Abstract
Purpose
Several studies have examined the association between glucokinase (GCK)-30G > A polymorphism and gestational diabetes mellitus (GDM). However, the results are still controversial. We performed the case–control study to investigate whether GCK-30G > A polymorphism correlates with the susceptibility of GDM in Chinese populations, and then conducted a meta-analysis by combining the previous studies.
Methods
We recruited 948 GDM patients and 975 controls from May 2011 to August 2013. All the subjects were genotyped using the PCR-based invader assay. The differences of allelic frequencies and genotype distributions between GDM patients and controls were investigated in case–control study. A systematic search of all relevant studies was conducted. The observational studies that were related to an association between the glucokinase (GCK)-30G > A polymorphism and GDM were identified. The association between the glucokinase (GCK)-30G > A polymorphism and GDM susceptibility was assessed using genetic models.
Results
The case–control study showed that GCK-30G > A polymorphism was associated with the susceptibility of GDM in a Chinese population. Furthermore, other six previously reported studies were included to perform meta-analysis. The meta-analysis showed that GCK-30G > A polymorphism was associated with GDM in Caucasian and Asian.
Conclusions
This study suggested that GCK-30G > A polymorphism may be associated with the susceptibility of GDM in a Chinese population. The further meta-analysis provides additional evidence supporting the above result that the risk allele of the GCK-30G > A polymorphism may increase GDM risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gestational diabetes mellitus (GDM) is the most common metabolic disorder during pregnancy, and is defined as glucose intolerance with onset or first recognition during pregnancy [1]. The prevalence of GDM varies in different ethnic populations. GDM affects about 1–3 % of all pregnancies in the western world but 5–10 % of Asian women [2, 3]. The frequency has increased in several populations during the last decade [4, 5]. Impaired beta cell function and insulin resistance characterize pregnancy complicated by GDM [6]. However, when insulin secretion is adjusted for the degree of insulin resistance, women with GDM have a severe reduction in beta cell function compared with normal pregnant women [7]. This beta cell dysfunction seems to persist in women with a history of GDM post-partum [6, 8].
In spite of much investigation, the causes of the development and progression of GDM have not been fully elucidated, and several evidence suggests that multiple genetic and environmental factors, as well as the interaction between these factors, determine the phenotype [9, 10]. Low et al. [11] reported that adiponectin SNP45TG is associated with GDM. Beltcheva et al. [12] showed that adiponectin promoter polymorphism rs266729 is associated with GDM.
Glucokinase (GCK), encoded by the GCK gene on chromosome 7p. GCK is a sensor and a key regulatory enzyme in the pancreatic beta cell, thus playing an important role in glucose homeostasis [13]. Because of its role in the regulation of insulin secretion, the GCK gene is an attractive candidate gene for both GDM and T2DM risk.
More than 600 mutations in the GCK gene have been described. Among these, the -30G > A (rs1799884) polymorphism in the GCK promoter region was found associated with T2DM [14], GDM [15] and obesity [16], although other studies failed to confirm these associations [14, 17]. The variant G to A at position -30 might lead to a decrease in GCK activity and to an increase in the threshold for glucose-stimulated insulin secretion [18]. Because this variant is located within a highly conserved region of the GCK promoter, it might alter transcriptional regulation of the gene [13]. If this variant reduced the transcription of the GCK gene, the cellular activity of GCK would likely decrease and lead to impaired glucose sensing, insulin secretion of beta cells and eventually diabetes [19].
Although several studies have investigated the role of the GCK-30G > A polymorphism in the development of GDM among various populations, results are still conflicting. To confirm the association between the GCK-30G > A polymorphism and GDM, we performed a case–control study for the association of rs1799884 with GDM in Chinese population, and then conducted a meta-analysis to derive a relatively comprehensive picture of the relationship between the GCK-30G > A polymorphism and the risk of GDM.
Materials and methods
Subjects
A total of 1,923 subjects were studied. All pregnant women were recruited from Obstetrics Department, Tianjin Central Hospital of Gynecology Obstetrics, Tianjin Medical University between May 2011 and August 2013. The present study was approved by the ethics committee of Tianjin Central Hospital of Gynecology Obstetrics, and all participants gave written, informed consent.
Eligible pregnant women underwent a 75-g OGTT at 24–32 weeks’ gestation (as close to 28 weeks as possible). Fasting, 1-h, and 2-h glucose levels were measured. Height, weight, and blood pressure were also measured using standardized procedures and calibrated equipment. A sample for random plasma glucose was collected at 34–37 weeks’ gestation as a safety measure to identify cases with hyperglycemia above a predefined threshold. Gestational age was determined as previously described. Demographic and lifestyle characteristics and age were collected via questionnaire. Race/ethnicity was self-identified. Participants and authors remained blinded to glucose values unless fasting plasma glucose was >5.8 mmol/l, 2-h OGTT plasma glucose was >11.1 mmol/l, random plasma glucose was >8.9 mmol/l, or any plasma glucose value was >2.5 mmol/l.
Genotyping
We selected the rs1799884 (GCK) single nucleotide polymorphisms (SNPs) for association analysis. Genomic DNA was extracted from peripheral blood leukocytes using genomic DNA isolation kits (Promega, Madison, WI) according to the manufacturer’s instructions. The primers, probes and reaction conditions were available upon request. SNPs were genotyped by the PCR-based invader assay (Third Wave Technologies) using ABI 7900 (Applied Biosystems, Foster City, CA, WI) [20]. Genotyping was done by laboratory personnel blinded to subject status. Of the samples, 10 % were tested twice to validate the genotyping results with 100 % reproducibility. Two authors independently reviewed the genotyping results, data entry, and statistical analysis.
Meta-analysis
Candidate studies had to meet the following criteria: (1) all patients meeting the diagnostic criteria for GDM, (2) case–control study focused on the relationship between the GCK-30G > A polymorphism and GDM, and (3) sufficient original data for calculating odds ratios (ORs) with corresponding 95 % confidence intervals (CIs). The major reasons for excluding studies were design other than a case–control study, duplicate publications, and no available data reported.
Two of the authors independently extracted the following data from each full-text report using a standard data extraction form. The data extracted from the studies included the title, authors, year of publication, study design, number of cases or controls, ethnicity, genotyping method, genotype distribution, and frequency of allele of the rs1799884 polymorphism in cases or controls.
Statistical analysis
Standard χ 2 analysis was used to examine differences of allelic frequencies and genotype distributions between GDM patients and controls in case–control study. Hardy–Weinberg equilibrium was tested by a goodness-of-fit χ 2 test.
Meta-analysis was performed with STATA 12.0 (Statacorp, college station, Tex). The association between the GCK-30G > A polymorphism and GDM susceptibility was assessed under the following genetic models, which were treated as a dichotomous variable: (a) A-allele versus G-allele for allele level comparison; (b) AA + AG versus GG for a dominant model of the A-allele; (c) AA versus AG + GG for a recessive model of the T-allele, and (d) AA versus GG for the extreme genotype. Statistical heterogeneity was assessed using Q statistics. A fixed-effects (inverse variance) model was used when the effects were assumed to be homogenous (P > 0.05). P < 0.05 implied statistical heterogeneity, and a random effects model was used in those circumstances. Two-sided P values less than 0.05 were considered statistically significant.
Results
A total of 1,923 subjects (948 cases and 975 controls) were successfully genotyped and subjected to statistical analysis. The distributions of the alleles and genotypes for rs1799884 are presented in Table 1. Genotype frequencies of GDM group and the control group were conformed to the Hardy–Weinberg equilibrium (P = 0.59 and 0.60, respectively).
Case–control association study
In accordance with the genome-wide association study (GWAS), the risk allele (A-allele) lead to a higher risk for GDM in Chinese population (Table 1). A-allele of the -30G > A polymorphism was significantly associated with increased risk of GDM.
Meta-analysis
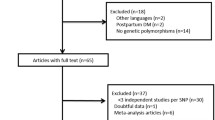
A total of 45 titles and abstracts were preliminarily reviewed, of which 5 of the published literature [15, 21–24] eventually satisfied the eligibility criteria (Fig. 1). All of the included studies investigated the relation between the rs1799884 polymorphism and GDM. Of these, one study contained data on two different groups [23]. We, therefore, performed meta-analysis between the previous studies [15, 21–24] and our case–control study. Ultimately, 6 studies (7 comparisons) investigated the relationship between -30G > A and GDM risk with a total of 2,959 GDM cases and 8,535 healthy controls. Characteristics of the studies that were included in the meta-analysis are presented in Table 2. First, we compared the allele frequency difference in GDM patients and controls. A significant relationship was observed between the A-allele and GDM susceptibility in all subjects (OR = 1.28, 95 % CI 1.17–1.39, P < 0.001). After stratification by ethnicity, subgroup analysis indicated that the A-allele frequency was significantly associated with GDM in Caucasian (OR = 1.24, 95 % CI 1.11–1.38, P < 0.001), and Asian (OR = 1.37, 95 % CI 1.18–1.60, P < 0.001), but not in African populations (OR = 1.12, 95 % CI 0.72–1.73, P = 0.618) (Fig. 2; Table 3). Summary ORs (95 % CI) and stratified analysis by ethnicity with various genetic models (AA vs. GG, AA + GA vs. GG, AA vs. GG + GA) are shown in Table 3.
Discussion
Gestational diabetes mellitus shares many risk factors with T2DM, and up to 50 % of women with GDM develop T2DM within 10 years after pregnancy [2]. However, the pathogenetic mechanisms of GDM is still unclear. GCK is the key glucose phosphorylation enzyme responsible for the first rate-limiting step in the glycolysis pathway and regulates glucose-stimulated insulin secretion from pancreatic beta cells and glucose metabolism in the liver [25]. Inactivating GCK mutations lead to maturity-onset diabetes of the young and neonatal diabetes [26], whereas activating GCK mutations cause persistent hyperinsulinemia and hypoglycemia [27, 28]. Therefore, GCK gene seems to play a key role in glucose homeostasis and is a determinant of diabetes risk in several populations. Although several case–control studies have evaluated the role of the GCK-30G > A polymorphism in the development of GDM among various populations, the results still remain controversial.
Genome-wide association study is a powerful method for the detecting genetic contributions to polygenic diseases, and has been increasingly used to study genetic predisposition in GDM. However, this method may produce spurious association [29, 30]. Therefore, replications of the associations in different ethnic groups and studies with large sample sizes are important to confirm the results of GWAS [31]. In the present study, we identified that GCK-30G > A polymorphism was associated with GDM susceptibility. The total number of GDM patients of the current study was more than that of previous studies, which provided more powerful evidence to support that rs1799884 may account for disease pre-disposition of GDM. Our study confirmed the earlier finding of positive association in Thailand population [23]. Moreover, the results of meta-analysis implicated that GCK-30G > A was associated with increased GDM susceptibility in the overall population. The present case–control study provides a more comprehensive summary of the currently available evidence on the association between the GCK-30G > A polymorphism and the risk of GDM.
Association studies are a useful tool to identify genetic factors conferring susceptibility to diseases [32]. However, the original association studies are not powerful enough to detect the genetic effects underlying the genetic susceptibility to develop diseases. This disadvantage has resulted in the reporting of inconsistent findings in the literature. The reasons for inconsistent results include the following items: limited sample size, poorly designed studies, false-positive studies, and different ethnicities. A meta-analysis method may permit the estimation of population-wide effects and the identification of sources of variability of genetic risk factors [33]. Therefore, we combined the genetic data from the included studies and present case–control study to evaluate the association of the rs1799884 polymorphism of the GCK gene to GDM with the help of a meta-analysis approach. Although seven comparisons were included in the present study, the study design of the original studies would be a limiting factor that exerts positive bias on the results. Genetic heterogeneity in the form of differences in the A-allele frequency of the rs1799884 polymorphism and the presence of admixture within the study populations could also be a source of heterogeneity influencing the conclusion of the present meta-analysis. Another potential explanation for the differences in the A-allele frequency is genotyping error, caused by different genotyping methods. To a certain degree, this could contribute to the variation in the A-allele frequency that we observed across the studies. Despite stratification by ethnicity, heterogeneity could not be absolutely omitted.
In the present study, stratification analyses were conducted to evaluate heterogeneity between studies. In the stratified analysis by ethnicity, a significant association was found in Caucasians and Asians for the polymorphism under most genetic models. However, no significant result was detected among Africans. The following items may account for such difference. (1) Different populations usually have different linkage disequilibrium patterns. The GCK-30G > A polymorphism may be in close linkage with different nearby causal variants in different populations. (2) The distribution of the A-allele varies extensively between different races. Further studies are still required to validate ethnic differences in the effect of this polymorphism on GDM. (3) Other clinical heterogeneity such as age, body mass index, years from onset, and disease severity may also explain the difference. Because only one study of Africans was included in the present meta-analysis. The exact conclusions of the meta-analysis in the African populations were doubtful due to the limited number of included studies.
Although stratification analyses were conducted, clinical heterogeneity cannot be resolved completely. Some degree of clinical heterogeneity was induced by the different genotyping method, severity of GDM, medical co-morbidities, nutritional status of patients and diagnostic criteria for GDM. Heterogeneity may also have been caused by different study design. Because of limited information got from original studies, heterogeneity cannot be completely resolved. Accordingly, although the results of the meta-analysis should be considered appropriate, methodological quality defects and clinical heterogeneity should be considered when interpreting the findings.
The limitations of this meta-analysis mainly include the following items: (1) The efficacy of the statistics may be further improved by including more studies in the future. The statistical power may be lower after subgroup analysis. Only one included study conducted in Africa cannot provide stable evidence. Further studies are still required in the future. (2) In the current meta-analysis, we only included published the English-language association studies of GCK-30G > A polymorphism and GDM. Thus, language bias may be an issue in the present study because it is exclusively based on the English-language reports. In addition, we may have missed some grey studies, including negative results or unpublished studies. Our reasons for the reluctance to include grey literature included the absence of peer review of such unpublished literature. Meta-analysis of unpublished data from interested sources is a controversial issue. (3) Genetic association studies have generated some confusion over the years. Although a meta-analysis can be useful for obtaining a sufficient sample size, controversies may not be resolved without suitable genetic models and standardized genotyping. (4) Although a meta-analysis can extract several similar studies to increase the statistical power, heterogeneity among studies can introduce some bias. Stratification by ethnicity may help to improve homogeneity among studies, but it may also influence statistical power. (5) Only published studies were included, and as a result, publication bias may have occurred.
Conclusions
This study suggested that GCK-30G > A polymorphism was associated with the susceptibility of GDM in a Chinese population. The further meta-analysis provides additional evidence supporting the above result that the risk allele of the GCK-30G > A polymorphism may increase GDM risk. Due to the limited data currently available for Africans and Asians, further studies with large sample sizes are required.
References
Reece EA, Leguizamon G, Wiznitzer A (2009) Gestational diabetes: the need for a common ground. Lancet 373(9677):1789–1797
Ben-Haroush A, Yogev Y, Hod M (2004) Epidemiology of gestational diabetes mellitus and its association with Type 2 diabetes. Diabet Med 21(2):103–113
Engelgau MM, Geiss LS, Saaddine JB, Boyle JP, Benjamin SM, Gregg EW, Tierney EF et al (2004) The evolving diabetes burden in the United States. Ann Intern Med 140(11):945–950
Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM (2004) An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol 103(3):526–533
Dabelea D, Snell-Bergeon JK, Hartsfield CL, Bischoff KJ, Hamman RF, McDuffie RS (2005) Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care 28(3):579–584
Buchanan TA (2001) Pancreatic B-cell defects in gestational diabetes: implications for the pathogenesis and prevention of type 2 diabetes. J Clin Endocrinol Metab 86(3):989–993
Xiang AH, Peters RK, Trigo E, Kjos SL, Lee WP, Buchanan TA (1999) Multiple metabolic defects during late pregnancy in women at high risk for type 2 diabetes. Diabetes 48(4):848–854
Osei K, Gaillard TR, Schuster DP (1998) History of gestational diabetes leads to distinct metabolic alterations in nondiabetic African-American women with a parental history of type 2 diabetes. Diabetes Care 21(8):1250–1257
Klein K, Haslinger P, Bancher-Todesca D, Leipold H, Knofler M, Handisurya A, Kautzky-Willer A et al (2012) Transcription factor 7-like 2 gene polymorphisms and gestational diabetes mellitus. J Matern Fetal Neonatal Med 25(9):1783–1786
Kim JY, Cheong HS, Park BL, Baik SH, Park S, Kim S, Shin HD et al (2013) Putative association between UBE2E2 polymorphisms and the risk of gestational diabetes mellitus. Gynecol Endocrinol 29(10):904–908
Low CF, Mohd TE, Chong PP, Idris F (2011) Adiponectin SNP45TG is associated with gestational diabetes mellitus. Arch Gynecol Obstet 283(6):1255–1260
Beltcheva O, Boyadzhieva M, Angelova O, Mitev V, Kaneva R, Atanasova I (2014) The rs266729 single-nucleotide polymorphism in the adiponectin gene shows association with gestational diabetes. Arch Gynecol Obstet 289(4):743–748
Rose CS, Ek J, Urhammer SA, Glumer C, Borch-Johnsen K, Jorgensen T, Pedersen O et al (2005) A -30G > A polymorphism of the beta-cell-specific glucokinase promoter associates with hyperglycemia in the general population of whites. Diabetes 54(10):3026–3031
Holmkvist J, Almgren P, Lyssenko V, Lindgren CM, Eriksson KF, Isomaa B, Tuomi T et al (2008) Common variants in maturity-onset diabetes of the young genes and future risk of type 2 diabetes. Diabetes 57(6):1738–1744
Shaat N, Karlsson E, Lernmark A, Ivarsson S, Lynch K, Parikh H, Almgren P et al (2006) Common variants in MODY genes increase the risk of gestational diabetes mellitus. Diabetologia 49(7):1545–1551
Gomez-Zumaquero JM, Rojo-Martinez G, Garcia-Escobar E, Martin-Nunez GM, Haro J, Esteva I, Ruiz DAM et al (2008) The -30G > A polymorphism of the glucokinase gene promoter is associated with obesity in a population from southern Spain. Obesity (Silver Spring). 16(8):1973–1975
Yamada K, Yuan X, Ishiyama S, Ichikawa F, Koyama KI, Koyanagi A, Koyama W et al (1997) Clinical characteristics of Japanese men with glucokinase gene beta-cell promoter variant. Diabetes Care 20(7):1159–1161
Rissanen J, Saarinen L, Heikkinen S, Kekalainen P, Mykkanen L, Kuusisto J, Deeb SS et al (1998) Glucokinase gene islet promoter region variant (G→A) at nucleotide -30 is not associated with reduced insulin secretion in Finns. Diabetes Care 21(7):1194–1197
Murad AS, Smith GD, Lewis SJ, Cox A, Donovan JL, Neal DE, Hamdy FC et al (2010) A polymorphism in the glucokinase gene that raises plasma fasting glucose, rs1799884, is associated with diabetes mellitus and prostate cancer: findings from a population-based, case-control study (the ProtecT study). Int J Mol Epidemiol Genet 1(3):175–183
Ohnishi Y, Tanaka T, Ozaki K, Yamada R, Suzuki H, Nakamura Y (2001) A high-throughput SNP typing system for genome-wide association studies. J Hum Genet 46(8):471–477
Zaidi FK, Wareham NJ, McCarthy MI, Holdstock J, Kalloo-Hosein H, Krook A, Swinn RA et al (1997) Homozygosity for a common polymorphism in the islet-specific promoter of the glucokinase gene is associated with a reduced early insulin response to oral glucose in pregnant women. Diabet Med 14(3):228–234
Santos IC, Frigeri HR, Rea RR, Almeida AC, Souza EM, Pedrosa FO, Fadel-Picheth CM et al (2010) The glucokinase gene promoter polymorphism -30G > A (rs1799884) is associated with fasting glucose in healthy pregnant women but not with gestational diabetes. Clin Chim Acta 411(11–12):892–893
Freathy RM, Hayes MG, Urbanek M, Lowe LP, Lee H, Ackerman C, Frayling TM et al (2010) Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study: common genetic variants in GCK and TCF7L2 are associated with fasting and postchallenge glucose levels in pregnancy and with the new consensus definition of gestational diabetes mellitus from the International Association of Diabetes and Pregnancy Study Groups. Diabetes 59(10):2682–2689
Chiu KC, Go RC, Aoki M, Riggs AC, Tanizawa Y, Acton RT, Bell DS et al (1994) Glucokinase gene in gestational diabetes mellitus: population association study and molecular scanning. Diabetologia 37(1):104–110
Matschinsky FM (1990) Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes 39(6):647–652
Njolstad PR, Sovik O, Cuesta-Munoz A, Bjorkhaug L, Massa O, Barbetti F, Undlien DE et al (2001) Neonatal diabetes mellitus due to complete glucokinase deficiency. N Engl J Med 344(21):1588–1592
Christesen HB, Jacobsen BB, Odili S, Buettger C, Cuesta-Munoz A, Hansen T, Brusgaard K et al (2002) The second activating glucokinase mutation (A456V): implications for glucose homeostasis and diabetes therapy. Diabetes 51(4):1240–1246
Gloyn AL, Noordam K, Willemsen MA, Ellard S, Lam WW, Campbell IW, Midgley P et al (2003) Insights into the biochemical and genetic basis of glucokinase activation from naturally occurring hypoglycemia mutations. Diabetes 52(9):2433–2440
Kingsmore SF, Lindquist IE, Mudge J, Beavis WD (2007) Genome-wide association studies: progress in identifying genetic biomarkers in common, complex diseases. Biomark Insights 2:283–292
McCarthy MI, Abecasis GR, Cardon LR, Goldstein DB, Little J, Ioannidis JP, Hirschhorn JN (2008) Genome-wide association studies for complex traits: consensus, uncertainty and challenges. Nat Rev Genet 9(5):356–369
Siontis KC, Patsopoulos NA, Ioannidis JP (2010) Replication of past candidate loci for common diseases and phenotypes in 100 genome-wide association studies. Eur J Hum Genet 18(7):832–837
Lohmueller KE, Pearce CL, Pike M, Lander ES, Hirschhorn JN (2003) Meta-analysis of genetic association studies supports a contribution of common variants to susceptibility to common disease. Nat Genet 33(2):177–182
Ioannidis JP, Ntzani EE, Trikalinos TA, Contopoulos-Ioannidis DG (2001) Replication validity of genetic association studies. Nat Genet 29(3):306–309
Conflict of interest
Each author certifies that he or she has no commercial associations that might pose a conflict of interest related to the submitted article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Han, X., Cui, H., Chen, X. et al. Association of the glucokinase gene promoter polymorphism -30G > A (rs1799884) with gestational diabetes mellitus susceptibility: a case–control study and meta-analysis. Arch Gynecol Obstet 292, 291–298 (2015). https://doi.org/10.1007/s00404-015-3635-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-015-3635-z