Abstract
Introduction
Cigarette smoking is an established risk factor for adverse perinatal outcomes. The purpose of this study is to examine the association between maternal smoking in pregnancy and the occurrence of placental-associated syndromes (PAS).
Methods
We analyzed data from a population-based retrospective cohort of singleton deliveries that occurred in the state of Missouri from 1989 through 2005 (N = 1,224,133). The main outcome was PAS, a composite outcome defined as the occurrence of placental abruption, placenta previa, preeclampsia, small for gestational age, preterm or stillbirth. We used logistic regression models to generate adjusted odd ratios and their 95 percent confidence intervals. Non-smoking gravidas served as the referent category.
Results
The overall prevalence of prenatal smoking was 19.6%. Cigarette smoking in pregnancy was associated with the composite outcome of placental syndromes (odds ratio, 95% confidence interval = 1.59, 1.57–1.60). This association showed a dose–response relationship, with the risk of PAS increasing with increased quantity of cigarettes smoked. Similar results were observed between smoking in pregnancy and independent risks for abruption, previa, SGA, stillbirth, and preterm delivery.
Conclusion
Maternal smoking in pregnancy is a risk factor for the development of placenta-associated syndrome. Smoking cessation interventions in pregnancy should continue to be encouraged in all maternity care settings.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The placenta-associated syndromes (PAS) are an informal classification of disease states arising from diseased placental spiral arteries, placental ischemia, and endothelial dysfunction [1–4]. The syndromes encompass preeclampsia, a disease state characterized by hypertension and proteinuria after 20 weeks’ gestation; placental abruption or infarction; and the associated complications of preterm birth, fetal growth restriction, or intrauterine fetal death [5, 6]. Although the entities in PAS share some common mechanisms, their etiology is believed to be multifactorial and poorly defined [1]. Their classification into a syndrome arises as a result of suspected shared placental vasculopathy that significantly compromises placental function leading to sustained morbidity in the developing fetus or fetal demise.
Smoking during pregnancy is a recognized risk factor for a wide range of adverse fetal outcomes, including low birth weight, preterm birth, premature rupture of the membranes, placenta previa and placental abruption among others [7–9]. Cigarette smoke is also known to contain substances that affect placental endothelial function leading to the development of ischemic vascular changes that impact placental growth and functions [8, 10, 11]. Although the association between cigarette smoke and individual entities of placental syndromes have not been reported, it is likely that cigarette smoke is associated with the components of PAS through similar vascular/ischemic pathways. The exact relationship between maternal smoking and the occurrence of the placental syndromes has not, to our knowledge, been investigated. Accordingly, we undertook this study to assess the association between maternal smoking in pregnancy and the occurrence of PAS based on the following hypotheses:
-
1.
prenatal smoking is associated with placental syndromes;
-
2.
the association between smoking during pregnancy and placental syndromes show a dose-gradient effect, with the risk of PAS increasing with increase in the number of cigarettes smoked by the pregnant mother.
Methods
We utilized the Missouri maternally linked cohort data files covering the period 1989 through 2005. The Missouri vital record system is a reliable one that has been adopted as “gold standard” to validate US national datasets that involve matching and linking procedures [12].
For the purpose of this study, we selected singleton births within the gestational age range of 20–44 weeks. Based on the previously published reports [13], we assigned women to the following smoking categories: class I smoker (0–9 number of cigarettes per day); class 2 smoker (10–19 number of cigarettes per day); and class 3 smoker (≥20 number of cigarettes per day). We used non-smoking mothers as the referent category.
The main outcome of interest was PAS, a composite outcome defined as the occurrence of either placental abruption, placenta previa, preeclampsia, small for gestational age, preterm, or stillbirth. Placental abruption was defined as a condition where all or part of the placenta has pulled away from the uterine wall, disrupting the flow of blood and oxygen to the fetus. Placenta previa was defined as an obstetric complication in which the placenta is attached to the uterine wall close to or covering the cervix. Preeclampsia was defined as a diastolic blood pressure of at least 90 mmHg accompanied by proteinuria (≥0.3 g day or ≥1 on a urine dipstick) [14, 15]. Small for gestational age was defined as less than tenth percentile of birth weight for gestational age using population-based national reference curves for singletons [16]. Preterm was defined as births at <37 weeks. Stillbirth was defined as in utero fetal death at ≥20 weeks’ gestation.
Gestational age was largely based on the interval between the last menstrual period and the date of delivery of the baby (95% cases). When the menstrual estimate of gestational age was inconsistent with the birth weight (e.g., very low birth weight at term), a clinical estimate of gestational age on the vital records was used instead [17, 18].
The distribution of the following selected maternal sociodemographic characteristics was compared between smoking and non-smoking mothers to assess differences in baseline characteristics: maternal age, parity, race, education, marital status, and adequacy of prenatal care. Adequacy of prenatal care was assessed using the revised graduated index algorithm, which has been found to be more accurate than several others, especially in describing the level of prenatal care utilization among groups that are high risk [19, 20]. This index assesses the adequacy of care based on when the trimester prenatal care begun, number of visits, and the gestational age of the infant at birth. In this study, inadequate prenatal care utilization refers to women who either had missing prenatal care information, had prenatal care but the level was considered sub-optimal, or mothers who had no prenatal care at all. We performed crude frequency comparisons between the two groups for the presence of common obstetric complications, namely, anemia, insulin-dependent diabetes mellitus, other types of diabetes mellitus, and chronic hypertension, preeclampsia, eclampsia, abruption placenta, and placenta previa.
Statistical analysis
Chi-square test was used to assess differences in sociodemographic characteristics and maternal pregnancy complications between the two groups (smoking/non-smoking). We used logistic regression models to generate adjusted odd ratios and their 95% confidence intervals. The covariates included in our model are maternal age, parity, race, education, marital status, adequacy of prenatal care, gender of the infant, and year of birth. We constructed the regression models and assessed goodness-of-fit of the regression models using the −2 log likelihood ratio test. We estimated the significance of main effects by means of the Wald test, and assessed dose–response using the χ2 test for linear trend [21].
Adjusted estimates were derived in all cases using non-smoking gravidas as the referent category. All tests of hypothesis were two-tailed with a type-1 error rate fixed at 5%. SAS version 9.1 (SAS Institute, Cary, NC) was used to perform all analyses. This study was approved by the Office of the Institutional Review Board at the University of South Florida.
Results
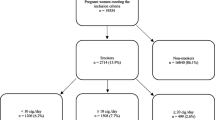
A total of 1,312,505 singleton births were available for analysis. We excluded pregnancies before 20 weeks or beyond 44 weeks of gestation (70,803 or 5.4%) and records for which prenatal smoking (9,646 or 0.77%), gestational age (7,595 or 0.61%), or birth weight (328 or 0.03%) values were missing (total = 17,569 or 1.4%). The final dataset comprised a total of 1,224,133 singleton records, consisting of smokers (240,247 or 19.6%) and non-smokers (983,886 or 80.4%; the referent group).
Overall, the prevalence of prenatal smoking was 19.6%. Figure 1 illustrates the trend of prevalence of prenatal smoking during pregnancy and PAS in the study population over the course of the study. Table 1 shows frequency comparison between smoking and non-smoking mothers with respect to selected sociodemographic characteristics. Smoking gravidas were more likely to be younger, white, multiparous and to have achieved a lower level of education than non-smokers. Smoking mothers were also less likely to be married and to have received adequate prenatal care than their non-smoking counterparts.
Table 2 displays the prevalence of common medical and obstetric complications among mothers in the study. The overall prevalence of pregnancy complications was 32.3% (N = 395,218) distributed as follows: smokers (N = 100,051 or 25.3%) and non-smokers (295,167 or 74.7%). Of the obstetric complications, anemia, placental abruption, placenta previa, SGA, preterm, and stillbirth were more likely among smoking gravidas. Preeclampsia, insulin-dependent diabetes, other forms of diabetes, and chronic hypertension were more common among mothers who did not engage in prenatal smoking. Infants born to non-smoking mothers had slightly greater mean gestational age [mean = 38.9 weeks, standard deviation (SD): ±2.5] than those born to smoking mothers [mean = 38.7 ± 2.8] (P < 0.01). Infants of non-smoking mothers were also found to have higher mean birth weight (3,387 ± 592 vs. 3,132 ± 593) (P < 0.01).
Figure 2 is an illustration of the distribution of rates of placenta syndromes by prenatal smoking status. There were 276,918 (22.6%) infants born to mothers who were diagnosed with placenta-associated syndrome in the study population. Infants born to mothers diagnosed with placenta-associated syndrome had lower mean gestational age [mean = 36.6 weeks, standard deviation (SD): ±3.8] than those born to mothers without placenta-associated syndrome (mean = 39.5 ± 1.5) (P < 0.01). Infants of mothers diagnosed with placenta-associated syndrome were also found to have lower mean birth weight (2,714 ± 708 vs. 3,520 ± 416) (P < 0.01).
The adjusted estimates for the association between prenatal smoking and placenta-associated syndrome are summarized in Table 3. Overall, mothers who engaged in smoking during pregnancy had a 59% increased risk for placental syndromes. A dose–response relationship was evident between the number of cigarettes smoked and the risk for placenta-associated syndrome. The increased risk of PAS ranged from 44% in class 1 smokers to 78% in class 3 smokers. The findings were similar for abruption, previa, SGA, stillbirth, and preterm. Preeclampsia had reduced risk levels for smokers, with the risk level decreasing, as the number of cigarettes smoked increased.
Discussion
In this large population-based sample, we observed an increased risk for PAS among women who engaged in smoking during pregnancy. The elevated risk showed a dose–response gradient in relation to the number of cigarettes smoked daily, with heavy smokers (at least 1 pack per day) being almost twice as likely to develop PAS than their counterparts who did not smoke in pregnancy. This finding is relevant because the effect of maternal cigarette use in pregnancy varies across the different disease states that comprise PAS. Although smoking in pregnancy is an established risk factor for placental abruption and placenta previa [22–25], in the case of preeclampsia, the evidence points to a protective effect, especially in primiparous pregnancies [26]. Our results corroborate these findings from the previous literature and support the assertion that placenta previa and abruption do not share a common etiology with preeclampsia in relation to cigarette smoking [22]. Understanding the association of smoking in pregnancy and the risk of PAS represents a first step toward enhancement of our knowledge on the role of intrauterine nicotine exposure in mediating these and other adverse pregnancy outcomes.
Previous studies suggest that women with a history of placental syndrome have a twofold increased risk of developing remote cardiovascular disease [1]. Cardiovascular disease is the leading cause of mortality in females [27]. The association between smoking in pregnancy and PAS shown in this study implies that the benefits of smoking cessation before pregnancy may extend not just to lowering the risk of placental syndromes and other documented poor birth outcomes, but also to a lower likelihood of subsequent development of cardiovascular dysfunction in affected mothers. This information can be used in counseling women of reproductive age as part of the efforts to enhance quitting rates among smokers. Knowledge of other benefits of not smoking during pregnancy may serve as an additional incentive for smokers to quit.
The prevalence of PAS reported here (>30% among smokers and 22.6% in the entire cohort of mothers) is much higher than previous estimates. Ray et al. [1] reported a prevalence of 7% among a large sample of mothers from Canada. The difference could be attributable to variation in the definition of our composite measure (PAS). Although Ray et al. defined maternal placental syndromes to include preeclampsia, gestational hypertension, placental abruption or placental infarction, we used a much broader definition in our study population. In addition to the conditions considered by Ray et al. our definition of PAS included fetal birth outcomes (small for gestational age, preterm or stillbirth). Other factors that could explain the lack of agreement in prevalence estimates include differences in demographics (especially maternal age and race) and in the occurrence of obesity.
Maternal smoking can predispose to PAS through different pathologic mechanisms culminating in a state of chronic intrauterine hypoxia. Nicotine and carbon monoxide generated by cigarette smoke act as potent vasoconstrictors of placental vessels, thereby compromising placental blood flow [10]. Carbon monoxide in tobacco smoke also interferes with fetal oxygenation by forming carboxyhemoglobin [28]. In addition, nicotine can raise maternal blood pressure and heart rate, further impairing blood flow to the fetus [10, 11]. The net effect of these insults is increased gestational bleeding, premature rupture of the membranes, placenta previa, and placental abruption [29, 30].
There are limitations to our study findings. First, previous investigators have highlighted the importance of implementing smoking cessation programs as early as possible in pregnancy, preferably in the preconception period [7, 31]. Unfortunately, our inability to obtain information on the trimester in which smoking occurred during pregnancy or whether the mothers quit smoking at any time during their pregnancy precludes us from commenting on the association between timing of smoking cessation and occurrence of PAS in our cohort. Second, cigarette smoking status was based on maternal self-report and was not validated by more objective assessments, such as serum cotinine levels. However, any misclassification resulting from this limitation is likely to skew our findings toward the null, as pregnant mothers are more likely to underreport smoking during pregnancy [32]. That we are still able to demonstrate significant association between maternal smoking and risk of PAS highlights the strength of the relationship. Third, we were unable to account for the possible effects of exposure to second-hand smoke, including paternal smoking habits, information that is not documented in vital records. Finally, cocaine use has been shown to predispose to placental abruption [33] and women who use cocaine are also more likely to be smokers. However, information on cocaine use was not available in our database.
The strengths of this study include a large sample size that improved the generalizability of our findings. Our use of a composite for PAS reflects the idea that the various disease conditions that comprise PAS share a common pathogenesis involving a disruption in placental function. Another strength is the relative completeness of our data source, with only about 0.8% missing data on prenatal smoking habit over the period of the study.
In summary, we found that maternal smoking in pregnancy is a risk factor for the development of PAS. Our findings highlight the need for ensuring that smoking cessation interventions in pregnancy continue to be encouraged in all maternity care settings.
References
Ray JG, Vermeulen MJ, Schull MJ, Redelmeier DA (2005) Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet 366:1797–1803
Zhang P, Schmidt M, Cook L (2006) Maternal vasculopathy and histologic diagnosis of preeclampsia: poor correlation of histologic changes and clinical manifestation. Am J Obstet Gynecol 194:1050–1056
Granger JP, Alexander BT, Llinas MT, Bennett WA, Khalil RA (2001) Pathophysiology of hypertension during preeclampsia linking placental ischemia with endothelial dysfunction. Hypertension 38:718–722
Brosens JJ, Pijnenborg R, Brosens IA (2002) The myometrial junctional zone spiral arteries in normal and abnormal pregnancies: a review of the literature. Am J Obstet Gynecol 187:1416–1423
Jaffe R (2001) Development of early uteroplacental circulation. Early Preg 5:34–35
Redman CW, Sargent IL (2001) The pathogenesis of pre-eclampsia. Gynecol Obstet Fertil 29:518–522
Polakowski LL, Akinbami LJ, Mendola P (2009) Prenatal smoking cessation and the risk of delivering preterm and small-for-gestational-age newborns. Obstet Gynecol 114:318–325
Aliyu MH, Salihu HM, Wilson RE, Kirby RS (2007) Prenatal smoking and risk of intrapartum stillbirth. Arch Environ Occup Health 62:87–92
[No authors listed] (2002) Women and smoking: a report of the Surgeon General. Executive Summary. MMWR Recomm Rep 51:1–13
Lambers DS, Clark KE (1996) The maternal and fetal physiologic effects of nicotine. Semin Perinatol 20:115–126
Cogswell ME, Weisberg P, Spong C (2003) Cigarette smoking, alcohol use and adverse pregnancy outcomes: implications for micronutrient supplementation. J Nutr 133:1722–1731
Martin J, Curtin S, Saulnier M, Mousavi J (2003) Development of the matched multiple birth file: 1995–1998 matched multiple birth dataset. NCHS CD-ROM series 21, no. 13a. National Center for Health Statistics, Hyattsville
Salihu HM, Aliyu MH, Bosny JP, Alexander GR (2003) Levels of excess infant deaths attributable to maternal smoking during pregnancy in the United States. Matern Child Health J 7:219–227
Aliyu MH, Luke S, Kristensen S, Alio A, Salihu HM. Joint effect of obesity and teenage pregnancy on the risk of preeclampsia: a population-based study. J Adolesc Health (in press). doi:10.1016/j.jadohealth.2009.06.006
Hernández-Díaz S, Toh S, Cnattingius S (2009) Risk of pre-eclampsia in first and subsequent pregnancies: prospective cohort study. BMJ 18(338):b2255. doi:10.1136/bmj.b2255
Alexander GR, Kogan M, Martin J, Papiernik E (1998) What are the fetal growth patterns of singletons, twins, and triplets in the United States? Clin Obstet Gynecol 41:114–125
Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M (1996) A United States national reference for fetal growth. Obstet Gynecol 87:163–168
Taffel S, Johnson D, Heuser R (1982) A method of imputing length of gestation on birth certificates. Vital Health Stat 2:1–11
Alexander GR, Cornely DA (1987) Prenatal care utilization: its measurement and relationship to pregnancy outcome. Am J Prev Med 3:243–253
Alexander GR, Kotelchuck M (1996) Quantifying the adequacy of prenatal care: a comparison of indices. Public Health Rep 111:408–418
Clayton D, Hills M (1993) Statistical models in epidemiology. Oxford University Press, Oxford
Ananth CV, Savitz DA, Luther ER (1996) Maternal cigarette smoking as a risk factor for placental abruption, placenta previa, and uterine bleeding in pregnancy. Am J Epidemiol 144:881–889
Williams MA, Mittendorf R, Lieberman E et al (1991) Cigarette smoking during pregnancy in relation to placenta previa. Am J Obstet Gynecol 165:28–32
Williams MA, Lieberman E, Mittendorf R et al (1991) Risk factors for abruptio placentae. Am J Epidemiol 134:965–972
Monica G, Lilja C (1995) Placenta previa, maternal smoking and recurrence risk. Acta Obstet Gynecol Scand 74:341–345
Xiong X, Wang FL, Davidge ST, Demianczuk NN, Mayes DC, Olson DM, Saunders LD (2000) Maternal smoking and preeclampsia. J Reprod Med 45:727–732
Mosca L, Ferris A, Fabunmi R, Robertson RM, American Heart Association (2004) Tracking women’s awareness of heart disease: an American Heart Association national study. Circulation 109:573–579
Salihu HM, Aliyu MH, Kirby RS (2005) In utero nicotine exposure and fetal growth inhibition among twins. Am J Perinatol 22:421–427
Meyer MB, Tonascia JA (1977) Maternal smoking, pregnancy complications, and perinatal mortality. Am J Obstet Gynecol 128:494–502
Berkowitz GS, Papiernik E (1993) Epidemiology of preterm birth. Epidemiol Rev 15:414–443
McCowan LM, Dekker GA, Chan E, Stewart A, Chappell LC, Hunter M, Moss-Morris R, SCOPE consortium (2009) Spontaneous preterm birth and small for gestational age infants in women who stop smoking early in pregnancy: prospective cohort study. BMJ 338:b1081
Pickett KE, Kasza K, Biesecker G, Wright RJ, Wakschlag LS (2009) Women who remember, women who do not: a methodological study of maternal recall of smoking in pregnancy. Nicotine Tob Res
Handler AS, Mason ED, Rosenberg DL et al (1994) The relationship between exposure during pregnancy to cigarette smoking and cocaine use and placenta previa. Am J Obstet Gynecol 170:884–889
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aliyu, M.H., Lynch, O., Wilson, R.E. et al. Association between tobacco use in pregnancy and placenta-associated syndromes: a population-based study. Arch Gynecol Obstet 283, 729–734 (2011). https://doi.org/10.1007/s00404-010-1447-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-010-1447-8