Abstract
Purpose
To study the association between the numbers of oocytes retrieved and the cumulative live birth rates (LBR) in women aged 35–40 years undergoing long GnRH agonist IVF/ICSI cycles.
Methods
A total of 931 women aged 35–40 years who underwent their first cycle of IVF/ICSI treatment between January 2010 and December 2013 at Nanjing Drum Tower Hospital were identified and reviewed. The main endpoint of this study was the cumulative LBR after one complete oocyte retrieval, which included fresh and all subsequent frozen–thaw embryo transfer cycles. Odds ratios (OR) and 95% confidence interval (CI) for live birth were estimated by multivariate logistic regression analysis. Furthermore, all the women were divided into four groups based on the number of oocytes retrieved: 0–4, 5–9, 10–14 or ≥15 oocytes group. Variables were then compared among groups.
Results
We found that 634 out of the 931 patients (68.1%) achieved at least one live birth. The number of oocytes retrieved was an independent predictive factor for live birth, with OR 1.20 (95% CI 1.15–1.26) when adjusted for age (years), duration of infertility and Gn (gonadotrophin) doses. The cumulative LBR in the four different oocyte groups was 35.6, 68.8, 83.4 and 89.2%, respectively. When the 1–4 oocytes group was issued as a reference, the ORs for cumulative LBR gradually increased to 3.66, 6.74 and 11.77 in other three oocytes groups, respectively. The moderate–severe ovarian hyperstimulation syndrome (OHSS) rate was dramatically increased in the ≥15 oocytes group (6.9%) when compared to that in the 10–14 oocytes group (0.8%), while the cumulative LBR only increased 5.8% (from 83.4 to 89.2%).
Conclusions
The ideal number of oocytes retrieved in women aged 35–40 years is 10–14 oocytes, which achieves a high cumulative LBR while maintaining an acceptable low OHSS rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Achieving a term live birth is the aim of in vitro fertilization (IVF) treatment. Cumulative live birth rate (LBR) is considered to be a suitable indicator to assess the success of an IVF treatment, which showed results following the fresh embryo transfer and all frozen–thawed embryo transfers (FET) [1]. Studies showed that the number of oocytes retrieved positively correlated to the chance of achieving a live birth [2,3,4,5], thus the number of oocytes retrieved was considered to be an important predictive factor for cumulative LBR [3,4,5]. However, getting more oocytes resulted in a higher rate of cycle cancellation to prevent ovarian hyperstimulation syndrome (OHSS) in young IVF treatment patients [3]. One previous study suggested that the optimal number of oocytes retrieved for getting a live birth was 6–15 oocytes in young patients under age 35 years [4].
Studies have demonstrated that the implantation rate remains constant until the age of 35 at which time a linear decrease of 2.77% per year is observed, which may be due to the euploid embryos number decreases linearly after 35 years old [6, 7]. Age is a negative factor for live birth starting from the age of 35 years, with a rapid decrease in live birth rate beyond 40 years old [8]. Age is a critical factor in response to controlled ovarian hyperstimulation [9], as women older than 35 years are at risk of poor ovarian response [10, 11]. Age is also a negative risk factor for OHSS with OR 0.9 (95% CI 0.81–0.99), as age increases the rate of OHSS decreases [12]. However, whether the preferred number of oocytes in advanced maternal age should be much more than that in younger patients by balancing between the maximum cumulative LBR and the minimum risk of OHSS has seldomly been studied.
Taking into consideration the fact of less oocytes retrieved and lower OHSS risk in women aged 35–40 years, we aimed to investigate whether there was a preferred oocytes number retrieved that would maximize the cumulative LBR while minimize the OHSS rate. This will bring insights into IVF practice, and hopefully will improve the safety of IVF without sacrificing clinical outcomes in advanced age patients.
Materials and methods
Study population
It is a retrospective cohort study. All patients aged 35–40 years were identified and reviewed which were stimulated with a long agonist protocol between January 2010 and December 2013 at the Reproductive Medicine Center of Nanjing Drum Tower Hospital. Exclusion criteria: patients who have not been stimulated with the long agonist protocol, had underwent IVF treatment previously, or the patients without a live birth which still have frozen embryos left over a 2 year period, BMI >30 kg/m2 or oocyte donation cycles. Thus, 931 patients were studied. The primary outcome of this study is a live birth, which was defined as an infant born alive after 28 weeks’ gestation. The cumulative LBR was calculated by including only the first live birth generated during the complete first IVF cycle including fresh and all subsequent frozen–thaw cycles as the numerator. The denominator was defined as all women allocated to treatment [13]. The patients without a live birth but still had frozen embryos left in 2 years after oocytes retrieval were excluded in this study. The value of patients’ age, duration of infertility, BMI (kg/m2), type of infertility, insemination method, Gn (gonadotrophin) doses and the number of oocytes retrieved in predicting the cumulative LBR was evaluated, respectively. The OHSS rate was evaluated using Golan’s criteria [14]. The study was approved by the Nanjing Drum Tower Hospital Research Ethics Committee.
IVF and fresh embryo transfer
All the patients received recombinant FSH for ovarian stimulation under pituitary suppression using an GnRH agonist (triptorelin; Ferring Pharmaceuticals, Germany) according to a protocol used routinely in our center [15, 16]. In all women, pituitary desensitization was achieved by subcutaneous injection of triptorelin which started in the mid-luteal phase. Ovarian stimulation was started with 150–300 IU/day of rFSH (Gonal F, Serono, Switzerland). Gonadotropin dosages were adjusted according to the E2 level changes and follicular growth. Intramuscular administration of 10,000 IU of human chorionic gonadotropin (hCG, Ferring Pharmaceuticals, Germany) was used for the trigger when more than two follicles larger than 18 mm in diameter were formed. Oocytes were picked up at 36 h after the hCG trigger and fertilization was achieved by IVF or ICSI, according to the state of the sperm. One or two good-quality embryos were transferred under abdominal ultrasound guidance on Day 3. Intramuscular administration of 60 mg progesterone daily for luteal support was used for 10 weeks after oocytes retrieval.
Frozen–thawed embryo transfer
For Day 3 embryos, the consensus scoring system was applied for embryo assessment [18]. Surplus good quality embryos were cryopreserved using a vitrification protocol as described earlier [17]. FET cycles were performed through hormone replacement therapy (HRT) cycles or natural cycles. Luteal support was also provided for 10 weeks after FET by intramuscular injection of progesterone (60 mg daily).
Statistical analysis
Univariate logistic regression was conducted between cumulative LBR and variables. Finally, four factors (the number of oocytes retrieved, age, Gn doses and duration of infertility) were chosen to contribute the multivariable logistic regression model. Odds ratios (OR) and 95% confidence interval (CI) for live birth associated with variables was estimated by multivariate logistic regression analysis. Then, all the women were divided into four groups based on the number of oocytes retrieved: 0–4, 5–9, 10–14 or ≥15 oocytes group. Variables between two groups were compared using the Chi-squared test, the independent-samples Student’s t test or Mann–Whitney tests, respectively. One-way ANOVA and Chi-squared tests were used to compare the differences among these four groups. P < 0.05 was considered as statistically significant. Analyses were performed using SPSS 14.0 (Chicago, IL, USA).
Results
Baseline characteristics
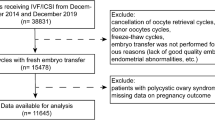
A total of 2562 patients aged 35–40 years were reviewed and, 931 were found to be eligible for analysis. The data selection process and reasons for exclusion are provided in Fig. 1. We observed that the live birth rate in the fresh embryo transfer cycle was 50.7% (472/931), while the cumulative LBR was 68.1% (634/931) during the first complete IVF cycle which included fresh and all subsequent frozen–thaw embryo transfer cycles (Table 1). Women who tended to get a live birth were much younger, had a shorter duration of infertility and required less gonadotrophins. There was no significant difference in insemination method or BMI between live birth group and no live birth group (Table 2). The number of oocytes retrieved was significantly higher in the live birth group, as was the number of transferable embryos (Table 2). Out of 931 patients only 11 patients underwent the moderate–severe OHSS (rate 1.2%) and all were in the live birth group. The distribution of retrieved oocyte numbers and transferable embryo numbers per IVF/ICSI cycle are shown in Figs. 2 and 3.
Multiple logistic regression analysis for cumulative LBR
Multiple logistic regression analysis was used to establish independent predictive factors related to the cumulative LBR. The number of oocytes retrieved (Fig. 4) was an independent predictive factor for the live birth rates when adjusted for age, duration of infertility and Gn doses, with OR 1.20, 95% CI (1.15–1.26) (the live birth group vs. no live birth group, Table 3). We also found that women’s age was also an independent predictive factor with OR 0.74 (95% CI 0.67–0.82) (Table 3).
Cumulative LBR and OHSS rate in four oocytes categories
Table 4 shows the adjusted ORs and 95% CIs of the cumulative LBR in 0–4, 5–9, 10–14 or ≥15 oocytes groups. The 1–4 oocytes group was used as a reference. When adjusted for age, duration of infertility and Gn doses, the number of oocytes retrieved remained as an independent prognostic factor (P < 0.001) for cumulative LBR. The ORs for cumulative LBR increased from 3.66 (95% CI 2.50–5.37) in the 4–9 oocytes group to 6.74 (95% CI 4.08–11.11) in the 10–14 oocytes group and 11.77 (95% CI 5.32–26.03) in the ≥15 oocytes group (Table 4). Cumulative LBR for the four groups was 35.6, 68.8, 83.4 and 89.2%, respectively (Fig. 5), while the moderate–severe OHSS rate also increased from 0, to 0.5, 0.8, 6.9% respectively (Table 5).
Discussion
Cumulative LBR per oocyte retrieval is a suitable indicator of the quality and success in an IVF treatment, as cryopreservation has become an integral part of one complete cycle of IVF treatment. Reporting the cumulative LBR will be more appropriate while comparing the IVF treatment outcome between different centers and making political and economic decisions regarding treatment efficacy and cost effectiveness, instead of reporting live birth rate based on fresh embryo transfer.
However, there is no consensus on the definition of cumulative LBR. Maheshwari et al. recommended a triple outcome strategy for reporting the short-term, medium-term and long-term cumulative LBR [13]. In this study, we adopted the short-term cumulative LBR, which was presented as live birth episodes per woman 2 years after one oocyte retrieval to account for the first live birth. In our study, the live birth rate in fresh embryo transfer cycle is 50.7% and the cumulative LBR is 68.1% in women aged 35–40 years, which is consistent with some other studies. Abuzeid et al. found their live birth rate in fresh embryo transfer was 40% and the cumulative LBR was 66% in women aged 35–39 years [19], while Wu et al. found their live birth rate in fresh embryo transfer was 51.8% and the cumulative LBR was 69.8% in women aged 35–39 years [20]. However, other studies found the cumulative LBR was 31.2–52.6% [5, 21, 22], which was much lower than the 68.1% in our study.
Previous studies have found that the optimal number of oocytes retrieved for the maximum cumulative LBR was between 6 and 15 oocytes in women aged 20–34 years when balancing the risk of OHSS [4]. Age is a negative predictive factor for live births during IVF treatment as the patients achieving a live birth were younger than those who had not achieved a live birth [23]. Poor ovarian response was also associated with a parallel decline in both the oocyte quality and quantity as the miscarriage rate fell from 20 to 13% with an increased number of oocytes retrieved [24]. Studies showed that the implantation rate linearly decreased from the age of 35 years. Whether these advanced aged women should get more than 15 oocytes to get the maximum cumulative LBR is seldom studied. The poor ovarian response rate was relatively high and live birth rate was relatively low in the women over 40 years of age who were using a long GnRH agonist protocol [25, 26], Thus, this study only investigated the optimum number of oocytes in women aged 35–40 years who underwent a long GnRH agonist IVF treatment.
In accordance with other studies, younger and shorter duration of infertility among patients achieved better outcomes and also required less amount of total gonadotrophins. Furthermore, we found that the number of oocytes retrieved was an independent predictive factor for cumulative LBR, with OR of 1.20, 95% CI 1.15–1.26. In general, this means with each additional oocyte retrieved increment, cumulative LBR would increase 1.20 times. The OR shows stronger correlation between oocyte retrieved and live birth rate than expected. Subgroups analysis performed in different number of oocytes retrieved groups showed the cumulative LBR was correlated with number of oocytes retrieved. The percentage of patients in different oocyte retrieval groups was 20.2, 43.0, 25.8 and 11.0% for the four groups 0–4, 5–9, 10–14 and ≥15 oocytes categories, respectively. The proportion of different oocyte retrieval groups was similar with that in the young patients [4]. The cumulative LBR in these four groups was 35.6, 68.8, 83.4 and 89.2%, respectively, suggesting that the cumulative LBR significantly increased with the number of oocytes retrieved, which was consistent with previous studies [27, 28]. These results showed that a high ovarian response predicted a high cumulative LBR, which incorporated fresh and thawed frozen embryo transfers.
Furthermore, the multivariate logistic step wise regression was performed to identify other influential factors which were significantly associated to the cumulative LBR such as total dosage of Gn, years of infertility and age. We found that the age of patients was negatively correlated with the cumulative LBR. This multivariate regression model may be a useful tool to consult the patients before IVF treatment to inform the chance of having a live baby in one single IVF treatment.
Getting more oocytes resulted in higher cumulative LBR, but more oocytes will also lead to higher OHSS rates. The overall moderate–severe OHSS rate was 1.2% in our study, while the rate was 0, 0.5, 0.8 and 6.9%, in 0–4, 5–9, 10–14 and ≥15 oocytes retrieved groups, respectively. These results showed that the risk of OHSS increases proportionally with the number of oocytes retrieved, which was consistent with previous studies [29,30,31]. The OHSS rate was dramatically increased in the ≥15 oocytes retrieved group when compared to that in the 10–14 oocytes retrieved group, while the cumulative LBR only increased 5.8% (from 83.4 to 89.2%). So the optimum number of oocytes retrieved in women aged 35–40 years is 10–14 oocytes, achieving the maximum cumulative LBR while maintaining an acceptable low OHSS rate.
There were some limitations in our study. First, the proportion of excluded patients who without a live birth still had frozen embryos left 2 years after oocytes retrieval was high, which might result in increased cumulative LBR. Second, it is a retrospective study; more prospective studies are needed to confirm the result. Third, the age of these women in this study was limited to 35–40 years, even older age patients should be investigated in further studies.
In conclusion, this study showed that the number of oocytes retrieved was positively correlated to the cumulative LBR in women aged 35–40 years. While the preferred number of oocytes retrieved in women aged 35–40 years is 10–14 oocytes, achieving a high cumulative LBR while maintaining an acceptable low OHSS rate.
References
Germond M, Primi MP, Urner F, Chanson A, Wirthner D, Senn A (2004) Number of transferred embryos: how to reduce multiple pregnancies. Ann N Y Acad Sci 1034:93–100. doi:10.1196/annals.1335.011
van der Gaast MH, Eijkemans MJ, van der Net JB, de Boer EJ, Burger CW, van Leeuwen FE (2006) Optimum number of oocytes for a successful first IVF treatment cycle. Reprod Biomed Online 13(4):476–480
Chen YH, Xu XH, Wang Q, Zhang SD, Jiang LL, Zhang CL, Ge ZJ (2015) Optimum oocyte retrieved and transfer strategy in young women with normal ovarian reserve undergoing a long treatment protocol: a retrospective cohort study. J Assist Reprod Genet 32(10):1459–1467. doi:10.1007/s10815-015-0571-6
Ji J, Liu Y, Tong XH, Luo L, Ma J, Chen Z (2013) The optimum number of oocytes in IVF treatment: an analysis of 2455 cycles in China. Hum Reprod 28(10):2728–2734. doi:10.1093/humrep/det303
Drakopoulos P, Blockeel C, Stoop D, Camus M, de Vos M, Tournaye H (2016) Conventional ovarian stimulation and single embryo transfer for IVF/ICSI. How many oocytes do we need to maximize cumulative live birth rates after utilization of all fresh and frozen embryos? Hum Reprod 31(2):370–376. doi:10.1093/humrep/dev316
Demko ZP, Simon AL, McCoy RC, Petrov DA, Rabinowitz M (2016) Effects of maternal age on euploidy rates in a large cohort of embryos analyzed with 24-chromosome single-nucleotide polymorphism-based preimplantation genetic screening. Fertil Steril 105(5):1307–1313. doi:10.1016/j.fertnstert.2016.01.025
Spandorfer SD, Chung PH, Kligman I, Liu HC, Davis OK, Rosenwaks Z (2000) An analysis of the effect of age on implantation rates. J Assist Reprod Genet 17(6):303–306
Garrido N, Bellver J, Remohí J, Simón C, Pellicer A (2011) Cumulative live-birth rates per total number of embryos needed to reach newborn in consecutive in vitro fertilization (IVF) cycles: a new approach to measuring the likelihood of IVF success. Fertil Steril 96(1):40–46. doi:10.1016/j.fertnstert.2011.05.008
Templeton A, Morris JK, Parslow W (1996) Factors that affect outcome of in vitro fertilisation treatment. Lancet 348(9039):1402–1406. doi:10.1016/S0140-6736(96)05291-9
Committee on Gynecologic Practice (2015) Committee opinion no. 618: ovarian reserve testing. Obstet Gynecol 125(1):268–273. doi:10.1097/01.AOG.0000459864.68372.ec
Vuong TN, Phung HT, Ho MT (2015) Recombinant follicle-stimulating hormone and recombinant luteinizing hormone versus recombinant follicle-stimulating hormone alone during GnRH antagonist ovarian stimulation in patients aged ≥35 years: a randomized controlled trial. Hum Reprod 30(5):1188–1195. doi:10.1093/humrep/dev038
Ashrafi M, Bahmanabadi A, Akhond MR, Arabipoor A (2015) Predictive factors of early moderate/severe ovarian hyperstimulation syndrome in non-polycystic ovarian syndrome patients: a statistical model. Arch Gynecol Obs 292(5):1145–1152. doi:10.1007/s00404-015-3723-0
Maheshwari A, McLernon D, Bhattacharya S (2015) Cumulative live birth rate: time for a consensus? Hum Reprod 30(12):2703–2707. doi:10.1093/humrep/dev263
Golan A, Ron-el R, Herman A, Soffer Y, Weinraub Z, Caspi E (1989) Ovarian hyperstimulation syndrome: an update review. Obstet Gynecol Surv 44(6):430–440
Zhou J, Wang S, Wang B, Wang J, Chen H, Zhang N, Hu Y, Sun H (2015) The value of HCG serum concentrations after trigger in predicting pregnancy and live birth rates in IVF–ICSI. Reprod Biomed Online 30(6):667–673. doi:10.1016/j.rbmo.2015.02.013
Ding LJ, Wang B, Shen XY, Yan GJ, Zhang NY, Hu YL, Sun H (2013) Withdrawal of GnRH agonist decreases oestradiol and VEGF concentrations in high responders. Reprod Biomed Online 27(2):131–139. doi:10.1016/j.rbmo.2013.04.014
Van Landuyt L, Verpoest W, Verheyen G, De Vos A, Van de Velde H, Liebaers I (2011) Closed blastocyst vitrification of biopsied embryos: evaluation of 100 consecutive warming cycles. Hum Reprod 26(2):316–322. doi:10.1093/humrep/deq338
Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology (2011) The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Reprod 26(6):1270–1283. doi:10.1093/humrep/der037
Abuzeid MI, Bolonduro O, La Chance J, Abozaid T, Urich M, Ullah K, Ali T, Ashraf M, Khan I (2014) Cumulative live birth rate and assisted reproduction: impact of female age and transfer day. Facts Views Vis Obgyn. 6(3):145–149
Wu CH, Lee TH, Chen HH, Chen CI, Huang CC, Lee MS (2015) The influence of female age on the cumulative live-birth rate of fresh cycles and subsequent frozen cycles using vitrified blastocysts in hyper-responders. Taiwan J Obstet Gynecol 54(5):567–571. doi:10.1016/j.tjog.2015.08.009
Toftager M, Bogstad J, Løssl K, Prætorius L, Zedeler A, Bryndorf T, Nilas L, Pinborg A (2017) Cumulative live birth rates after one ART cycle including all subsequent frozen–thaw cycles in 1050 women: secondary outcome of an RCT comparing GnRH-antagonist and GnRH-agonist protocols. Hum Reprod 32(3):556–567. doi:10.1093/humrep/dew358
De Vos A, Van Landuyt L, Santos-Ribeiro S, Camus M, Van de Velde H, Tournaye H, Verheyen G (2016) Cumulative live birth rates after fresh and vitrified cleavage-stage versus blastocyst-stage embryo transfer in the first treatment cycle. Hum Reprod 31(11):2442–2449. doi:10.1093/humrep/dew219
Hornstein MD (2016) State of the ART: assisted reproductive technologies in the US. Reprod Sci 23(12):1630–1633. doi:10.1177/1933719116667227
Sunkara SK, Khalaf Y, Maheshwari A, Seed P, Coomarasamy A (2014) Association between response to ovarian stimulation and miscarriage following IVF: an analysis of 124 351 IVF pregnancies. Hum Reprod 29(6):1218–1224. doi:10.1093/humrep/deu053
Tigges J, Godehardt E, Soepenberg T, Maxrath B, Friol K, Gnoth C (2016) Determinants of cumulative ART live-birth rates in a single-center study: age, fertilization modality, and first-cycle outcome. Arch Gynecol Obstet 294(5):1081–1089. doi:10.1007/s00404-016-4162-2
Du XF, Yang XH, Li J, Hao M, Guo YH (2016) Growth hormone co-treatment within a GnRH agonist long protocol improves implantation and pregnancy rates in patients undergoing IVF-ET. Arch Gynecol Obstet 294(4):877–883. doi:10.1007/s00404-016-4163-1
Tannus S, Turki R, Cohen Y, Son WY, Shavit T, Dahan MH (2017) Reproductive outcomes after a single dose of gonadotropin-releasing hormone agonist compared with human chorionic gonadotropin for the induction of final oocyte maturation in hyper-responder women aged 35–40 years. Fertil Steril 107(6):1323–1328.e2. doi:10.1016/j.fertnstert.2017.04.014
Vlaisavljević V, Kovačič B, Knez J (2017) Cumulative live birth rate after GnRH agonist trigger and elective cryopreservation of all embryos in high responders. Reprod Biomed Online S1472–6483(17):30151–30157. doi:10.1016/j.rbmo.2017.03.017
Verwoerd GR, Mathews T, Brinsden PR (2008) Optimal follicle and oocyte numbers for cryopreservation of all embryos in IVF cycles at risk of OHSS. Reprod Biomed Online 17(3):312–317
Reljic M, Vlaisavljević V, Gavrić V, Kovacic B (1999) Number of oocytes retrieved and resulting pregnancy. Risk factors for ovarian hyperstimulation syndrome. J Reprod Med 44(8):713–718
Steward RG, Lan L, Shah AA, Yeh JS, Price TM, Goldfarb JM, Muasher SJ (2014) Oocyte number as a predictor for ovarian hyperstimulation syndrome and live birth: an analysis of 256,381 in vitro fertilization cycles. Fertil Steril 101(4):967–973. doi:10.1016/j.fertnstert.2013.12.026
Author information
Authors and Affiliations
Contributions
ZJJ: data management/analysis, manuscript writing/editing, WB: manuscript writing/editing, HYL: study design, data collection, manuscript editing, SHX: study design, data management/analysis, manuscript writing/editing.
Corresponding author
Ethics declarations
Funding
This study was supported by Chinese National Natural Science Foundation (81571504, 81200450), Nanjing Medical Science and technique Development Foundation (QRX11166), Maternal and fetus medicine Key Lab of Jiangsu Province (XK 201102,BL2014003).
Conflict of interest
Authors declare no conflict of interest with respect to the authorship and/or publication of this article.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the Nanjing Drum Tower Hospital Research Ethics Committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Jianjun Zhou and Bin Wang contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhou, J., Wang, B., Hu, Y. et al. Association between the number of oocytes retrieved and cumulative live birth rate in women aged 35–40 years undergoing long GnRH agonist IVF/ICSI cycles. Arch Gynecol Obstet 296, 1005–1012 (2017). https://doi.org/10.1007/s00404-017-4503-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-017-4503-9