Abstract
Purpose
To assess which is the optimal protocol in terms of endometrial preparation prior to frozen-thawed embryo transfer (FET) in women with polycystic ovarian syndrome (PCOS) and to explore the effect in stimulated cycle with the addition of vaginal 17-β oestradiol.
Methods
Five hundred and seventy-six patients with PCOS were prepared for FET using artificial cycle induced with oestradiol and progesterone supplementation (n = 291) and stimulated cycle induced by human menopausal gonadotrophin (HMG) within or without the addition of vaginal 17-β oestradiol (n = 285). Then the FET was performed in a receptive endometrium.
Results
Endometrial thickness was similar (9.03 ± 1.65 vs. 9.12 ± 1.58, P > 0.05) in artificial and stimulated cycle. The two protocols resulted in clinical pregnancy rate (41.0 % vs. 41.6 %, P > 0.05), ongoing pregnancy rate (36.6 % vs. 34.7 %, P > 0.05), live birth rate (30.0 % vs. 31.7 %, P > 0.05), which were not statistically different. Nevertheless, the cancelled cycle rate made a significant difference (2.2 % vs. 5.4 %, P < 0.05). There is no significant difference in the clinical pregnancy rate in HMG, HMG added with vaginal oestradiol and HMG switch to vaginal oestradiol group (42.6 %, 41.1 %, and 33.3 %, respectively).
Conclusions
The mean endometrial thickness, clinical pregnancy rate, ongoing pregnancy rate, live birth rate and implantation rate were similar in artificial and stimulated cycle for endometrial preparation prior to FET in PCOS. It was fine to add vaginal 17-β oestradiol to stimulated cycle when necessary. However, stimulated cycles had a significantly higher cancelled cycle rate. We should follow the principles of individualization, securitization and optimization in endometrial preparation of the FET in patients with PCOS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the world first frozen-thawed embryo transfer (FET) achieved the clinical pregnancy in 1983 [1], FET had become an essential part of in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI) treatment. FET enables the surplus embryos generated by IVF/ICSI treatment to be stored and transferred at a later date. FET increases the cumulative pregnancy rate, reduces cost, prevents ovarian hyperstimulation syndrome (OHSS), is relatively simple to undertake compared with repeated “fresh” cycles. Furthermore, FET is a common procedure in women with polycystic ovarian syndrome (PCOS). The PCOS patients have high responses to controlled ovarian hyperstimulation (COH), therefore there is a high risk of developing OHSS. In order to avoid the OHSS, the high cancellation rates occur in fresh cycles [2].
In order to perform the FET, endometrial preparation should be accomplished. For the regularly ovulating patients, natural cycle may be a good choice. But for the patients with PCOS, the protocol to prepare the endometrium seems to be more complex. PCOS, a common endocrine disorder, is characterized by menstrual irregularities, infertility, clinical and biochemical hyperandrogenism, ovarian enlargement, abnormal gonadotropin secretory dynamics, obesity and insulin resistance, which do not coexist in all women diagnosed with PCOS [3, 4]. The endometrium in patient with PCOS should be well prepared prior to FET to synchronize the development of embryo with the endometrium. To overcome this problem, the endometrium can be prepared using either artificial priming with exogenous oestradiol followed by progesterone or mild ovarian stimulation with human menopausal gonadotrophin (HMG) [5].
Oestradiol valerate is widely used to prime the endometrium. The oral route of administration is simple and well tolerated, whereas it is extensively metabolized by the intestinal mucosa and subsequently the liver. The vaginal 17-β oestradiol could bypass the first-pass hepatic metabolism. Thus, vaginal administration of oestradiol results in high serum levels and even higher endometrial tissue levels [6]. Induced ovulation is an another method to prime the endometrium. HMG is an alternative follicle-stimulating drug for FET preparation [7]. The granulosa cells of the developing follicle produce oestradiol in response to HMG stimulation, so that the endometrium could be prepared. In the stimulated cycles, we noticed that there were an increased number of patients with the thin endometrium or the failure to develop mature follicle.
In view of the above, we designed a prospective study to explore the effect in stimulated cycle with the addition of vaginal 17-β oestradiol and assess which is the optimal protocol between artificial cycle induced with oestradiol and progesterone supplementation and stimulated cycle induced by HMG within or without the addition of vaginal 17-β oestradiol in terms of endometrial preparation prior to FET in women with PCOS.
Materials and methods
Patients
A total of 638 patients with PCOS undergoing FET cycles between 1 January 2010 and 31 December 2012 were invited. The Rotterdam criteria define PCOS by the presence of at least two out of three criteria: oligo- and/or anovulation, clinical and/or biochemical hyperandrogenism and polycystic ovaries. Clinical hyperandrogenism was assessed as the self-reported degree of hirsutism using the modified Ferriman–Gallwey (mF–G) scoring method [8]. The women compared the amount of body hair they had before hair removal with a chart displaying degree of hair growth in nine regions. Clinical hyperandrogenism was defined by a mF-G score ≥8. Biochemical hyperandrogenemia was defined as the finding of elevated androgens, the specific cut-off values for testosterone and DHEAS were 65 ng/dL and 2,800 μg/L [9]. Polycystic ovaries were identified by vaginal ultrasound, conducted in the follicular phase or when hormonal assessment showed no follicular activity. Polycystic ovaries means ≥12 follicles measuring 2–9 mm in diameter, or ovarian volume >10 mL in at least one ovary [10]. To establish, the diagnosis of PCOS is important to exclude the other androgen excess disorders with a similar clinical presentation but with different etiologies (congenital adrenal hyperplasia, androgen-secreting tumours, Cushing’s syndrome, hyperprolactinemia, thyroid dysfunction). In order to exclude other pathologies, we considered the following parameters: serum thyroid stimulating hormone (TSH), fT3, fT4, PRL, 17OH-progesterone (17-OHP) and plasma cortisol. The cut-off points for prolactin, TSH and free thyroxine were 30 ng/mL, 0.35–4.94 μIU/mL and 10–23 pmol/L, respectively. When basic 17-OHP levels were >1.5 ng/mL, the ACTH test was conducted to rule out congenital adrenal hyperplasia. Other causes of hyperandrogenism, including prolactinoma, Cushing’s syndrome and androgen-secreting tumours, were also excluded.
Inclusion criteria for participation in this study were maternal age <35 years, the first FET cycle, previous conventional IVF/ICSI with two or more good embryo cryopreservation on day 3 and normal intrauterine cavity after pretreatment assessment. Exclusion criteria were the use of testicular sperm for ICSI, the use of preimplantation genetic diagnosis (PGD), early (day 3) follicular phase follicle-stimulating hormone (FSH) levels ≥12 IU/L, endometriosis of stage III or higher, hydrosalpinges and body mass index(BMI) ≥28 kg/m2.
The research project was accepted by our Institutional Review Board. All patients signed informed consent before the treatment procedure.
Procedures
These 576 women with PCOS, who met the inclusion criteria, were assigned randomly to either the artificial cycle (n = 291) or the HMG cycle (n = 285). Participants were allocated randomly into one of the two study groups. The random allocation was performed by a study nurse at consultation, using a computer generated randomization list. From day 3 of the menstrual cycle or progesterone withdrawal, patients underwent cycle were monitored by a series of transvaginal ultrasound examination, urine luteinizing hormone (LH) analysis and serum hormone assay for FSH, LH, oestradiol and progesterone. Endometrial thickness and follicle diameter were measured consistently using a 6.5-MHz vaginal probe (Sonoline, Adara, Siemens). All ultrasound examinations were performed by a single physician. No women reported use of any medication during the last semester that could interfere with the normal function of the hypothalamic–pituitary–gonadal axis, including metformin. The protocols used prior to FET were the following.
Artificial cycle group
Artificial cycles were commenced on the third day of menstrual cycle or progesterone withdrawal without prior downregulation of gonadotrophin releasing hormone agonist (GnRHa). The dose of oestradiol valerate (Progynova®, Schering AG, Berlin, Germany) was 2 mg twice daily. Vaginal ultrasound and hormone assays were performed on day 10. If the endometrial thickness reached ≥8 mm, 40-mg progesterone injection was given i.m. for 3 days (20 mg per ampoule; Shanghai General Pharmaceutical Factory, China) and oral oestradiol was continued, then embryos were transferred on the fourth day of progesterone treatment. If the endometrial thickness was <7 mm on day 10, the dose of oestradiol valerate was increased to 4 mg twice daily. If the endometrium reached a minimum thickness of 7 mm on day 14, progesterone treatment was started and the FET procedure was followed. If the endometrial thickness had not reached 7 mm on day 14, patients were switched to vaginal 17-β oestradiol (Femoston®, Abbott Biologicals B.V., The Netherlands) in addition to oral oestradiol valerate. Using the Progynova 8 mg/day orally and Femoston 2 mg/day vaginally to support the endometrial development until the endometrium was ≥7 mm, at which point they began progesterone treatment. If the endometrial thickness was not ≥7 mm by day 20, the cycle was cancelled.
HMG group
In the HMG group, 75 IU/day of HMG (75 IU; Livzon Pharmaceutical Factory, China) was injected as a low-dose regimen from the day 5 to day 9 of menstrual cycle or progesterone withdrawal. Ultrasound and hormone assays were performed on day 10, and the dose of HMG was increased in 75–150 IU increments for another 5–10 days when needed to stimulate the development of follicle and endometrial proliferation. Transvaginal ultrasound and hormonal surveillance were continued. When the endometrial thickness of 7 mm or more was reached and follicle diameter was above 18–20 mm and urine LH was <25 IU/L, 5,000–10,000 IU of HCG (5,000 IU per ampoule, Profasi; Serono) was used to trigger final oocyte maturation, and the thawed embryos were transferred 5 days later. If urine LH was ≥25 IU/L, the same dose of hCG was given and FET was performed 4 days later. If urine LH was ≥25 IU/L and endometrial thickness did not reach 7 mm, the cycle was cancelled. If the endometrial thickness is <7 mm on day 15, the patients were added with the vaginal 17-β oestradiol (Femoston®, 2 mg per day). If no follicle diameter above 18–20 mm was found on day 20, this protocol was actually switched to vaginal 17-β oestradiol 2 mg per day to prime endometrium. Once the endometrial thickness is ≥7 mm on day 20 within or without the mature follicle ovulation, the FET was performed. Cycles with endometrial thickness continuously <7 mm by day 20 were cancelled due to poor endometrial response. Cycles with a premature progesterone rise were cancelled as well. Thawed embryo transfer was performed after beginning progesterone supplementation 72 h in advance for embryos frozen on day 3.
Embryo freezing and thawing
The fresh cleavage-stage embryos had been frozen according to the vitrification protocol on day 3 in a previous IVF/ICSI treatment. All embryos were assigned grades in accordance with strict criteria [11]. Cleaved embryos with at least six cells on day 3 and less than 10 % fragmentation were considered type A. If the percentage of anucleate fragments was between 10 and 30 % and/or 4–6 cells were present on day 3, the embryos were considered type B. If >50 % anucleate fragments were present and/or if less than four cells were present on day 3, the embryos were classified type C. Embryos were selected for cryopreservation if they had reached 6–8 cells on day 3 (64–66 h) of in vitro culture, had symmetrical component blastomeres devoid of multinucleation, and showed no more than 10 % cytoplasmic fragmentation. The transferred embryos had at least 50 % intact blastomeres after thawing. The number of embryos transferred in each case depended on the number of previous treatments, the number of embryos frozen in the same straw, and the quality of available embryos.
Embryo transfer
Patients presented with a full bladder, which would provide an acoustic window for the visualization of the uterus, in preparation for the cavity measurements and ultrasound-guided ET. All ETs were performed using Cook catheter (Soft Pass, J-SPPE; Cook Ob/Gyn, Spencer, IN, USA), while the ultrasonographer performed the abdominal ultrasound using a 5-MHz probe (Sonoline, Adara, Siemens). The tip of the catheter was loaded with the embryos and was placed to a level of 1.0–2.0 cm below the apex of the endometrial cavity as ascertained by transabdominal ultrasound. After embryo transfer, the patient will lie in bed for 30 min. The three operators participating in this trial all had similar results during the last 6 months (approximately 2,000 transfers).
Luteal supplementation
The luteal supplementation was administrated by vaginal progesterone. Utrogestan® (Lab Besins Internat S.A., Paris, France) was dosed 200 mg in the morning and 200 mg in the evening from the day of embryo transfer for 14 days. If the serum β-HCG was >30 IU/L 14 days after ET, the patients were advised to continue the luteal support until the 8–10th gestational week. In artificial cycle group, the same dose of oestradiol was continued until the 8–10th gestational week and then decreased step by step. In HMG with vaginal oestradiol addition group, the vaginal oestradiol was decreased step by step from the assurance of clinical pregnancy with the mature follicle ovulation, whereas the vaginal oestradiol was decreased a third every 3 days step by step from the 8–10th gestational week without the mature follicle ovulation.
Definition of clinical outcomes
The serum β-HCG >30 IU/L indicated the positive of pregnancy test 14 days after ET. A clinical pregnancy was defined as the observation of a gestational sac with foetal heartbeat on ultrasound scanning between 4 and 5 weeks of gestation after the positive pregnancy test. An ongoing pregnancy was a pregnancy that reached a viable gestational age of ≥24 weeks. Implantation rate was defined as the number of gestational sacs observed on ultrasound compared with the number of embryos transferred. A miscarriage was defined as the cessation or lack of detection of cardiac activity in the gestational sac or the inability to detect a previously confirmed gestational sac until week 28 of gestation. The live birth rate was the number of live births per embryo transfer cycle.
Hormone measurement and biochemical assays
Serum levels of FSH, LH, total testosterone (T), β-HCG, dihydroepiandrosterone sulphate (DHEAS), 17-hydroxyprogesterone (17-OHP), insulin and plasma glucose were measured. Analyses were performed at the Department of Clinical Laborator, The First People’s Hospital of Yunnan Province. Serum FSH, LH, total T and β-HCG levels were assessed by a chemiluminescent method using the Access® hFSH kit, Access® hLH kit, Access® Testosterone Calibrators kit and Access® Total β-HCG kit, respectively (Beckman Coulter, Inc., USA) with the UniCel Dxi 800 Access analyzer (Beckman Coulter, Inc., USA). The sensitivity and the intra- and interassay coefficients of variation were FSH 0.2 IU/L, 3.5 and 4.5 %, respectively; LH 0.2 IU/L, 3.6 and 4.6 %, respectively. Total T 0.5 ng/mL, 3.7 and 4.7 %, respectively, β-HCG 0.5 mIU/mL, 3.5 and 4.7 %, respectively. DHEAS and 17-OHP were online extracted from serum and separated by reversed phase chromatography and detected by tandem mass spectrometry. The sensitivity and the intra- and inter-assay coefficients of variation were DHEAS 35 nmol/L, 9 and 10 %, respectively, and 17-OHP 0.4 nmol/L, 9 and 10 %, respectively. Serum insulin was assayed using a competitive chemiluminescent immunoassay performed on the manufacturer’s DPC Immulite 2000 analyser (Euro/DPC). The analytical sensitivity of the insulin assay was 2 μU/mL, 3.8 and 4.4 % for insulin. Plasma glucose was measured on fluoride oxalate samples by a glucose oxidase method on a Roche Modular clinical chemistry analyzer with a between batch coefficient of variation of <2.5 %. Urine LH was measured by semi-quantitative LH urine test paper (Kunming Yunda biotechnology Co. Ltd.). It was important that the samples collected were first morning urine samples, because such samples result in optimal uniformity and maximum concentration. The insulin resistance index (HOMA-IR) was calculated using the formula: HOMA-IR = fasting serum insulin (microunits per millilitre) × fasting plasma glucose (micromoles per litre)/22.5. Day 1 after the urine LH rise, the disappearance of the mature follicle, a drop of oestradiol and concomitant rise of progesterone >1.5 nmol/L confirmed the ovulation.
Statistical analysis
Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. Results of continuous variables were given as means with standard deviations (SDs) if normally distributed, and as medians with interquartile ranges (IQR) if not normally distributed. Categorical variables were presented as percentages (%). Statistical comparison was carried out by Student’s t test, Mann–Whitney U test, one-way analysis of variance (ANOVA), where appropriate. Chi-square test or Fisher’s exact test for categorical variables, where appropriate. Parameters in the different groups were initially assessed by Chi-square analysis and analysis of variance with Bonferroni’s post hoc test. P values are two sided, and values <0.05 represent statistical significance. With the number of patients included in our study and a power of 80 %, the study is able to demonstrate a difference in pregnancy rate of about 15 % between both groups. A multiple logistic regression was used to evaluate further the association between cycle outcome and those factors that might potentially influence outcome. The independent factors studied were type of protocol, age, BMI, baseline serum FSH, Basal serum LH, serum total T, Serum DHEAS, HOMA-IR, No. of oocytes retrieved, No. of oocytes fertilized (2PN), endometrial thickness, No. of embryos transferred. The model of logistic regression was gained by a stepwise procedure, and specific interactions between parameters of interest were also investigated. Models were compared by the likelihood ratio test. The statistical analysis was performed with the Statistical Package for Social Sciences version 11.0 (SPSS, Chicago, IL, USA). A P value of <0.05 was considered to indicate statistical significant.
Results
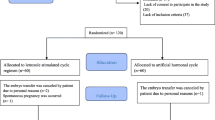
Of the initial 638 women invited to participate, 576 women satisfied the study criteria and were included in this analysis (Fig. 1). In the artificial cycle group, FET was cancelled in 18 of 291 patients, 6 for the thin endometrium and 12 for the failure of the embryos to survive thawing. In the HMG group, FET was not performed in 23 of 285 patients, 5 were due to the thin endometrium, 10 were due to premature LH surge or progesterone rise and 8 were due to the failure of thawing. The cancelled cycle rate (exclude the failure of embryos to survive thawing) between artificial cycle and HMG cycle made a significant difference (2.2 vs. 5.4 %, P = 0.043). Overall, 273 artificial cycles and 262 HMG cycles were included in the final analysis of implantation and pregnancy rate.
As demonstrated in Table 1, patients in the two groups had similar demographic characteristics and fresh cycle characteristics. Age in artificial and stimulated cycles (30.51 ± 3.35 vs. 30.48 ± 3.34, P = 0.913) and BMI (22.74 ± 2.09 vs. 22.58 ± 2.17, P = 0.357) were similar. There were no significant differences between the two groups in terms of the basal serum FSH, basal serum LH, serum total T, serum DHEAS, serum 17-OHP, plasma glucose, serum insulin, HOMA-IR value, glucose/insulin ratio, number of retrieved oocytes and number of fertilized oocytes (2PN) in the fresh cycles.
FET cycle outcomes between the two groups are presented in Table 2. Post-thawing embryo survival rate and the number of transferred embryos were not significantly different. Endometrial thickness on the day of progesterone supplementation was similar (9.03 ± 1.65 vs. 9.12 ± 1.58, P = 0.376). The two protocols resulted in clinical pregnancy rate (41.0 vs. 41.6 %, P = 0.892), ongoing pregnancy rate (36.6 vs. 34.7 %, P = 0.647), live birth rate (30.0 vs. 31.7 %, P = 0.681), which were not statistically different. Nevertheless, the cancelled cycle rate (exclude the failure of embryos to survive thawing) made a significant difference (2.2 vs. 5.4 %, P = 0.043).
In artificial cycle, the baseline characteristics in these subgroups were similar. The endometrial thickness in oral and additional vaginal oestradiol group was significantly lower than in oral oestradiol-alone group (9.75 ± 1.46 vs. 9.04 ± 1.35, P < 0.01). However, the clinical pregnancy rate (40.4 vs. 42.9 %, P = 0.718), ongoing pregnancy rate (36.5 vs. 37.1 %, P = 0.918) and live birth rate (30.5 vs. 28.6 %, P = 0.756) were similar (Table 3).
Table 4 described the cycle outcomes in the stimulated group. The differences did not reach the statistical significance in endometrial thickness, clinical pregnancy rate (42.6 %, 41.1 %, and 33.3 %, P = 0.718, respectively), ongoing pregnancy rate and live birth rate in HMG, HMG added with vaginal oestradiol and HMG switch to vaginal oestradiol group.
Figure 2 showed the association between the clinical pregnancy rates and the endometrial thickness recorded on the day of P supplementation. The lowest rates were associated with endometrial thicknesses of 7 mm (20 %) and >15 mm (0 %). Higher clinical pregnancy rates were maintained at endometrial thicknesses ranging from 9 to 14 mm. There was a significant difference in the clinical pregnancy rate between FET cycles with an endometrial thickness of 7–8 mm (26.9 %) and those with an endometrial thickness of 9–10 mm (56.1 %; P < 0.01) or 11–14 mm (51.2 %; P < 0.01). However, the clinical pregnancy rates in cycles with 9–10 mm thickness and in those with 11–14 mm thickness were not different (56.1 vs. 51.2 %, P = 0.32).
In the logistic regression model, age, the number of embryos transferred and endometrial thickness were significantly associated with pregnancy outcome. Pregnancy rate improved with increased endometrial thickness; the estimated odds ratio (OR) for successful pregnancy with each additional millimetre of endometrial thickness was 1.12 (95 % CI 1.05–1.2, P = 0.002). Age was negatively associated with pregnancy outcome (95 % CI 0.93–0.99, P = 0.016), while the number of embryos transferred was positively associated with pregnancy outcome (Table 5). The interactions between type of protocol and age (χ 2 = 0.051, df = 1; P = 0.821), type of protocol and BMI (χ 2 = 0.074, df = 1; P = 0.786), type of protocol and baseline serum FSH (χ 2 = 0.037, df = 1; P = 0.834), type of protocol and basal serum LH (χ 2 = 0.023, df = 1; P = 0.898), type of protocol and serum total T (χ 2 = 0.151, df = 1; P = 0.694), type of protocol and serum DHEAS (χ 2 = 0.009, df = 1; P = 0.924), type of protocol and HOMA-IR (χ 2 = 0.137, df = 1; P = 0.712), type of protocol and No. of oocytes retrieved (χ 2 = 0.031, df = 1; P = 0.873), type of protocol and No. of oocytes fertilized (2PN) (χ 2 = 0.108, df = 1; P = 0.748), type of protocol and endometrial thickness (χ 2 = 0.043, df = 1; P = 0.847), type of protocol and the number of embryos transferred (χ 2 = 0.165, df = 1; P = 0.676) were examined and tested by the likelihood ratio test. None of these interactions was significant.
Discussion
The results of this research demonstrated the similar implantation and pregnancy rates between artificial and stimulated cycles for endometrial preparation prior to FET in women with PCOS. There are many studies to research which is the most effective policy in infertility patients, but there are very few studies that aim at the PCOS patients only. A recent meta-analysis showed that there were no differences in the clinical pregnancy rate, ongoing pregnancy rate or live birth rate between the nature cycle, modified natural cycle using hCG, artificial cycle and artificial cycle supplemented with GnRHa in infertility patients [12]. However, there is little consensus on the most effective method of endometrium preparation prior to FET in women with PCOS.
A conclusion is in agreement that pregnancies can be achieved in women without ovarian function and that oestradiol and progesterone are apparently the only hormones that need to be provided to generate a receptive endometrial environment [13]. Each of these stages is associated with a specific sequence of hormonal signals that travel through the circulation and stimulate the endometrium to first proliferate and then transform to a receptive state. Oestradiol priming results in endometrial proliferation and induction of progesterone receptors in most cases. Oestradiol and progesterone can be supplemented by exogenously administered or generated from the developing follicle by induced ovulation. Based on the theory above, PCOS patients characterized by chronic anovulation [4] need to be performed to prepare the endometrium prior to FET using the programmed cycle.
In artificial cycle, oestradiol and progesterone are given in a sequential protocol which aims to mimick the endocrine exposure of the endometrium in the normal cycle. In the beginning, oestradiol is administered to cause proliferation of the endometrium, while suppressing the development of the dominant follicle. This is continued until the endometrial thickness is observed to be 7 mm or more, at which time progesterone is added to initiate the transformation to secretory. Sometimes, the artificial cycle is prepared within GnRHa. A prospective randomized study found that endometrial preparation with an artificial cycle using oestradiol and progesterone alone is simpler, less expensive and more convenient for patients, with similar pregnancy rates, when compared with GnRHa-primed cycles [14].
If the endometrium fails to respond to a short course of oral oestradiol valerate, the duration of stimulation should be extended and the dose of oral oestradiol valerate should be increased. If the endometrium had not reached a thickness of 7 mm on day 14, patients were switched to vaginal 17-β oestradiol in addition to oral oestradiol valerate. The greater efficiency of oestradiol delivery to the endometrium after vaginal administration makes this route a good option for patients who fail to achieve adequate endometrial thickness with oral oestradiol administration [15]. A recent study showed that vaginal oestradiol supplementation improved implantation rates [16]. Furthermore, if a patient failed to reach the thickness in HMG group, the vaginal oestradiol was added to help developing the endometrium.
17-β oestradiol has increased bioavailability compared with oestradiol valerate [17]. Vaginal administration of oestradiol improves endometrial proliferation and uterine perfusion compared with oral oestradiol, probably because of combined local and systemic effects [18]. Tourgeman et al. [6] reported that vaginal administration of oestradiol produced 10-fold higher serum oestradiol levels and 70-fold higher endometrial oestradiol levels than those achieved after equivalent doses administered by the oral route. Evidence suggests that the administration of vaginal oestradiol results in preferential absorption of oestradiol into the endometrium, consistent with a “uterine first pass” effect [19]. Furthermore, if the vaginal route of oestradiol administration is considered for artificial cycle, much lower doses of the standard oral quantities should be used. Once the uterus is prepared, the progesterone must be used to compensate for the high tissue levels of oestradiol.
A phenomenon was noted that the endometrial thickness in oral oestradiol group was significantly higher than in oral and additional vaginal oestradiol group. The reason for them to switch to additional vaginal oestradiol was their thin endometrial thickness. There may be several reasons needed to explore, such as some diseases in endometrium or the poor response to exogenous oestradiol. Therefore, it was reasonable to get the thinner endometrium in oral and additional vaginal oestradiol group. However, the pregnancy rate was similar. We make the subdividing groups to see the association between the clinical pregnancy rates and the endometrial thickness recorded on the day of P supplementation. The lowest rates were associated with endometrial thicknesses of 7 mm (20 %) and >15 mm (0 %). Higher clinical pregnancy rates were maintained at endometrial thicknesses ranging from 9 to 14 mm. However, the clinical pregnancy rates in cycles with 9–10 mm thickness and in those with 11–14 mm thickness were not different (56.1 % vs. 51.2 %, P = 0.32). Numerous studies have previously indicated that a thin endometrium (usually <7 mm) is associated with implantation failure [20, 21].
Mild ovarian stimulation is another choice to prime the endometrium. In terms of FET, we try our best to mimick a normal ovulatory cycle, endometrial development is controlled by the hormonal secretions of the ovary, which undergoes a series of predictable changes associated with follicle development, ovulation, and transformation to a corpus luteum. Based on the thesis described above, there would be a corpus luteum of pregnancy to support the further embryonic development. Nevertheless, patients in the HMG group did receive supplemental transvaginal progesterone to offset any potential for poor endogenous luteal phase progesterone secretion.
In our study, the protocols of HMG alone, HMG added with vaginal estradiol and HMG switching to vaginal estradiol that achieved the similar clinical pregnancy rate, ongoing pregnancy rate and live birth rate. Based on the results, we could do the protocols more flexible. We may change our protocols of endometrial preparation when the process of using HMG alone went in a poor situation. We could add vaginal estradiol to help developing the thin endometrium. When the women who were using mild ovarian stimulation with low-dose HMG failed to develop the mature follicle or ovulation, in order to decrease the cycle cancelled rate, we actually switched HMG cycle to artificial cycle using vaginal estradiol to develop the endometrium. In fact, we just need the hormones to react and generate a receptive endometrial. In this regard, the vaginal oestradiol for luteal supplementation was decreased step by step from the 8–10th gestational week because of the lack of corpus luteum. However, we received the similar pregnancy rate.
A result was noted that the cancelled cycle rate in HMG cycle was higher than the artificial cycle. There were 3.5 % women in HMG group who cancelled the cycle because of the premature LH surge or progesterone rise. The detrimental effect seems to be related to endometrial receptivity impairment according to lack of detrimental effect on oocytes competence [22]. A recent meta-analysis of over 60,000 cycles definitively shows that premature progesterone elevation has a detrimental effect on pregnancy rates after in vitro fertilization [23].
Besides, there are a variety of drugs for mild ovulation induction. Clomiphene citrate (CC) is a first-line drug for ovulation induction in PCOS [24], but it is not the first and appropriate choice for FET, probably because of its anti-oestrogenic effects on the endometrium [25, 26]. HMG is the conventional drug for ovulation induction in FET. However, HMG may stimulate multiple follicle growth and therefore increase the chances of OHSS. Recently, letrozole, an aromatase inhibitor, has been widely used as a potential ovulation induction agent for PCOS [27]. A pilot study was reported that the letrozole stimulation group had a significantly higher maximal endometrial thickness and significantly higher rates of clinical pregnancy, ongoing pregnancy and implantation, compared with the artificial and HMG stimulation groups [28].
Multiple factors influence the success rate of FET cycle [29]. Top quality embryo characteristics, endometrial preparation protocol, number of embryos transferred and BMI affected independently the LBR in FET. To control for confounding variables, our research excluded women with age ≥35 years, BMI ≥28 kg/m2 and so on. Age could be a prediction of ongoing pregnancy in embryo transfer [30]. Age was the significant factor on the clinical pregnancy rates [31]. It is also reported that women with higher BMI have a lower chance of pregnancy rate, require higher dose of gonadotrophins and have an increased miscarriage rate [32]. There is insufficient evidence on the effect of BMI on live birth, cycle cancellation, oocyte recovery and ovarian hyperstimulation syndrome [33].
For successful implantation to occur and to continue to progress to further embryonic development, the endometrium must be in the “window of implantation” [34, 35], a synchronized dialogue between a receptive endometrium and a functionally normal embryo. From proliferative to secretory, in response to progesterone, the endometrium undergoes profound conformational and biochemical changes, with a concomitant induction of endometrial receptivity and opening of the window of implantation [36, 37]. Adequate endometrial development can have a significant impact on the outcome of FET with thinner endometrium associated with lower implantation rate [38, 39]. Endometrial thickness alone is not predictive of pregnancy or pregnancy outcome, it was found to be positively associated with pregnancy rates [40]. Furthermore, the measurement of endometrial thickness may have sufficient predictive value to be used alone as an indicator of adequate oestradiol priming [41]. Hofmann et al. [41] thought the threshold of endometrial thickness that was reported as “adequate thickness”, which generally means more than 7 or 8 mm. Different studies chose the different threshold of endometrial thickness [42–44]. We chose 7 mm as minimum thickness for the endometrium.
Based on the current literatures, it is not clear to show which protocol would lead to a better pregnancy outcome in FET [45]. In artificial cycles, progesterone may be added at any time that is convenient once oestradiol stimulation has resulted in an endometrial thickness ≥7 mm. The drawback is the prolonged dual hormonal treatment required in case of pregnancy. Conversely, stimulated cycles require a more number of transvaginal ultrasound examination, the urine LH analysis, a precise timing of HCG administration, and subsequent FET, to avoid missing the implantation window. Both the ectopic pregnancy rates in our study were higher than the other studies reported. The reasons for most of the patients were PCOS combined with tubal factor; many of them had laparoscopic operation. These reasons played a potential or apparent influence. This issue needs a further study.
However, the present study was a study with a small size of the sample. It is better to do a large sample and multicentre randomized controlled trial.
In summary, similar clinical pregnancy rate, ongoing pregnancy rate, live birth rate and implantation rate in artificial and stimulated cycles for endometrial preparation prior to FET had been demonstrated in this study. Mean endometrial thickness was also equivalent between the two protocols; however, stimulated cycles had a significantly higher cancelled cycle rate. It was fine to add vaginal 17-β oestradiol to stimulated cycle when necessary. Therefore, ovulatory status, convenience (schedule and timing of transfer) and the protocol used in last FET determine the choice of protocol used for endometrial preparation in present FET cycle. We should follow the principles of individualization, securitization and optimization.
References
Trounson A, Mohr L (1983) Human pregnancy following cryopreservation, thawing and transfer of an eight-cell embryo. Nature 305:707–709
Broekmans FJ, Verweij PJ, Eijkemans MJ, Mannaerts BM, Witjes H (2014) Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Hum Reprod (Oxford, England). doi:10.1093/humrep/deu090
March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, Davies MJ (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod (Oxford, England) 25:544–551
Diamanti-Kandarakis E, Dunaif A (2012) Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr Rev 33:981–1030
Muasher SJ, Kruithoff C, Simonetti S, Oehninger S, Acosta AA, Jones GS (1991) Controlled preparation of the endometrium with exogenous steroids for the transfer of frozen-thawed pre-embryos in patients with anovulatory or irregular cycles. Hum Reprod (Oxford, England) 6:443–445
Tourgeman DE, Gentzchein E, Stanczyk FZ, Paulson RJ (1999) Serum and tissue hormone levels of vaginally and orally administered estradiol. Am J Obstet Gynecol 180:1480–1483
Van der Auwera I, Meuleman C, Koninckx PR (1994) Human menopausal gonadotrophin increases pregnancy rate in comparison with clomiphene citrate during replacement cycles of frozen/thawed pronucleate ova. Hum Reprod (Oxford, England) 9:1556–1560
Hatch R, Rosenfield RL, Kim MH, Tredway D (1981) Hirsutism: implications, etiology, and management. Am J Obstet Gynecol 140:815–830
Livadas S, Pappas C, Karachalios A, Marinakis E, Tolia N, Drakou M et al (2014) Prevalence and impact of hyperandrogenemia in 1,218 women with polycystic ovary syndrome. Endocrine. doi:10.1007/s12020-014-0200-7
Balen AH, Laven JS, Tan SL, Dewailly D (2003) Ultrasound assessment of the polycystic ovary: international consensus definitions. Hum Reprod Update 9:505–514
Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG (1992) The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in vitro fertilization and embryo transfer programme. Hum Reprod (Oxford, England) 7:117–119
Groenewoud ER, Cantineau AE, Kollen BJ, Macklon NS, Cohlen BJ (2013) What is the optimal means of preparing the endometrium in frozen-thawed embryo transfer cycles? A systematic review and meta-analysis. Hum Reprod Update 19:458–470
Paulson RJ, Hatch IE, Lobo RA, Sauer MV (1997) Cumulative conception and live birth rates after oocyte donation: implications regarding endometrial receptivity. Hum Reprod (Oxford, England) 12:835–839
Simon A, Hurwitz A, Zentner BS, Bdolah Y, Laufer N (1998) Transfer of frozen-thawed embryos in artificially prepared cycles with and without prior gonadotrophin-releasing hormone agonist suppression: a prospective randomized study. Hum Reprod (Oxford, England) 13:2712–2717
Tourgeman DE, Slater CC, Stanczyk FZ, Paulson RJ (2001) Endocrine and clinical effects of micronized estradiol administered vaginally or orally. Fertil Steril 75:200–202
Wright KP, Guibert J, Weitzen S, Davy C, Fauque P, Olivennes F (2006) Artificial versus stimulated cycles for endometrial preparation prior to frozen-thawed embryo transfer. Reprod Biomed Online 13:321–325
Paulson RJ (2011) Hormonal induction of endometrial receptivity. Fertil Steril 96:530–535
Fanchin R, Righini C, Schonauer LM, Olivennes F, Cunha Filho JS, Frydman R (2001) Vaginal versus oral E(2) administration: effects on endometrial thickness, uterine perfusion, and contractility. Fertil Steril 76:994–998
Tourgeman DE, Boostanfar R, Chang L, Lu J, Stanczyk FZ, Paulson RJ (2001) Is there evidence for preferential delivery of ovarian estradiol to the endometrium? Fertil Steril 75:1156–1158
Gonen Y, Casper RF, Jacobson W, Blankier J (1989) Endometrial thickness and growth during ovarian stimulation: a possible predictor of implantation in in vitro fertilization. Fertil Steril 52:446–450
Coulam CB, Bustillo M, Soenksen DM, Britten S (1994) Ultrasonographic predictors of implantation after assisted reproduction. Fertil Steril 62:1004–1010
Manno M, Tomei F (2014) Can we prevent premature luteinization in IVF cycles? Med Hypotheses 82:122–123
Venetis CA, Kolibianakis EM, Bosdou JK, Tarlatzis BC (2013) Progesterone elevation and probability of pregnancy after IVF: a systematic review and meta-analysis of over 60 000 cycles. Hum Reprod Update 19:433–457
Abu Hashim H (2012) Clomiphene citrate alternatives for the initial management of polycystic ovary syndrome: an evidence-based approach. Arch Gynecol Obstet 285:1737–1745
Eden JA, Place J, Carter GD, Jones J, Alaghband-Zadeh J, Pawson ME (1989) The effect of clomiphene citrate on follicular phase increase in endometrial thickness and uterine volume. Obstet Gynecol 73:187–190
Gonen Y, Casper RF (1990) Sonographic determination of a possible adverse effect of clomiphene citrate on endometrial growth. Hum Reprod (Oxford, England) 5:670–674
Mitwally MF, Casper RF (2001) Use of an aromatase inhibitor for induction of ovulation in patients with an inadequate response to clomiphene citrate. Fertil Steril 75:305–309
Hu YJ, Chen YZ, Zhu YM, Huang HF (2014) Letrozole stimulation in endometrial preparation for cryopreserved-thawed embryo transfer in women with polycystic ovarian syndrome: a pilot study. Clin Endocrinol 80:283–289
Veleva Z, Orava M, Nuojua-Huttunen S, Tapanainen JS, Martikainen H (2013) Factors affecting the outcome of frozen-thawed embryo transfer. Hum Reprod (Oxford, England) 28:2425–2431
Kim JH, Jee BC, Suh CS, Kim SH (2014) Nomogram to predict ongoing pregnancy using age of women and serum biomarkers after in vitro fertilization cycles. Eur J Obstet Gynecol Reprod Biol 172:65–69
Shapiro DB, Pappadakis JA, Ellsworth NM, Hait HI, Nagy ZP (2014) Progesterone replacement with vaginal gel versus i.m. injection: cycle and pregnancy outcomes in IVF patients receiving vitrified blastocysts. Hum Reprod (Oxford, England). doi:10.1093/humrep/deu121
Singh N, Gupta P, Mittal S, Malhotra N (2012) Correlation of body mass index with outcome of in vitro fertilization in a developing country. Arch Gynecol Obstet 285:259–263
Maheshwari A, Stofberg L, Bhattacharya S (2007) Effect of overweight and obesity on assisted reproductive technology–a systematic review. Hum Reprod Update 13:433–444
Navot D, Scott R, Droesch K, Veeck L, Liu H-C, Rosenwaks Z (1991) The window of embryo transfer and the efficiency of human conception in vitro. Fertil Steril 55:114–118
Bergh PA, Navot D (1992) The impact of embryonic development and endometrial maturity on the timing of implantation. Fertil Steril 58:537–542
Kodaman PH, Taylor HS (2004) Hormonal regulation of implantation. Obstet Gynecol Clin North Am 31:745–766
Critchley HO, Saunders PT (2009) Hormone receptor dynamics in a receptive human endometrium. Reprod Sci (Thousand Oaks, Calif) 16:191–199
Noyes N, Liu H, Sultan K, Schattman G, Rosenwaks Z (1995) Implantation: endometrial thickness appears to be a significant factor in embryo implantation in in vitro fertilization. Hum Reprod 10:919–922
El-Toukhy T, Coomarasamy A, Khairy M, Sunkara K, Seed P, Khalaf Y et al (2008) The relationship between endometrial thickness and outcome of medicated frozen embryo replacement cycles. Fertil Steril 89:832–839
Konc J, Kanyo K, Varga E, Kriston R, Cseh S (2010) The effect of cycle regimen used for endometrium preparation on the outcome of day 3 frozen embryo transfer cycle. Fertil Steril 94:767–768
Hofmann GE, Thie J, Scott RT Jr, Navot D (1996) Endometrial thickness is predictive of histologic endometrial maturation in women undergoing hormone replacement for ovum donation. Fertil Steril 66:380–383
Weissman A, Horowitz E, Ravhon A, Steinfeld Z, Mutzafi R, Golan A et al (2011) Spontaneous ovulation versus HCG triggering for timing natural-cycle frozen-thawed embryo transfer: a randomized study. Reprod Biomed Online 23:484–489
Kyrou D, Fatemi HM, Popovic-Todorovic B, Van den Abbeel E, Camus M, Devroey P (2010) Vaginal progesterone supplementation has no effect on ongoing pregnancy rate in hCG-induced natural frozen-thawed embryo transfer cycles. Eur J Obstet Gynecol Reprod Biol 150:175–179
Shi Y, Wei D, Liang X, Sun Y, Liu J, Cao Y et al (2014) Live birth after fresh embryo transfer vs elective embryo cryopreservation/frozen embryo transfer in women with polycystic ovary syndrome undergoing IVF (FreFro-PCOS): study protocol for a multicenter, prospective, randomized controlled clinical trial. Trials 15:154
Dal Prato L, Borini A (2006) Best protocol for frozen-thawed embryo transfer-cost benefit analysis needed. Fertil Steril 86:1554–1555 (author reply 5–6)
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yu, J., Ma, Y., Wu, Z. et al. Endometrial preparation protocol of the frozen-thawed embryo transfer in patients with polycystic ovary syndrome. Arch Gynecol Obstet 291, 201–211 (2015). https://doi.org/10.1007/s00404-014-3396-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-014-3396-0