Abstract
Background
Morgagni hernia presents a rare type of congenital diaphragmatic hernia (CDH, about 2–5 %) (Herman, J Perinatol 21:343–344, 2001), which is characterized by an anterior mainly right-sided defect of the diaphragm. Infrequently, this is combined with a herniation of the liver into the pericardial cavity (Aké, Prenat Diagn 11:719–724, 1991; Stevens, Pediatr Radiol 26:791–793, 1996). This may cause massive pericardial effusion and subsequently lung hypoplasia (Pober et al., Congenital diaphragmatic hernia overview, University of Washington, Seattle, 2015; Ikeda, J Perinat Med 30:336–340, 2002; Hara, J Obstet Gynaecol Res 33:561–565, 2007). So far only few cases have been reported in fetal life.
Case
We report a case of Morgagni hernia with pericardiodiaphragmatic aplasia, complicated by two-compartment effusions (massive pericardial effusion and mild ascites), diagnosed in the second trimester. The case was successfully managed in utero with thoraco-amniotic shunting and late tracheal occlusion, followed by corrective surgery after birth.
Discussion
A review of the literature was performed, identifying 13 cases of prenatally diagnosed Morgagni hernia. The diagnosis was established by the sonographic findings of pericardial effusion und intrathoracic herniation of the liver. In only two cases a prenatal intervention was carried out. All neonates were operated postnatally with excellent final outcome.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Case
A 39-year-old G3, P1 was referred at 23 weeks’ for detailed ultrasound evaluation because of hydrothorax. Our examination demonstrated a fetus with concordant growth, mild ascites, polyhydramnios and massive thoracic effusion. It was difficult to identify first the origin of the effusion, whether pleural or pericardial, due to the practically non visible fetal lungs (Fig. 1a). There was no evidence of further fetal abnormalities including cardiac defects. The karyotype was normal (46, XY) and TORCH serology was negative. Thoraco-amniotic shunting was advised to the patient due to the pronounced “hydrops” to improve neonatal outcome on account of highly possible pulmonary hypoplasia. The shunt (Somatex Intra-Uterine-Shunt, 18G, Somatex Medical Technologies GmbH, Germany) was placed into the right thoracic cavity, resulting in the complete drainage of the thoracic effusion and subsequently resolution of the ascites. After the drainage both lungs were seen compressed into an extremely posterior position (Fig. 1b). They expanded only slightly. Follow-up scans demonstrated a mediastinal shift to the left and a progressive, right-sided protrusion of the liver into the thorax with further distinctly compressed lungs into an extremely posterior position (Fig. 1c, d). The diagnosis of intrathoracic liver herniation was established by the demonstration of hepatic vessels. An anterior, right diaphragmatic hernia (Morgagni) combined with pericardiodiaphragmatic aplasia was suspected at 26 weeks’ gestation. Between 26 and 32 weeks’ gestation, the estimated o/e lung to head ratio (LHR) of the left lung was 21–23 %. To strengthen our diagnosis and to differentiate from diaphragmatic eventration, a fetal MRI was performed at 32 weeks’ (Fig. 2). The o/e total fetal lung volume (TVLV) was 38 %. Because of the suspected pulmonary hypoplasia, the option of a fetal endoscopic tracheal occlusion (FETO) was considered. The tracheal balloon was placed at 32 weeks’ and removed 4 weeks later without complications.
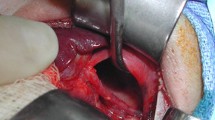
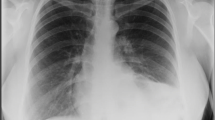
A male infant of 3600 g was delivered by cesarean section at 38 weeks’ gestation. The slightly deprived infant was intubated immediately and admitted to the intensive care unit. (APGAR: 6/6/7, pH 7.17, pCO2 71 mmHg, pO2 48 mmHg, BE −4.8 mmol/l). Despite very small lungs visible at chest X-ray (Fig. 3), the baby received only mild ventilation and underwent corrective surgery on day 6. During operation the prenatal diagnosis was confirmed (Fig. 4). The herniated hepatic lobes were relocated into the abdominal cavity and the anterior diaphragmatic hernia was closed primarily with the small rim of the diaphragmatic muscle and the hernial sac. Reconstruction of the pericardium was also achieved with a layer of the hernial sac. The postoperative course was complicated by two incidents of respiratory insufficiency with the need of intubation and mechanical ventilation. Because of suspected recurrence, a surgical re-intervention was necessary on day 59. A relaxation of the diaphragm was seen, and therefore, the remaining diaphragm was just strengthened. The child was released home 3 months after birth in good health, without any breathing support.
Discussion
We present an especially rare form of Morgagni hernia, complicated by intrapericardial liver herniation, two-compartment effusions (pericardial, ascites) with the necessity of two prenatal interventions to improve the perinatal outcome.
At the first examination, the lungs were barely visible due to the massive thoracic effusion. In general, the origin of effusion is easily determined by the position of the lungs. While in pericardial effusion they are displaced posteriorly in the thoracic cavity, in pleural effusions they are located centrally with the fluid around them [7]. Our suspected diagnosis was confirmed by the complete drainage of the effusion after the placement of just one thoraco-amniotic shunt, followed by the limited expansion and persisting posterior location of the compressed lungs and the later apparent liver herniation. In the era of high-resolution ultrasound, it has become feasible to differentiate the herniated liver from other masses originating from thoracic tissues, by the same echogenicity as the subdiaphragmatic liver and the presence of hepatic vessels [7–9].
A review of the literature (1991–2015, Table 1) revealed 13 other cases of prenatal diagnosed Morgagni hernia [2, 3, 5–14]. The majority was detected in the second or third trimester and presented mainly with large pericardial effusion. However, a cardiac tamponade was never observed. Prenatal pericardiocentesis was performed in just two cases due to feared lung hypoplasia [9, 10]. In the rest of the cases no prenatal interventions were undertaken either due to advanced gestational age or lack of fetal deterioration. In our case, thoraco-amniotic shunting aimed to reduce intrathoracic pressure allowing better development of the lungs. On the first ultrasound evaluation the effusions were interpreted as “hydrops fetalis”, which was incorrect because in fetuses with diaphragmatic hernia the thorax and abdominal cavity should be considered as a single communicating cavity and the presentation of hydrops indicates an overflow of fluid from one compartment to the other [15]. The underlying cause of the effusions is heterogeneously discussed. Similar to fetuses with diaphragmatic hernia and fluid effusions, locally disturbed lymphatic drainage may lead to pericardial effusion due to an embryonic insult of the thoracic duct at the level of the diaphragma because of their close proximity [15]. Mechanical irritation with reactive effusion and liver congestion because of kinking of the hepatic veins could present another mechanism [16]. Other differential diagnoses of pericardial effusion should include diaphragmatic eventration, cardiac diverticulum or pericardial teratoma [16]. Like in our case, MRI might especially aid to distinguish from eventration.
Morgagni hernia during infancy is frequently associated with further congenital abnormalities, particularly congenital heart defects and syndromes [4]. Among 14 prenatally described cases (Table 1), 10 (71.4 %) were isolated and 4 (28.6 %) presented with additional structural abnormalities [6, 7, 11, 12]. Defects of the septum transversum are one of the features of Cantrell’s pentalogy and some of the above mentioned non-isolated cases can be considered as variants of that entity.
Except for two cases, where the parents opted for termination of pregnancy [6, 8], the reported outcome after corrective surgery showed to be excellent. Some of the neonates showed respiratory distress, but all were discharged at home without breathing support. In the presented case, the fetal lungs remained hypoplastic despite the insertion of a thoraco-amniotic shunt, probably due to the large amount of intrathoracic fluid that suppressed the expansion of the lungs at that early stage of the pregnancy. Unlike previous published data [17] a rather large discrepancy was observed between the estimated o/e LHR and the o/e TFVL. The rather small ultrasound based measurement could be attributed to the right anterior diaphragmatic defect and a more posterior displacement of the lungs, resulting in a small visible lung area at the reference level of the four chamber view. The low estimated o/e LHR and TFLV with the distinct liver herniation into the right hemithorax led us to the consideration of FETO to possibly improve the prognosis.
In conclusion, intrapericardial Morgagni hernia is a rare condition that should be considered in fetuses with large pericardial effusions. Given the potential for pulmonary hypoplasia and progression to hydrops as in our case, intrauterine fetal therapy in its various forms should be considered to improve the perinatal outcome.
References
Herman TE, Siegel MJ (2001) Bilateral congenital Morgagni hernias. J Perinatol 21(5):343–344. doi:10.1038/sj.jp.7200077
Aké E, Fouron JC, Lessard M, Boisvert J, Grignon A, van Doesburg NH (1991) In utero sonographic diagnosis of diaphragmatic hernia with hepatic protrusion into the pericardium mimicking an intrapericardial tumour. Prenat Diagn 11(9):719–724
Stevens RL, Mathers A, Hollman AS, MacKenzie JR, Galea P, Macdonald PD, Wilson N (1996) An unusual hernia: congenital pericardial effusion associated with liver herniation into the pericardial sac. Pediatr Radiol 26(11):791–793
Pober BR, Russell MK, Ackerman KG (2015) Congenital diaphragmatic hernia overview. In: Pagon RA, Adam MP, Ardinger HH, Bird TD, Dolan CR, Fong C-T, Smith RJ, Stephens K (eds) GeneReviews(®), University of Washington, Vol Seattle (WA), Seattle 1993–2016
Ikeda K, Hokuto I, Tokieda K, Nishimura O, Ishimoto H, Morikawa Y (2002) A congenital anterior diaphragmatic hernia with massive pericardial effusion requiring neither emergency pericardiocentesis nor operation. A case report and review of the literature. J Perinat Med 30(4):336–340. doi:10.1515/JPM.2002.050
Hara K, Kikuchi A, Takagi K, Kaneko S, Yasukochi S, Ogiso Y (2007) Massive pericardial effusion in an early gestational fetus having intrapericardial diaphragmatic hernia. J Obstet Gynaecol Res 33(4):561–565. doi:10.1111/j.1447-0756.2007.00571.x
Robnett-Filly B, Goldstein RB, Sampior D, Hom M (2003) Morgagni hernia: a rare form of congenital diaphragmatic hernia. J Ultrasound Med 22(5):537–539
Jain KK, Sen J, Rathee SK, Saini J (2008) Antenatal diagnosis of a Morgagni hernia in the second trimester. J Clin Ultrasound 36(2):116–118. doi:10.1002/jcu.20396
Antiñolo G, De Agustin JC, Losada A, Marenco ML, Garcia-Diaz L, Morcillo J (2010) Diagnosis and management of fetal intrapericardial Morgagni diaphragmatic hernia with massive pericardial effussion. J Pediatr Surg 45(2):424–426. doi:10.1016/j.jpedsurg.2009.11.009
Kanamori Y, Hashizume K, Sugiyama M, Tomonaga T, Goishi K, Yokoyama Y, Igarashi T, Kikuchi A, Kawana Y, Kozuma S, Taketani Y (2005) A case of intrapericardial diaphragmatic hernia with a massive pericardial effusion: fetal diagnosis and therapy. J Pediatr Surg 40(11):e43–e45. doi:10.1016/j.jpedsurg.2005.07.044
Grethel EJ, Hornberger LK, Farmer DL (2007) Prenatal and postnatal management of a patient with pentalogy of Cantrell and left ventricular aneurysm. A case report and literature review. Fetal Diagn Ther 22(4):269–273. doi:10.1159/000100788
Slone T, Emil S, Meissner N, Behjatnia B, Fairbanks T, Romansky S (2007) Sternal cleft, Morgagni hernia, and ectopic liver: a unique chest wall anomaly. J Pediatr Surg 42(12):2132–2135. doi:10.1016/j.jpedsurg.2007.08.053
Haino K, Serikawa T, Itsukaichi M, Numata M, Kikuchi A, Takakuwa K, Sakakibara S, Hirayama Y, Tanaka K (2011) Morgagni hernia with massive pericardial effusion diagnosed in the second trimester: prenatal diagnosis and perinatal management. Fetal Diagn Ther 29(1):108–110. doi:10.1159/000317272
Kalelioğlu IH, Karamustafaoğlu B, Has R, Gün F, Çelik A, Yüksel A (2012) Intrapericardial diaphragmatic hernia: report of 2 cases with prenatal diagnosis. J Ultrasound Med 31(11):1825–1828
Van Mieghem T, Cruz-Martinez R, Allegaert K, Dekoninck P, Castanon M, Sandaite I, Claus F, Devlieger R, Gratacos E, Deprest J (2012) Outcome of fetuses with congenital diaphragmatic hernia and associated intrafetal fluid effusions managed in the era of fetal surgery. Ultrasound Obstet Gynecol 39(1):50–55. doi:10.1002/uog.10097
Jeanty C, Nien JK, Espinoza J, Kusanovic JP, Gonçalves LF, Qureshi F, Jacques S, Lee W, Romero R (2007) Pleural and pericardial effusion: a potential ultrasonographic marker for the prenatal differential diagnosis between congenital diaphragmatic eventration and congenital diaphragmatic hernia. Ultrasound Obstet Gynecol 29(4):378–387. doi:10.1002/uog.3958
Sandaite I, Claus F, De Keyser F, Done` E, Van Mieghem T, Gucciardo L, DeKononck P, Jani J, Deprest JA (2011) Examining the relationship between the lung-to-head ratio measured on ultrasound and lung volumetry by magnetic resonance in fetuses with isolated congenital diaphragmatic hernia. Fetal Diagn Ther 29(1):80–87. doi:10.1159/000320204
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The institutional review board of the Universities of Bonn does not require formal approval for retrospective archive studies. Therefore, an ethical approval was not sought.
There were no sponsors for our study.
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from the participant included in the study.
Rights and permissions
About this article
Cite this article
Zamprakou, A., Berg, C., Strizek, B. et al. Morgagni hernia presenting with massive pericardial effusion and ascites: prenatal management by thoraco-amniotic shunting and fetal endoscopic tracheal occlusion (FETO) and review of the literature. Arch Gynecol Obstet 294, 953–958 (2016). https://doi.org/10.1007/s00404-016-4103-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-016-4103-0