Abstract
Purpose
Curcumin (Cur), a yellow-colored dietary flavor from the plant (Curcuma longa), has been demonstrated to potentially resist diverse diseases, including ovarian cancer, but drug resistance becomes a major limitation of its success clinically. The key molecule or mechanism associated with curcumin resistance in ovarian cancer still remains unclear. The aim of our study was to investigate the effects of curcumin on autophagy in ovarian cancer cells and elucidate the underlying mechanism.
Methods
In our study, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), EdU proliferation assay and colony-forming assay were used to assess cell viability. Apoptosis was detected by western blot and flow cytometric analysis of apoptosis. Autophagy was defined by both electron microscopy and immunofluorescence staining markers such as microtubule-associated protein 1 light chain 3 (LC3). Plasmid construction and shRNA transfection helped us to confirm the function of curcumin.
Results
Curcumin reduced cell viability and induced apoptotic cell death by MTT assay in human ovarian cancer cell lines SK-OV-3 and A2780 significantly. Electron microscopy, western blot and immunofluorescence staining proved that curcumin could induce protective autophagy. Moreover, treatment with autophagy-specific inhibitors or stable knockdown of LC3B by shRNA could markedly enhance curcumin-induced apoptosis. Finally, the cells transiently transfected with AKT1 overexpression plasmid demonstrated that autophagy had a direct relationship with the AKT/mTOR/p70S6K pathway.
Conclusions
Curcumin can induce protective autophagy of human ovarian cancer cells by inhibiting the AKT/mTOR/p70S6K pathway, indicating the synergistic effects of curcumin and autophagy inhibition as a possible strategy to overcome the limits of current therapies in the eradication of epithelial ovarian cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ovarian cancer is one of the most deadly gynecological malignant tumors due to the difficulties in early detection [1,2,3]. It is estimated that nearly 14,070 women are expected to die of ovarian cancer in the United States in 2018 [2]. Standard treatment for ovarian cancer contains cytoreductive surgery and chemotherapeutic drugs [4]. Although adjuvant and neoadjuvant chemotherapy with surgery are performed, the recurrence rate is still over 85% [4]. The success of subsequent chemotherapy is limited due to the continuous development of drug resistance. Therefore, it is imperative to develop more targeted molecular therapies for ovarian cancer.
Curcumin, a common Chinese medicine, a yellow-colored dietary flavor from the plant (Curcuma longa), has been reported to treat ovarian cancer effectively [5] and put into several clinical trials [6,7,8] owing to its apoptosis effect [9,10,11]. Although several studies reported that curcumin could induce apoptotic cell death to perform anti-cancer effect, drug resistance of curcumin confined its clinical use in the treatment of ovarian cancer patients alone [12,13,14]. Recent studies have shown that curcumin-induced drug resistance is closely related to autophagy [6, 8, 15]. However, its possible effects on ovarian cancer cells are still unknown.
Autophagy is mainly to maintain the homeostasis within cells [16]. Increasing evidences have shown that curcumin-induced autophagy is not only a pro-death signal [17,18,19], but also an adaptation to stress to avoid cell death and cell apoptosis [20], resulting in resistance to chemotherapy. Majority of the studies suggest that drug resistance in ovarian cancer is associated with protective autophagy [21, 22]. Furthermore, curcumin has been identified as an autophagy inducer in various cancer studies, such as melanoma [23], gliomas [17], breast cancer [24] and oral cancer [18]. Therefore, inhibition of autophagy may be a promising way to overcome the barrier of curcumin resistance to ovarian cancer. Autophagy inhibitors, such as chloroquine (CQ), have been shown to enhance the anti-tumor effects when combined with chemotherapy drugs and radiation, suggesting its vital role in anti-cancer therapy [25, 26]. Therefore, the autophagy inhibitor is promising to resolve the drug resistance of curcumin to ovarian cancer [27]. However, the underlying mechanism is still unknown.
We herein focused on the effects of curcumin on autophagy in ovarian cancer cells and tried to elucidate the underlying mechanism. Thus, our findings suggest that protective autophagy plays a key role on curcumin resistance. Inhibition of autophagy may provide a new perspective for clinical intervention to ovarian cancer with respect to curcumin therapy.
Materials and methods
Cell lines and culture
The human ovarian cancer cell lines, SK-OV-3 and A2780, were acquired from the American Type Culture Collection (ATCC, Manassas, VA, USA). HO-8910 and HEK-293T cell lines were purchased from the Chinese Academy of Sciences (Shanghai, China). Both A2780 and HO-8910 cells were cultured in RPMI-1640 medium (GE Healthcare Life Sciences, HyClone Laboratories, Logan, UT, USA). SK-OV-3 and HEK-293T cells were cultured in McCoy’s 5A medium and DMEM(GE Healthcare Life Sciences, HyClone Laboratories, Logan, UT, USA), respectively. All media contained 10% FBS (Biological Industries, Kibbutz Beit Haemek, Israel). They were maintained at 37 °C in a humidified atmosphere containing 5% CO2.
Reagents and antibodies
Curcumin, dimethyl sulfoxide (DMSO) and MTT were purchased from Sigma-Aldrich (St. Louis, MO, USA). CQ was obtained from Abcam (Cambridge, UK). Curcumin and CQ were prepared at a concentration of 10 mmol/l stock solution. The used primary antibodies: the antibodies of GAPDH (5174), Caspase-9 (9508), Cleaved Caspase-9 (Asp330) (D2D4), PARP (poly ADP-ribose polymerase) (9542), Cleaved PARP (Asp214) (D64E10), LC3B (3868), Atg3 (3415), Beclin 1 (3495), p-AKT (4060), AKT (4691), p-mTOR (5536), mTOR (2983), p-p70S6K (9234), p-4EBP1 (2855), HRP-linked anti-rabbit IgG, and HRP-linked anti-mouse IgG antibodies were all purchased from Cell Signaling Technology (Danvers, MA, USA). Crystal violet staining solution and RIPA lysis buffer were purchased from Beyotime Biotechnology (Shanghai, China). LY294002 (10 mM) was acquired from Selleck Chemicals (HOU Houston, Texas).
Cell viability and proliferation assays
Cell viabilities of SK-OV-3, A2780 and HO-8910 cells were assessed with MTT assay. The cells were seeded at a density of (3–5) × 103 cells/well on 96-well plates overnight, and then treated with different concentrations (0, 10, 20, 40 μM) of curcumin for 24, 48 and 72 h. At indicated time-points, cells were incubated with MTT solution (5 mg/ml) for another 4 h at 37 °C. The formazan product was dissolved in 100 μl DMSO and measured at 490 nm using a microplate reader (Infinite M200 PRO, Bio-Rad Laboratories, Hercules, CA, USA). Proliferation was examined using the 5-ethynyl-2′-deoxyuridine (EdU) incorporation (C103103, Ribobio, Guangzhou, China) assay, which was performed according to the manufacturer’s protocol, examining the cells under a fluorescence microscope (CKX41, Olympus, Tokyo, Japan). Three independent experiments were performed for statistical analysis.
Colony-forming assay
Colony formation assay was used to conduct an investigation of the long-term inhibitory effect of curcumin on SK-OV-3 and A2780 cell proliferation. SK-OV-3 and A2780 cells were seeded (1 × 103 cells/well) in six well plates, treated with different concentrations (0, 10, 20, 40 μM for SKOV-3 cell line and 0, 7.5, 15, 30 μM for A2780 cell line) of curcumin for 48 h, and then were planned to grow for 10 days. Subsequently, the cells were washed with phosphate buffer saline (PBS) and fixed in 4% paraformaldehyde solution for 15 min and stained with 1% crystal violet solution. Colonies (> 50 cells) were counted under an optical microscope. Three independent experiments were performed.
Flow cytometric analysis of apoptosis
Flow cytometry analysis was utilized to measure the levels of cellular apoptosis. SK-OV-3 and A2780 cells were seeded (1 × 105 cells/well) in six well plates, treated with different concentrations (0, 10, 20, 40 μM for SKOV-3 cell line and 0, 7.5, 15, 30 μM for A2780 cell line) of curcumin for 48 h. Then, the cells were harvested and resuspended in 100-μl binding buffer to achieve a concentration of 1 × 106 cells/ml. SK-OV-3 and A2780 cells were then stained with 5-μl Annexin V-FITC and 10-μl propidium iodide (PI) (20 μg/ml) (BD bioscience) following the instructions from the manufacturer and analyzed by a flow cytometry (Novocyte, ACEA). The data were analyzed by Flowjo software (Tree Star, Ashland, OR, USA). Three independent experiments were performed for statistical analysis.
Electron microscopy
Electron microscopy was performed to detect the induction of autophagy in EOC cells. SK-OV-3 and A2780 cells were treated with 15 μM curcumin for 48 h. SK-OV-3 and A2780 cells and these control groups were harvested by trypsinization and then fixed with ice-cold fixative containing 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 M cacodylate buffer. Next, post-fixation was done in 1% osmium tetroxide buffer (OsO4) for 2 h, washed twice with PBS and a series of dehydration was carried out by gradient concentration of ethanol ranging from 70 to 100%. The samples were embedded in epoxy resin and cut into thin sections stained with saturated uranyl acetate and lead citrate. We observed the representative areas for ultrathin sectioning with a Hitachi 7700 electron microscope (Japan) at 120 kV.
Immunofluorescence staining
Immunofluorescence staining was used to detect autophagosome. SK-OV-3 cells were plated in 24-well plates and treated with 40-μM curcumin for 48 h. The SK-OV-3 cells were fixed with 4% paraformaldehyde for 15 min at room temperature and permeabilized with 0.2% Triton X-100 (200 μl) in PBS for 10 min. Then, the SK-OV-3 cells were blocked with indicated anti-LC3B antibody (1:200) overnight at 4 °C, followed by incubation with related secondary antibody (1:50) for 1 h. After that, the SK-OV-3 cells were stained with 4,6-diamidino-2-phenylindole (DAPI) for 5 min in dark at room temperature. The fluorescence images were observed by the above-mentioned fluorescence microscope. Three independent experiments were performed for statistical analysis.
Western blot
Curcumin-treated SK-OV-3 and A2780 cells were harvested and lysed in a mixed buffer contained RIPA, NaF and PMSF (100:1:1). The supernatants were separated, mixed with loading buffer (5 ×), and boiled for 5 min. The equal amounts of total proteins from each sample were separated by 12% SDS-PAGE through electrophoresis and transferred onto PVDF membranes (Immobilon-P; Millipore, Bedford, MA, USA). After blocking for 2 h using 5% non-fat milk, the stripes were incubated with the indicated primary antibodies (1:1000) overnight at 4 °C, followed by incubation with HRP-linked anti-rabbit or anti-mouse IgG secondary antibodies (1:3000) at room temperature for 2 h. A chemiluminescent substrate (ECL, Amersham Biosciences, UK) was used to detect the immunoreactive bands by ImageQuent LAS 4000 (GE Healthcare Life Sciences, Logan, UT, USA). The gray values were analyzed by ImageJ software. Three independent experiments were performed for statistical analysis.
Plasmid construction, transient transfection of plasmids, lentivirus production and shRNA transfection
The SK-OV-3 and A2780 ovarian cancer cells were transiently transfected with AKT1 overexpression plasmid for 4 h using Lipofectamine 2000 (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer’s instruction. Control plasmid served as a control. Human AKT1 cDNA was purchased from the Genechem Company (Shanghai, China). After transfection, the cells were exposed to curcumin for 48 h and lysed for a western blot assay. LC3B shRNA in pLKO.1-puro was obtained in Sigma-Aldrich. The CDS (Coding sequence) of LC3B was amplified by PCR and cloned into pLenti-C-Myc-DDK-IRES-Puro (PCMV) vector (Origene, USA). Lentivirus were produced in HEK-293T cells packaged with psPAX2 and pMD 2.G. SK-OV-3 and A2780 cells were infected with lentivirus for 24 h and then selected for 7 days in medium containing 2 μg/ml puromycin (Merck Millipore, USA) to acquire stable expression cells, establishing SK-OV-3-plko.1-shLC3B and SK-OV-3-plko.1-NC and A2780-plko.1-shLC3B, A2780-plko.1-NC cell lines. Sequences of shRNAs (F:CCGGCGCTTACAGCTCAATGCTAATCTCGAGATTAGCATTGAGCTGTAAGCGTTTTTG; R:AATTCAAAAACGCTTACAGCTCAATGCTAATCTCGAGATTAGCATTGAGCTGTAAGCG).
Statistical analysis
The Student’s t test was performed to determine significance by using SPSS 23.0 and Graphpad Prism 5 software. Statistical significance was determined at P < 0.05. All the experiments were performed in triplicate.
Results
Curcumin repressed proliferation of ovarian cancer cells
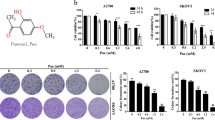
To investigate the anti-cancer activity of curcumin in EOC cells, we examined the effect of curcumin on cell viability in three ovarian cancer cell lines (SK-OV-3, A2780 and HO-8910 cell lines). The cells were, respectively, treated with different concentrations (0, 5, 10, 20, 40, 80 μM) of curcumin for 24, 48 and 72 h. Then, the cell viability was estimated by using the MTT assay. Our study showed that curcumin could reduce cell viability in all three ovarian cancer cell lines in a dose-dependent and time-dependent manner, as shown in Fig. 1a. The IC50 values of curcumin were 41.05, 31.84 and 30.29 μM for SKOV-3 cell line at 24, 48 and 72 h, respectively; 18.37, 13.26 and 13.83 μM for A2780 cell line at 24, 48 and 72 h, respectively; and 18.93, 14.46, and 13.92 μM for HO-8910 cell line at 24, 48 and 72 h, respectively. Furthermore, we utilize the colony formation assay to observe the long-term inhibitory effect of curcumin on EOC cell proliferation. Over 10 μM, curcumin suppressed colony-forming ability of EOC cells significantly for SKOV-3 cell line (Fig. 1b). To test whether curcumin had a direct impact on cell proliferation, we used EdU staining assay to find that treatment with curcumin for 48 h significantly inhibited EdU uptake rate in a dose-dependent manner in SKOV-3 cells (Fig. 1c). Collectively, these results suggested that curcumin might be a potential anti-cancer agent for all three ovarian cancer cell lines.
Curcumin repressed proliferation of ovarian cancer cells. a A MTT assay was performed to evaluate cell viability in SK-OV-3, A2780 and HO8910 human ovarian cancer cell lines treated with different concentrations (0, 10, 20, 40 μM) of curcumin for 24, 48 and 72 h. b Inhibition of colony formation ability by curcumin in human EOC cells. Cells were treated with the indicated concentrations of curcumin for 48 h and then incubated in normal medium for the remaining 10 days. Representative Giemsa staining pictures of SK-OV-3 and A2780 cells formed colonies are shown on the left. Colony number ratio was shown on the right side (c). EdU proliferation of SK-OV-3 cells treated with curcumin at 0, 10, 20 and 40 μM, respectively, for 48 h. SK-OV-3 cells were treated as indicated and stained for EdU incorporation (red) or DAPI (blue) to highlight nuclei, and the red and blue images were merged. EdU positive SK-OV-3 cells ratio was shown on the right side (magnification, × 100). All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
Curcumin stimulated apoptosis in ovarian cancer cells
Since curcumin was previously reported to induce apoptosis-dependent cell death in different cancer cell lines, we next tested whether the cytotoxicity of curcumin is associated with apoptosis in EOC cells. To study the underlying mechanism by which curcumin inhibited cell growth in ovarian cancer cells, apoptosis assay was performed by flow cytometry. We detected that 40-μM curcumin could significantly induce apoptosis in SK-OV-3 cell line, while 30-μM curcumin triggered apoptosis in A2780 cell line (Fig. 2a). We next analyzed the effects of curcumin on activity of caspases and PARP. Western blotting assay showed that curcumin activated Caspase-9 and PARP (Fig. 2b). Taken together, these results suggested that curcumin-induced cell death might be dependent of apoptosis by caspases activation in EOC cells.
Curcumin stimulated apoptosis in ovarian cancer cells. a Flow cytometric analysis of PI (Y axis)-Annexin V-fluorescein isothiocyanate (FITC) (X axis) was used to quantify apoptosis in SK-OV-3 and A2780 cells. Percentage of apoptotic SK-OV-3 and A2780 cells were shown on the right side. b Western blot analysis revealed that curcumin could induce apoptosis in SK-OV-3 and A2780 cells. (cleaved-Capase-9 and cleaved-PARP proteins). All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
Curcumin induced autophagy in ovarian cancer cells
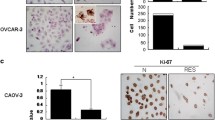
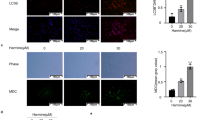
Although several studies reported that curcumin could perform anti-cancer effect, drug resistance of curcumin confined its clinical use in the treatment of ovarian cancer patients alone and was closely associated with autophagy. To confirm the effects of curcumin on autophagy, we used electron microscopy to observe the ultrastructure of A2780 cells. The results indicated the presence of a large number of autophagic vesicles in curcumin-treated A2780 cells as compared to the control A2780 cells (Fig. 3a). In addition, increased formation/expression of LC3B-I/II in response to curcumin was confirmed by immunofluorescent staining of SK-OV-3 cells in culture. SK-OV-3 cells treated with 40-μM curcumin exhibited increasing puncta formation and fluorescence intensity of LC3B compared with the control group significantly, suggesting that treatment of curcumin resulted in autophagy of SK-OV-3 cells (Fig. 3b). Given that curcumin could induce autophagy, we utilized western blot analysis to evaluate expression levels of certain proteins related to autophagy. Exposure to curcumin dramatically increased the expression levels of LC3B-II in a dose-dependent manner (Fig. 3c). Besides, curcumin increased the expression of Atg3 and Beclin1 in a concentration-dependent manner (Fig. 3d). Taken together, these results indicated that curcumin induced autophagy in ovarian cancer cells.
Curcumin induced autophagy in ovarian cancer cells. a Cells were treated with and without curcumin for 48 h and observed by electron microscopy. Black arrows pointed to the autophagosomes (magnification, × 8000). b Immunofluorescence staining shows immunoreactivity of LC3B-I/II in SK-OV-3 cells on the bottom. DAPI is the nuclear marker. Percentage of LC3B positive cells was shown on the top. Scale bar 50 μm. c SK-OV-3 and A2780 cells were treated with curcumin as described above. Western blotting assessed the protein levels of LC3B. Fold change of LC3B was shown on the top. d The protein levels of Atg3, Beclin-1, and p62 were determined by western blotting. All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
CQ–Curcumin combination therapy attenuated the viability of ovarian cancer cells
Given that curcumin had been confirmed to induce autophagy in SK-OV-3 and A2780 cancer cell lines, we next explored the role of curcumin-induced autophagy on SK-OV-3 and A2780 cell proliferation. Furthermore, we also observed the effect of combination of curcumin and autophagy inhibitor on cell viability in SK-OV-3 and A2780 cell lines. The cell viability examined by the MTT assay was significantly decreased in the combined treatment of CQ and curcumin, compared with curcumin treatment alone in SK-OV-3 and A2780 ovarian cancer cell lines (Fig. 4a). CQ has been widely used to inhibit lysosomal proteases and autophagosome–lysosomal fusion events. The result suggests that autophagy inhibitor enhances curcumin-induced apoptosis. Moreover, the apoptosis assay confirmed that CQ–curcumin-combined group had more apoptotic cell death than curcumin group (Fig. 4b). To determine whether CQ–curcumin combination has a direct impact on cell proliferation, we found that the combined treatment of CQ and curcumin significantly inhibited EdU uptake rate, compared with curcumin treatment alone in SK-OV-3 ovarian cancer cell line (Fig. 4c, d) [28]. Taken together, these results indicated that blockage of autophagy aggravated curcumin-induced apoptosis. Since autophagy is thought to act as a cell-survival pathway in cancer, CQ could be used in combination with curcumin drug to treat ovarian cancer
CQ–curcumin combination therapy attenuated the viability of ovarian cancer cells. a SK-OV-3 and A2780 cells were pretreated with CQ for 2 h before being treated with curcumin for the indicated time. The cell viability was measured using the MTT assay. b Flow cytometric analysis of PI-Annexin-V was used to quantify apoptosis in SK-OV-3 and A2780 cells after treatment as described in Materials and Methods. c EdU proliferation of SK-OV-3 cells exposed to curcumin with or without 10 μM CQ for 48 h as indicated as above. d Quantitative analysis of EdU proliferation in SK-OV-3 cells after curcumin treatment with or without 10 μM CQ for 48 h. All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
Suppression of LC3B enhanced curcumin-induced growth inhibition and apoptosis in ovarian cancer cells
To confirm the role of LC3B on autophagy in the curcumin-treated human ovarian cancer cells, we silenced the expression of LC3B and examined the modulation of LC3B expression by curcumin treatment combined with shRNA-induced LC3B knockdown in SK-OV-3 and A2780 ovarian cancer cells. As demonstrated in Fig. 5a, western blot assay showed that transfection with shLC3B blocked the autophagic effect of curcumin-treated SKOV3 and A2780 cells. LC3B protein expression levels were significantly increased in SK-OV-3 and A2780 cells treated with LC3B shRNAs and curcumin in comparison to cells treated with LC3B shRNAs alone. The result suggested that curcumin could induce autophagy in human ovarian cancer cell lines. Additionally, to assess the effects of LC3B shRNAs on curcumin-mediated cell apoptosis, we found that the protein expression of cleaved Caspase-9 and cleaved PARP increased consistently (Fig. 5b), demonstrating that combination group of LC3B shRNAs and curcumin had more apoptotic cell death than curcumin group. Collectively, these findings suggested that downregulation of LC3B enhanced the sensitivity of SK-OV-3 and A2780 cells toward curcumin treatment.
Suppression of LC3B enhanced curcumin-induced growth inhibitory effect and apoptosis in ovarian cancer cells. a LC3B knockdown sublines established stably to blocking autophagy (SK-OV3-shLC3B and A2780-shLC3B) and normal control sublines that contained a scrambled shRNA were treated with curcumin for 48 h. Then, cell lysates were collected and probed with antibodies against LC3B. Quantitative analysis of the expression levels of LC3B-II in EOC cells were shown on the right side. b Western blot analysis revealed that curcumin could induce apoptosis in SK-OV3-shLC3B and A2780-shLC3B cell lines. (cleaved-Capase-9 and cleaved-PARP proteins). All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
Curcumin inhibited the activity of AKT/mTOR/p70S6K signaling pathway
To further investigate the mechanisms of curcumin in increasing apoptosis and protective autophagy effect, we next explored whether AKT/mTOR signaling pathway was involved. As shown in Fig. 6a, the phosphorylation levels of AKT, mTOR as well as downstream factors of mTOR, such as p70S6K and 4E-BP1, were markedly decreased in the ovarian cancer cells treated with different concentrations (0, 10, 20, 40 μM for SKOV-3 cell line and 0, 7.5, 15, 30 μM for A2780 cell line) of curcumin, respectively. These data suggested that the curcumin-induced protective effects on ovarian cancer cells might depend on inhibition of AKT/mTOR pathway. Then, to demonstrate that autophagy had a direct relationship with the AKT/mTOR/p70S6K pathway, we transiently transfected SK-OV-3 and A2780 ovarian cancer cells with an AKT overexpression plasmid, which is a vector constitutively expressing the active form of AKT1. The overexpression of AKT1 reduced expression of LC3B-II, leading to the reversal of curcumin-induced cell death (Fig. 6b). Given the high level of activated AKT in SK-OV-3 and A2780 ovarian cancer cells, the pretreatment of cells with LY294002, an inhibitor of PI3K, suppressed the function of AKT, as demonstrated by higher expression of LC3B-II, contributing to curcumin-induced cell death markedly (Fig. 6c). These results indicated that the downregulation of AKT signaling pathway markedly contributed to curcumin-induced cell death and autophagy in SK-OV-3 and A2780 ovarian cancer cells.
Curcumin inhibited the activity of AKT/mTOR/p70S6K signaling pathway. a Western blotting analyses of SK-OV-3 and A2780 cells exposed to curcumin as described above. AKT, p-AKT (Ser473), p-mTOR (Ser2448), mTOR, p-4EBP1 (Thr37/46) and p-p70S6K (Thr389) levels were carried out. GAPDH served as a loading control. Fold changes of the proteins were shown on the right side. b SKOV3 and A2780 cells were transfected with the indicated vectors for 24 h, and then treated with curcumin for 48 h. Western blot analysis verified the overexpression of LC3B-II and AKT. Fold change of LC3B-II/GAPDH was shown in the middle. The cell death rate was measured by a MTT assay on the right side. c Effect of depletion of AKT on curcumin-mediated cell death and autophagy. SKOV3 and A2780 cells were incubated with the PI3K inhibitor (LY294002, 10 μM) for 2 h before treatment with curcumin for 48 h. Fold changes of the proteins were shown in the middle. Cell death rate was measured by MTT assay on the right side. All data are expressed as the mean ± SD of values from triplicate experiments. *P < 0.05, **P < 0.01 and ***P < 0.001 compared with control group
Discussion
Ovarian cancer is the most lethal gynecological malignancy in the world [1, 2]. Although curcumin has been confirmed to be secure in clinical trials in the treatment of ovarian cancer [29], resistance of curcumin may limit its clinical application. In this study, we demonstrated that curcumin could enhance apoptosis of ovarian cancer cells and play an anti-tumor role. However, at the same time, curcumin induced protective autophagy on ovarian cancer cells through AKT/mTOR/p70S6K signaling pathway. Therefore, we could use CQ, an autophagy inhibitor, to inhibit curcumin-induced protective autophagy and potentiate the cytotoxicity of curcumin on ovarian cancer [30,31,32].
Curcumin, an active component of turmeric, displays various pharmacological activities [33]. The promising role of curcumin against different diseases is widely publicized. Moreover, curcumin not only has attracted much attention for its anticancer properties, but also has been regarded to be safe in clinical trials owing to its low toxicity and good tolerance to human at doses of approximately 12 g/day [7, 12, 17, 29, 33,34,35,36,37,38]. Consistent with the past multiple researches, we demonstrated that curcumin stimulated apoptosis cell death in ovarian cancer cells. Caspases are apoptosis executioners, which is to form the active forms of the enzymes by launching proteolytic processing cascade [11, 39,40,41]. In our study, high expression of cleaved-Caspase-9 and cleaved-PARP indicated that curcumin induced apoptotic cell death of EOC cells.
Autophagy maintains cell homeostasis and is called “double-edge sword”. On one hand, it is a process of metabolic decomposition in cells, including digestion of long-lived proteins, damage of organelles and superfluously unwanted materials by lysosomes [39]. On the other hand, it helps us to provide a new thought to consider in clinical studies that may circumvent drug resistance in patients by targeting protective autophagy pathways, In this study, we found that curcumin induced protective autophagy and apoptosis of EOC cells in a concentration-dependent manner at the same time. Furthermore, curcumin-induced protective autophagy weakened anti-tumor effect on ovarian cancer cells. To further explore whether curcumin-induced autophagy was closely related to chemoresistance, we detected the underlying mechanism of curcumin-induced autophagy. Our study showed that curcumin could inhibit phosphorylation of mTOR as well as its downstream effectors phosphorylation of p70S6K and eukaryotic initiation factor 4E-binding protein 1 (4E-BP1). The AKT/mTOR signaling pathway is a classic pathway that not only promotes angiogenesis and cell progression, but also plays a pivotal role in various human malignancies [42, 43]. Inhibition of the AKT/mTOR pathway has been reported to promote autophagy and apoptosis to produce anticancer effect [44]. Our following study showed that autophagy could be induced in the ovarian cancer cells when AKT/mTOR/p70S6K pathway was blocked by LY294002, while overexpression of AKT could reverse curcumin-induced autophagy. Thus, curcumin could induce protective autophagy of human ovarian cancer cells by inhibiting the AKT/mTOR/p70S6K pathway.
Autophagy inhibition weakens the protective effects and sensitizes chemotherapy to augment anticancer effects in cancer cells. Therefore, autophagy inhibitors were used to explore this issue and classified as two categories based on their mechanisms of action on the pathway inducing autophagy: early stage inhibitors and late stage inhibitors. Wortmannin and 3-MA belong to the early-stage autophagy inhibitors which perform a suppressive effect on the formation of autophagosomes, while downstream of autophagosome formations is suppressed by CQ, hydroxychloroquine and Baf A1. These late-stage inhibitors not only inhibit the fusion of autophagosomes and lysosomes, but also block the degradation of autophagic cargo [45]. CQ has been approved to be an antimalarial drug used to treat rheumatoid arthritis and autoimmune diseases by FDA. On account of the safety of CQ, it can be used to inhibit autophagy in both cells and human patients. Furthermore, some funds have been put into other field, such as cancer therapy [30,31,32]. In our study, compared with curcumin treatment alone, the combined treatment of curcumin with CQ could increase SK-OV-3 and A2780 cells death. Consistent with the results of CQ, knocking down the expression level of LC3B to block curcumin-induced autophagy significantly enhanced apoptotic cell death at the genetic level in SK-OV-3 and A2780 cells.
Conclusion
To the best of our knowledge, curcumin could induce protective autophagy of human ovarian cancer cells by inhibiting the AKT/mTOR/p70S6K pathway. CQ, an autophagy inhibitor, enhanced the anti-tumor effect of curcumin on ovarian cancer cells by combination. Thus, the combined treatment of curcumin with autophagy inhibitor could lead to curcumin resistance reduction in ovarian cancer cells. It would be a possible clinical strategy to overcome the limits of current curcumin therapy in the eradication of EOC cells population. However, more studies are needed to demonstrate the responsible molecular mechanisms of curcumin inducing protective autophagy in human ovarian cancer cells and feasibility research in vivo.
References
Grossman DC, Curry SJ, Owens DK et al (2018) Screening for ovarian cancer: US Preventive Services Task Force recommendation statement. JAMA 319(6):588–594. https://doi.org/10.1001/jama.2017.21926
Siegel RL, Miller KD, Jemal A (2018) Cancer statistics. CA Cancer J Clin 68(1):7–30. https://doi.org/10.3322/caac.21442
Corrado G, Salutari V, Palluzzi E, Distefano MG, Scambia G, Ferrandina G (2017) Optimizing treatment in recurrent epithelial ovarian cancer. Expert Rev Anticancer Ther 17(12):1147–1158. https://doi.org/10.1080/14737140.2017.1398088
Bristow RE, Chi DS (2006) Platinum-based neoadjuvant chemotherapy and interval surgical cytoreduction for advanced ovarian cancer: a meta-analysis. Gynecol Oncol 103(3):1070–1076. https://doi.org/10.1016/j.ygyno.2006.06.025
Imran M, Ullah A, Saeed F, Nadeem M, Arshad MU, Suleria HAR (2018) Cucurmin, anticancer, and antitumor perspectives: a comprehensive review. Crit Rev Food Sci Nutr 58(8):1271–1293. https://doi.org/10.1080/10408398.2016.1252711
Qu W, Xiao J, Zhang H et al (2013) B19, a novel monocarbonyl analogue of curcumin, induces human ovarian cancer cell apoptosis via activation of endoplasmic reticulum stress and the autophagy signaling pathway. Int J Biol Sci 9(8):766–777. https://doi.org/10.7150/ijbs.5711
Panda AK, Chakraborty D, Sarkar I, Khan T, Sa G (2017) New insights into therapeutic activity and anticancer properties of curcumin. J Exp Pharmacol 9:31–45. https://doi.org/10.2147/JEP.S70568
Kantara C, O’Connell M, Sarkar S, Moya S, Ullrich R, Singh P (2014) Curcumin promotes autophagic survival of a subset of colon cancer stem cells, which are ablated by DCLK1-siRNA. Cancer Res 74(9):2487–2498. https://doi.org/10.1158/0008-5472.CAN-13-3536
O’Sullivan-Coyne G, O’Sullivan GC, O’Donovan TR, Piwocka K, McKenna SL (2009) Curcumin induces apoptosis-independent death in oesophageal cancer cells. Br J Cancer 101(9):1585–1595. https://doi.org/10.1038/sj.bjc.6605308
Banerjee S, Ji C, Mayfield JE, Goel A, Xiao J, Dixon JE, Guo X (2018) Ancient drug curcumin impedes 26S proteasome activity by direct inhibition of dual-specificity tyrosine-regulated kinase 2. Proc Natl Acad Sci USA 115(32):8155–8160. https://doi.org/10.1073/pnas.1806797115
Weir NM, Selvendiran K, Kutala VK, Tong L, Vishwanath S, Rajaram M, Tridandapani S, Anant S, Kuppusamy P (2007) Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol Ther 6(2):178–184
Saxena V, Hussain MD (2013) Polymeric mixed micelles for delivery of curcumin to multidrug resistant ovarian cancer. J Biomed Nanotechnol 9(7):1146–1154
Abouzeid AH, Patel NR, Sarisozen C, Torchilin VP (2014) Transferrin-targeted polymeric micelles co-loaded with curcumin and paclitaxel: efficient killing of paclitaxel-resistant cancer cells. Pharm Res 31(8):1938–1945. https://doi.org/10.1007/s11095-013-1295-x
Gou Q, Liu L, Wang C, Wu Q, Sun L, Yang X, Xie Y, Li P, Gong C (2015) Polymeric nanoassemblies entrapping curcumin overcome multidrug resistance in ovarian cancer. Colloids Surf B Biointerfaces 126:26–34. https://doi.org/10.1016/j.colsurfb.2014.12.012
Li X, Feng K, Li J, Yu D, Fan Q, Tang T, Yao X, Wang X (2017) Curcumin inhibits apoptosis of chondrocytes through activation ERK1/2 signaling pathways induced autophagy. Nutrients. https://doi.org/10.3390/nu9040414
Sun Y, Jin L, Liu JH, Sui YX, Han LL, Shen XL (2016) Interfering EZH2 expression reverses the cisplatin resistance in human ovarian cancer by inhibiting autophagy. Cancer Biother Radiopharm 31(7):246–252. https://doi.org/10.1089/cbr.2016.2034
Aoki H, Takada Y, Kondo S, Sawaya R, Aggarwal BB, Kondo Y (2007) Evidence that curcumin suppresses the growth of malignant gliomas in vitro and in vivo through induction of autophagy: role of Akt and extracellular signal-regulated kinase signaling pathways. Mol Pharmacol 72(1):29–39. https://doi.org/10.1124/mol.106.033167
Kim JY, Cho TJ, Woo BH, Choi KU, Lee CH, Ryu MH, Park HR (2012) Curcumin-induced autophagy contributes to the decreased survival of oral cancer cells. Arch Oral Biol 57(8):1018–1025. https://doi.org/10.1016/j.archoralbio.2012.04.005
Li B, Takeda T, Tsuiji K, Wong TF, Tadakawa M, Kondo A, Nagase S, Yaegashi N (2013) Curcumin induces cross-regulation between autophagy and apoptosis in uterine leiomyosarcoma cells. Int J Gynecol Cancer 23(5):803–808. https://doi.org/10.1097/IGC.0b013e31828c9581
Tork OM, Khaleel EF, Abdelmaqsoud OM (2015) Altered cell to cell communication, autophagy and mitochondrial dysfunction in a model of hepatocellular carcinoma: potential protective effects of curcumin and stem cell therapy. Asian Pac J Cancer Prev 16(18):8271–8279
Zhang SF, Wang XY, Fu ZQ et al (2015) TXNDC17 promotes paclitaxel resistance via inducing autophagy in ovarian cancer. Autophagy 11(2):225–238. https://doi.org/10.1080/15548627.2014.998931
Zou J, Liu L, Wang Q, Yin F, Yang Z, Zhang W, Li L (2017) Downregulation of miR-429 contributes to the development of drug resistance in epithelial ovarian cancer by targeting ZEB1. Am J Transl Res 9(3):1357–1368
Zhao G, Han X, Zheng S, Li Z, Sha Y, Ni J, Sun Z, Qiao S, Song Z (2016) Curcumin induces autophagy, inhibits proliferation and invasion by downregulating AKT/mTOR signaling pathway in human melanoma cells. Oncol Rep 35(2):1065–1074. https://doi.org/10.3892/or.2015.4413
Guan F, Ding Y, Zhang Y, Zhou Y, Li M, Wang C (2016) Curcumin suppresses proliferation and migration of MDA-MB-231 breast cancer cells through autophagy-dependent Akt degradation. PLoS One 11(1):e0146553. https://doi.org/10.1371/journal.pone.0146553
Maycotte P, Aryal S, Cummings CT, Thorburn J, Morgan MJ, Thorburn A (2012) Chloroquine sensitizes breast cancer cells to chemotherapy independent of autophagy. Autophagy 8(2):200–212. https://doi.org/10.4161/auto.8.2.18554
Sasaki K, Tsuno NH, Sunami E et al (2010) Chloroquine potentiates the anti-cancer effect of 5-fluorouracil on colon cancer cells. BMC Cancer 10:370. https://doi.org/10.1186/1471-2407-10-370
Ren F, Shen J, Shi H, Hornicek FJ, Kan Q, Duan Z (1866) Novel mechanisms and approaches to overcome multidrug resistance in the treatment of ovarian cancer. Biochim Biophys Acta 2:266–275. https://doi.org/10.1016/j.bbcan.2016.10.001
Shao FY, Du ZY, Ma DL et al (2015) B5, a thioredoxin reductase inhibitor, induces apoptosis in human cervical cancer cells by suppressing the thioredoxin system, disrupting mitochondrion-dependent pathways and triggering autophagy. Oncotarget 6(31):30939–30956. https://doi.org/10.18632/oncotarget.5132
Terlikowska KM, Witkowska AM, Zujko ME, Dobrzycka B, Terlikowski SJ (2014) Potential application of curcumin and its analogues in the treatment strategy of patients with primary epithelial ovarian cancer. Int J Mol Sci 15(12):21703–21722. https://doi.org/10.3390/ijms151221703
Solomon VR, Lee H (2009) Chloroquine and its analogs: a new promise of an old drug for effective and safe cancer therapies. Eur J Pharmacol 625(1–3):220–233. https://doi.org/10.1016/j.ejphar.2009.06.063
Boya P, González-Polo RA, Casares N et al (2005) Inhibition of macroautophagy triggers apoptosis. Mol Cell Biol 25(3):1025–1040. https://doi.org/10.1128/MCB.25.3.1025-1040.2005
Nilsson JR (1992) Does chloroquine, an antimalarial drug, affect autophagy in Tetrahymena pyriformis. J Protozool 39(1):9–16
Zhang C, Li B, Zhang X, Hazarika P, Aggarwal BB, Duvic M (2010) Curcumin selectively induces apoptosis in cutaneous T-cell lymphoma cell lines and patients’ PBMCs: potential role for STAT-3 and NF-kappaB signaling. J Investig Dermatol 130(8):2110–2119. https://doi.org/10.1038/jid.2010.86
Guo S, Long M, Li X, Zhu S, Zhang M, Yang Z (2016) Curcumin activates autophagy and attenuates oxidative damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep 13(3):2187–2193. https://doi.org/10.3892/mmr.2016.4796
Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C (2015) Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res 35(2):645–651
Wang J, Zhang J, Zhang CJ, Wong YK, Lim TK, Hua ZC, Liu B, Tannenbaum SR, Shen HM, Lin Q (2016) In situ proteomic profiling of curcumin targets in HCT116 colon cancer cell line. Sci Rep 6:22146. https://doi.org/10.1038/srep22146
He M, Wang D, Zou D, Wang C, Lopes-Bastos B, Jiang WG, Chester J, Zhou Q, Cai J (2016) Re-purposing of curcumin as an anti-metastatic agent for the treatment of epithelial ovarian cancer: in vitro model using cancer stem cell enriched ovarian cancer spheroids. Oncotarget 7(52):86374–86387. https://doi.org/10.18632/oncotarget.13413
Aggarwal BB, Kumar A, Bharti AC (2003) Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 23(1A):363–398
Dong Z, Liang S, Hu J, Jin W, Zhan Q, Zhao K (2016) Autophagy as a target for hematological malignancy therapy. Blood Rev 30(5):369–380. https://doi.org/10.1016/j.blre.2016.04.005
Zhang X, Zhang HQ, Zhu GH, Wang YH, Yu XC, Zhu XB, Liang G, Xiao J, Li XK (2012) A novel mono-carbonyl analogue of curcumin induces apoptosis in ovarian carcinoma cells via endoplasmic reticulum stress and reactive oxygen species production. Mol Med Rep 5(3):739–744. https://doi.org/10.3892/mmr.2011.700
Seo JA, Kim B, Dhanasekaran DN, Tsang BK, Song YS (2016) Curcumin induces apoptosis by inhibiting sarco/endoplasmic reticulum Ca2 + ATPase activity in ovarian cancer cells. Cancer Lett 371(1):30–37. https://doi.org/10.1016/j.canlet.2015.11.021
Vanhaesebroeck B, Guillermet-Guibert J, Graupera M, Bilanges B (2010) The emerging mechanisms of isoform-specific PI3K signalling. Nat Rev Mol Cell Biol 11(5):329–341. https://doi.org/10.1038/nrm2882
Li H, Zeng J, Shen K (2014) PI3K/AKT/mTOR signaling pathway as a therapeutic target for ovarian cancer. Arch Gynecol Obstet 290(6):1067–1078. https://doi.org/10.1007/s00404-014-3377-3
Hu JL, Hu XL, Guo AY, Wang CJ, Wen YY, Cang SD (2017) Endoplasmic reticulum stress promotes autophagy and apoptosis and reverses chemoresistance in human ovarian cancer cells. Oncotarget 8(30):49380–49394. https://doi.org/10.18632/oncotarget.17673
Lin JF, Lin YC, Tsai TF, Chen HE, Chou KY, Hwang TI (2017) Cisplatin induces protective autophagy through activation of BECN1 in human bladder cancer cells. Drug Des Dev Ther 11:1517–1533. https://doi.org/10.2147/DDDT.S126464
Funding
This study was supported by Grants from Natural Science Foundation of Shandong Province (ZR2016HM27) and Science Foundation of Qilu Hospital of Shandong University (no. 2016QLQN27).
Author information
Authors and Affiliations
Contributions
LL: protocol and project development, data management, data analysis, manuscript writing and manuscript editing. YP: protocol and project development, manuscript editing. XZ: data curation, formal analysis. RL: manuscript editing. CJ: manuscript editing. JX: manuscript editing. RD: resources, supervision. PL: protocol and project development, funding acquisition, supervision.
Corresponding authors
Ethics declarations
Conflict of interest
No conflict of interest exits in the submission of this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liu, Ld., Pang, Yx., Zhao, Xr. et al. Curcumin induces apoptotic cell death and protective autophagy by inhibiting AKT/mTOR/p70S6K pathway in human ovarian cancer cells. Arch Gynecol Obstet 299, 1627–1639 (2019). https://doi.org/10.1007/s00404-019-05058-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-019-05058-3