date decisions were made independent of patient characteristics, and patients with a low zygote number were also allowed to extend the culture to D5. Second, transfer cancellations in the D5 group were not excluded from the analysis, they were counted as transferred but not pregnant to compare pregnancy rates per patient rather than per embryo transfer.
Blastocyst transfers are associated with a sex imbalance, favoring men and monozygotic twinning [40,41,42]. They are also associated with increased birth weights [43]. However, they are as safe as cleavage transfers in terms of pregnancy complications, obstetric outcomes, and congenital abnormalities [27, 29, 42, 44]. Consequently, patients should be thoroughly counseled about the potential benefits and risks of blastocyst transfers.
This study has several limitations. Although the embryo transfers were planned independently of patient characteristics, the study’s retrospective nature introduces potential bias, as the patient characteristics were not completely matched between groups. The patients receiving blastocyst transfers were younger with more mature oocytes. Furthermore, the ongoing pregnancy and live birth rates were not available for analysis. Further prospective studies are needed to verify these results.
In conclusion, blastocyst transfers provide significantly better results for patients with many zygotes, suggesting that it is preferable to perform extended culture and delay embryo transfer to find or select competent embryos. Moreover, extended culture seems to have no or negligible influence on embryo viability. Patients with few zygotes undergoing either D3 or D5 transfer have similar clinical outcomes. This can guide practitioners and patients in avoiding cleavage transfers that will not be successful.
Availability of data and material
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Code availability
Not applicable.
References
Cutting R (2018) Single embryo transfer for all. Best Pract Res Clin Obstet Gynaecol 53:30–37. https://doi.org/10.1016/j.bpobgyn.2018.07.001
Wang X, Du M, Guan Y, Wang B, Zhang J, Liu Z (2017) Comparative neonatal outcomes in singleton births from blastocyst transfers or cleavage-stage embryo transfers: a systematic review and meta-analysis. Reprod Biol Endocrinol 15(1):36. https://doi.org/10.1186/s12958-017-0255-4
De Vos A, Dos Santos-Ribeiro S, Tournaye H, Verheyen G (2020) Birthweight of singletons born after blastocyst-stage or cleavage-stage transfer: analysis of a data set from three randomized controlled trials. J Assist Reprod Genet 37(1):127–132. https://doi.org/10.1007/s10815-019-01641-4
Li W, Xue X, Zhao W, Ren A, Zhuo W, Shi J (2017) Blastocyst transfer is not associated with increased unfavorable obstetric and perinatal outcomes compared with cleavage-stage embryo transfer. Gynecol Endocrinol 33(11):857–860. https://doi.org/10.1080/09513590.2017.1332175
Marconi N, Raja EA, Bhattacharya S, Maheshwari A (2019) Perinatal outcomes in singleton live births after fresh blastocyst-stage embryo transfer: a retrospective analysis of 67 147 IVF/ICSI cycles. Hum Reprod 34(9):1716–1725. https://doi.org/10.1093/humrep/dez133
Shi W, Zhang W, Li N, Xue X, Liu C, Qu P, Shi J, Huang C (2019) Comparison of perinatal outcomes following blastocyst and cleavage-stage embryo transfer: analysis of 10 years’ data from a single centre. Reprod Biomed Online 38(6):967–978. https://doi.org/10.1016/j.rbmo.2018.12.031
Krishnamoorthy K, Perlman BE, Morelli SS, Greenberg P, Jindal SK, Mcgovern P (2019) Ectopic/heterotopic pregnancy outcomes after blastocyst-stage frozen-thawed embryo transfers compared with cleavage stage: a SART-CORS study. Fertil Steril 112(3):E178. https://doi.org/10.1016/j.fertnstert.2019.07.582
Yin Y, Chen G, Li K, Liao Q, Zhang S, Ma N, Chen J, Zhang Y, Ai J (2017) Propensity score-matched study and meta-analysis of cumulative outcomes of day 2/3 versus day 5/6 embryo transfers. Front Med 11(4):563–569. https://doi.org/10.1007/s11684-017-0535-6
ESHRE Special Interest Group of Embryology and Alpha Scientists in Reproductive Medicine. Electronic address: coticchio.biogenesi@grupposandonato.it (2017) The Vienna consensus: report of an expert meeting on the development of ART laboratory performance indicators. Reprod Biomed Online 35(5):494–510. https://doi.org/10.1016/j.rbmo.2017.06.015
Practice Committee of the American Society for Reproductive Medicine, & Practice Committee of the Society for Assisted Reproductive Technology (2018) Blastocyst culture and transfer in clinically assisted reproduction: a committee opinion. Fertil Steril 110(7):1246–1252. https://doi.org/10.1016/j.fertnstert.2018.09.011
ALPHA Scientists in Reproductive Medicine, ESHRE Special Interest Group of Embryology (2011) Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Reprod Biomed Online 22(6):632–646. https://doi.org/10.1016/j.rbmo.2011.02.001
Haas J, Meriano J, Bassil R, Barzilay E, Casper RF (2019) What is the optimal timing of embryo transfer when there are only one or two embryos at cleavage stage? Gynecol Endocrinol 35(8):665–668. https://doi.org/10.1080/09513590.2019.1580259
Levi-Setti PE, Cirillo F, Smeraldi A, Morenghi E, Mulazzani G, Albani E (2018) No advantage of fresh blastocyst versus cleavage stage embryo transfer in women under the age of 39: a randomized controlled study. J Assist Reprod Genet 35(3):457–465. https://doi.org/10.1007/s10815-017-1092-2
Yang L, Cai S, Zhang S, Kong X, Gu Y, Lu C, Dai J, Gong F, Lu G, Lin G (2018) Single embryo transfer by Day 3 time-lapse selection versus Day 5 conventional morphological selection: a randomized, open-label, non-inferiority trial. Hum Reprod 33(5):869–876. https://doi.org/10.1093/humrep/dey047
Magli MC, Jones GM, Gras L, Gianaroli L, Korman I, Trounson AO (2000) Chromosome mosaicism in day 3 aneuploid embryos that develop to morphologically normal blastocysts in vitro. Hum Reprod 15(8):1781–1786. https://doi.org/10.1093/humrep/15.8.1781
Braude P, Bolton V, Moore S (1988) Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature 332(6163):459–461. https://doi.org/10.1038/332459a0
Xiao JS, Healey M, Talmor A, Vollenhoven B (2019) When only one embryo is available, is it better to transfer on Day 3 or to grow on? Reprod Biomed Online 39(6):916–923. https://doi.org/10.1016/j.rbmo.2019.08.003
De Croo I, De Sutter P, Tilleman K (2020) A stepwise approach to move from a cleavage-stage to a blastocyst-stage transfer policy for all patients in the IVF clinic. Human Reproduction Open 2020(3):hoaa34. https://doi.org/10.1093/hropen/hoaa034
Milki AA, Hinckley MD, Gebhardt J, Dasig D, Westphal LM, Behr B (2002) Accuracy of day 3 criteria for selecting the best embryos. Fertil Steril 77(6):1191–1195. https://doi.org/10.1016/s0015-0282(02)03104-7
Keefe D, Kumar M, Kalmbach K (2015) Oocyte competency is the key to embryo potential. Fertil Steril 103(2):317–322. https://doi.org/10.1016/j.fertnstert.2014.12.115
Karlıkaya G, Boynukalin FK, Gultomruk M, Kavrut M, Abalı R, Demir B, Ecemis S, Yarkiner Z, Bahceci M (2021) Euploidy rates of embryos in young patients with good and low prognosis according to the POSEIDON criteria. Reprod Biomed Online 42(4):733–741. https://doi.org/10.1016/j.rbmo.2021.01.001
Kahraman S, Çil AP, Oğur C, Semiz A, Yilanlioglu C (2016) Probability of finding at least one euploid embryo and the euploidy rate according to the number of retrieved oocytes and female age using FISH and array CGH. J Reprod Biotechnol Fertil 5:2058915816653277. https://doi.org/10.1177/2058915816653277
Tulay P, Gultomruk M, Findikli N, Bahceci M (2016) Number of embryos biopsied as a predictive indicator for the outcome of preimplantation genetic diagnosis by fluorescence in situ hybridisation in translocation cases. Zygote 24(1):107–114. https://doi.org/10.1017/S0967199414000793
Esteves SC, Carvalho JF, Bento FC, Santos J (2019) A novel predictive model to estimate the number of mature oocytes required for obtaining at least one euploid blastocyst for transfer in couples undergoing in vitro fertilization/intracytoplasmic sperm injection: the ART calculator. Front Endocrinol 10:99. https://doi.org/10.3389/fendo.2019.00099
Sunkara SK, Rittenberg V, Raine-Fenning N, Bhattacharya S, Zamora J, Coomarasamy A (2011) Association between the number of eggs and live birth in IVF treatment: an analysis of 400,135 treatment cycles. Hum Reprod 26(7):1768–1774. https://doi.org/10.1093/humrep/der106
Smeltzer S, Acharya K, Truong T, Pieper C, Muasher S (2019) Clinical pregnancy (CP) and live birth (LB) increase significantly with each additional fertilized oocyte up to nine, and CP and LB decline after that: an analysis of 15,803 first fresh in vitro fertilization cycles from the Society for Assisted Reproductive Technology registry. Fertil Steril 112(3):520-526.e1. https://doi.org/10.1016/j.fertnstert.2019.04.023
Zhu Q, Zhu J, Wang Y, Wang B, Wang N, Yin M, Zhang S, Lyu Q, Kuang Y (2019) Live birth rate and neonatal outcome following cleavage-stage embryo transfer versus blastocyst transfer using the freeze-all strategy. Reprod Biomed Online 38(6):892–900. https://doi.org/10.1016/j.rbmo.2018.12.034
Harlev A, Pariente M, Har-Vardi I, Friger M, Levitas E (2020) Pregnancy outcomes of fresh IVF conceived pregnancies after embryo transfer at different stages of early embryonic development. J Matern Fetal Neonatal Med. https://doi.org/10.1080/14767058.2020.1716215
Martins WP, Nastri CO, Rienzi L, van der Poel SZ, Racowsky GC (2017) Blastocyst vs cleavage-stage embryo transfer: systematic review and meta-analysis of reproductive outcomes. Ultrasound Obstet Gynecol 49(5):583–591. https://doi.org/10.1002/uog.17327
National Collaborating Centre for Women’s and Children’s Health (UK) (2013) Fertility: assessment and treatment for people with fertility problems. Royal College of Obstetricians & Gynaecologists, London
Adriaenssens T, Van Vaerenbergh I, Coucke W, Segers I, Verheyen G, Anckaert E, De Vos M, Smitz J (2019) Cumulus–corona gene expression analysis combined with morphological embryo scoring in single embryo transfer cycles increases live birth after fresh transfer and decreases time to pregnancy. J Assist Reprod Genet 36(3):433–443. https://doi.org/10.1007/s10815-018-01398-2
Glujovsky D, Farquhar C, Quinteiro Retamar AM, Alvarez Sedo CR, Blake D (2016) Cleavage stage versus blastocyst stage embryo transfer in assisted reproductive technology. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD002118.pub5
Irani M, Zaninovic N, Canon C, O’Neill C, Gunnala V, Zhan Q, Palermo G, Reichman D, Rosenwaks Z (2018) A rationale for biopsying embryos reaching the morula stage on Day 6 in women undergoing preimplantation genetic testing for aneuploidy. Hum Reprod 33(5):935–941. https://doi.org/10.1093/humrep/dey053
Desai N, Ploskonka S, Goodman L, Attaran M, Goldberg JM, Austin C, Falcone T (2016) Delayed blastulation, multinucleation, and expansion grade are independently associated with live-birth rates in frozen blastocyst transfer cycles. Fertil Steril 106(6):1370–1378. https://doi.org/10.1016/j.fertnstert.2016.07.1095
Neal SA, Morin SJ, Franasiak JM, Goodman LR, Juneau CR, Forman EJ, Werner MD, Scott RT Jr (2018) Preimplantation genetic testing for aneuploidy is cost-effective, shortens treatment time, and reduces the risk of failed embryo transfer and clinical miscarriage. Fertil Steril 110(5):896–904. https://doi.org/10.1016/j.fertnstert.2018.06.021
Drakopoulos P, Blockeel C, Stoop D, Camus M, deVos M, Tournaye H, Polyzos NP (2016) Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 31:370–376. https://doi.org/10.1093/humre3p/dev316
Patrizio P, Vaiarelli A, Levi Setti PE, Tobler KJ, Shoham G, Leong M, Shoham Z (2015) How to define, diagnose and treat poor responders? Responses from a worldwide survey of IVF clinics. Reprod Biomed Online 30:581–592. https://doi.org/10.1016/j.rbmo.2015.03.002
Vaiarelli A, Cimadomo D, Ubaldi N, Rienzi L, Ubaldi FM (2018) What is new in the management of poor ovarian response in IVF? Curr Opin Obstet Gynecol 30(3):155–162. https://doi.org/10.1097/GCO.0000000000000452
Kuang Y, Chen Q, Hong Q, Lyu Q, Ai A, Fu Y, Shoham Z (2014) Double stimulations during the follicular and luteal phases of poor responders in IVF/ICSI programmes (Shanghai protocol). Reprod Biomed Online 29:684–691. https://doi.org/10.1016/j.rbmo.2014.08.009
Ding J, Yin T, Zhang Y, Zhou D, Yang J (2018) The effect of blastocyst transfer on newborn sex ratio and monozygotic twinning rate: an updated systematic review and meta-analysis. Reprod Biomed Online 37(3):292–303. https://doi.org/10.1016/j.rbmo.2018.05.015
Hattori H, Kitamura A, Takahashi F, Kobayashi N, Sato A, Miyauchi N, Nishigori H, Mizuno S, Sakurai K, Ishikuro M, Obara T, Tatsuta N, Nishijima I, Fujiwara I, Kuriyama S, Metoki H, Yaegashi N, Nakai K, Arima T, Japan Environment and Children’s Study Group (2019) The risk of secondary sex ratio imbalance and increased monozygotic twinning after blastocyst transfer: data from the Japan Environment and Children’s Study. Reprod Biol Endocrinol 17(1):27. https://doi.org/10.1186/s12958-019-0471-1
Wang S, Chen L, Fang J, Jiang W, Zhang N (2019) Comparison of the pregnancy and obstetric outcomes between single cleavage-stage embryo transfer and single blastocyst transfer by time-lapse selection of embryos. Gynecol Endocrinol 35(9):792–795. https://doi.org/10.1080/09513590.2019.1594762
Litzky JF, Boulet SL, Esfandiari N, Zhang Y, Kissin DM, Theiler RN, Marsit CJ (2018) Birthweight in infants conceived through in vitro fertilization following blastocyst or cleavage-stage embryo transfer: a national registry study. J Assist Reprod Genet 35(6):1027–1037. https://doi.org/10.1007/s10815-018-1168-7
Zhu Q, Wang N, Wang B, Wang Y, Kuang Y (2018) The risk of birth defects among children born after vitrified blastocyst transfers and those born after fresh and vitrified cleavage-stage embryo transfers. Arch Gynecol Obstet 298(4):833–840. https://doi.org/10.1007/s00404-018-4870-x
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SO: Conceptualization, formal analysis, methodology, writing. MS: Formal analysis, supervision, writing. MC: Methodology, supervision, visualization.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
Approved by the Institutional Review Board of Akdeniz University, Faculty of Medicine (Approval Number: 27012021/71).
Consent to participate
Approved.
Consent for publication
Approved.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Dirican, E.K., Olgan, S., Sakinci, M. et al. Blastocyst versus cleavage transfers: who benefits?. Arch Gynecol Obstet 305, 749–756 (2022). https://doi.org/10.1007/s00404-021-06224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-021-06224-2