Abstract
Catecholamines (epinephrine, norepinephrine and dopamine) are considered toxic to the melanocytes and may play an important role in the development of depigmented patches on the skin. This study was done to evaluate the levels of catecholamines in skin and plasma samples of active vitiligo patients’ and gene expression changes in catecholamines’ metabolism regulatory genes (COMT and GTPCH1), immunoregulatory genes (CTLA4 and PTPN22), and Catalase in active vitiligo patients. In this single-centre, prospective, case–control study, 30 patients with active vitiligo were recruited and skin biopsies from the perilesional site and plasma samples were collected. Skin biopsies from the normal site in vitiligo patients and healthy controls (n = 15) and plasma samples from controls were also obtained. Catecholamines’ estimation was done via high-performance liquid chromatography. Gene expression variations were investigated via reverse transcription–polymerase chain reaction (PCR) and real-time PCR. Epinephrine, norepinephrine and dopamine levels were significantly higher in perilesional skin biopsies as compared to controls (P = 0.035, 0.024, and 0.006, respectively). However, epinephrine, norepinephrine and dopamine levels observed in patients’ plasma samples were comparable to controls. The mRNA expression level of the Catalase gene was found to be upregulated at the perilesional site of patients as compared to the non-affected site of same patients (P < 0.001) and healthy controls (P = 0.037). Transcriptional expression of GTPCH1 and COMT were observed to be increased significantly at the perilesional site of patients in comparison to controls (P = 0.004 and P = 0.046, respectively). Our results support the presence of oxidative stress, inflammation and induced immune response in vitiligo patients at the perilesional sites. The increased inflammatory response may lead to catecholamines upregulation resulting in oxidative stress and melanocyte damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vitiligo is a chronic non-contagious, multifactorial pigmentary skin disorder causing depigmented patches on the body due to melanocytes destruction. Several theories have been proposed explaining vitiligo pathogenesis but the exact cause and mechanism behind melanocyte destruction is still a matter to confirm. Autoimmunity, oxidative stress, melanocytorrhagy, genetic and environmental causes are some of the factors that have been associated with vitiligo pathogenesis [1]. Among these, oxidative stress is a debated factor to be involved in disease precipitation in spite of genetic predisposition. Another possible phenomenon to be involved in vitiligo progression is an inflammatory response as a consequence of induced or irregulated immune response. The inflammatory response is known to stimulate the secretion of catecholamines [2]. The main biological catecholamines are epinephrine (E), norepinephrine (NE) and dopamine (DA). Catecholamines are implicated in oxidative stress development via the accumulation of their oxidation products [quinones, semiquinone radicals and hydrogen peroxide (H2O2)] which are toxic for melanocytes [3]. Catalase is involved in the degradation of H2O2 and plays a crucial role in protecting cells against reactive oxygen species (ROS). Inflammatory cytokines and catecholamines can have a direct effect on melanocytes. Being possibly localised to the site of depigmentation, catecholamines’ accumulation due to inflammatory response may be a reason behind melanocyte damage in patches in vitiligo. The inflammatory response has been found to upregulate the expression of GTPCH1 and downregulate the COMT expression [4, 5]. GTPCH1 plays role in maintaining the biosynthesis of catecholamines, while COMT is a key enzyme for the degradation of catecholamines via methylation and prevents the formation of toxic quinones during melanin biosynthesis [6, 7]. Furthermore, CTLA-4 (CD152), a surface molecule of activated T cells, has an essential inhibitory function in maintaining the homeostasis of the immune system and increased expression is associated with higher severity in inflammatory diseases [8, 9]. While PTPN22 is expressed in T-cells, B-cells, NK cells and dendritic cells and encodes a tyrosine phosphatase that inhibits Src-family kinases responsible for Ag receptor signalling in lymphocytes [10].
To explore the catecholamines accumulation and metabolism in vitiligo patients with active disease, we analysed perilesional skin biopsies for the levels of catecholamines and its metabolism regulatory system genes, GTP cyclohydrolase 1 (GTPCH1) and catechol-O-methyltransferase (COMT) expression pattern and compared with levels of these seen in the non-affected site in vitiligo patients and healthy controls. Further expression changes of antioxidant system gene, Catalase and inflammatory response regulating gene, liver X receptor-alpha (LXR-α) were investigated. Expression changes of cytotoxic T-lymphocyte-associated protein 4 (CTLA4) and protein tyrosine phosphatase, non-receptor type 22 (PTPN22) were also observed to assess active immune response.
Materials and methods
Enrolment of patients and controls
In this single-centre prospective case–control study, 30 patients of non-segmental vitiligo having active disease visiting the Department of Dermatology of our tertiary care centre were enrolled for the study after obtaining written informed consent. The study was approved by the institutional ethics committee. Enrolled patients were not taking any medical treatment at least for the last 4 months. Patients aged < 18 years and pregnant women were not included in the study. Healthy donors (n = 15) of the same age group were enrolled for the study as controls.
Samples collection
Blood samples (4.0 ml) and only perilesional epidermal skin graft (up to 1.5 cm square) were taken from all 30 patients. An epidermal graft from a non-affected site was taken from six patients randomly out of all thirty patients. Blood samples and healthy skin grafts from non-tanned areas of 15 healthy controls were also taken.
Blood samples processing for high-performance liquid chromatography (HPLC) analysis
Blood samples collected in ice-cold EDTA tubes were processed to harvest plasma by spinning for 30 min at 3500 rpm and 4 °C immediately. Then 250 µl of harvested plasma from samples was taken and sodium heparin solution (5% in 0.15 M NaCl) was added to it. Up to 10% of plasma volume was taken and incubated for 10 min under ice-cold conditions to facilitate lipoprotein precipitation. After this, perchloric acid was added to make the final concentration 0.1 M in the processed sample and further incubated for 5 min for total protein precipitation followed by centrifugation at 15,000 rpm for 30 min at 4 °C. After centrifugation, the supernatant was harvested and filtered with a 0.2 µm filter for further HPLC injection.
Skin biopsies processing for HPLC analysis
Skin biopsies were frozen in liquid nitrogen immediately after collection and were broken into very small pieces in a frozen state after weighing. After washing with phosphate buffer saline, tissue was transferred to an ice-cold microcentrifuge tube containing 250 µl of 0.1 M perchloric acid and then homogenized by ultrasonic disintegration over ice. Perchloric acid was used for protein precipitation. Lipoprotein precipitation was done with sodium heparin solution similarly to plasma samples. After homogenization and protein precipitation, samples were centrifuged at 15,000 rpm for 30 min at 4 °C. After centrifugation supernatant was harvested and filtered with a 0.2 µm filter for further HPLC injection.
HPLC analysis for catecholamines level in patients’ plasma as well as perilesional skin biopsies
HPLC separation for E, NE and DA was performed on a reverse-phase pinnacle II C18 (5 µm, 250 × 4.6 mm) column with Hitachi HPLC system. A volume of 20 µl per injection was used for each blank, standards as well as samples. Further dilution of processed samples if required was done with distilled water filtered with 0.2 µm filter. Mobile phase containing 95% of 50 mM potassium dihydrogen phosphate buffer with 0.5 mM of 1-octane sulphonic acid sodium salt and 5% of acetonitrile was passed through the column with a flow rate of 1.0 ml per minute. The temperature was maintained at 4 °C to avoid the decomposition of samples. Detection was done with a UV–visible detector at 280 nm. (+ −)-Epinephrine, (−)-nor-Epinephrine and Dopamine hydrochloride were used as standards with working concentrations prepared in 0.1 M perchloric acid. Quantitative analysis was based on an external standard method, where calibration curves were prepared with various known concentrations of each standard. A straight-line graph was obtained for peak area vs. various known concentrations (from 0 to 5000 pg/ml) of each standard. The concentration of unknown samples was further estimated as unit/ml for plasma samples and unit/mg of tissue samples used initially for analysis after considering dilution factor as well.
RNA extraction
Total cellular RNA from skin and blood samples of patients and controls was extracted by TRI Reagent (Ambion) following the manufacturer's protocol. RNA yield and purity was determined spectrophotometrically by measuring absorbance at 260 nm and 280 nm with absorbance 260/280 ratio > 1.90. The integrity of RNA was verified by electrophoresis through 1.5% denaturing agarose gels stained with ethidium bromide.
cDNA synthesis
cDNA was synthesized with 1.0 µg of total cellular RNA using random hexamer primers and reverse transcriptase in a 20 µl reaction volume with RevertAid™ first-strand cDNA synthesis kit (Fermentas).
Gene amplification
Gene amplification was done with RT-PCR as well as with real-time PCR using RT-PCR kit (Invitrogen) and SYBR green master mix (Roche), respectively. Fold change was calculated based on real-time PCR data. β-Actin was taken as an internal reference gene. PCR for amplification was performed following pre-incubation at 95 °C for 10 min then amplification up to 35 cycles with denaturation at 95 °C for 30 s, annealing at annealing temperature for 30 s and extension at 72 °C for 45 s, and finally incubation at 72 °C for 10 min to stop the reaction. Primers used were as follows: β-actin (Ta: 60 °C), forward primer: 5′CATGTACGTTGCTATCCAGGC 3′ and reverse primer: 5′CTCCTTAATGTCACGCACGAT 3′; GTPCH1 (Ta: 55 °C), forward primer: 5′GGAGTTGCGGTTTTGTTTGT 3′ and reverse primer: 5′CCTGAACGAGCACAGAATGA 3′; COMT (Ta: 60 °C), forward primer: 5′CACGTCTGGCACCTTCAGTA 3′ and reverse primer: 5′ACACGCTTCTCTTGGAGGAA 3′; CTLA4 (Ta: 52 °C), forward primer: 5′CTTCAGTCACCTGGCTGTCA 3′ and reverse primer: 5′CTCAGCTGAACCTGGCTACC 3′; PTPN22 (Ta: 55 °C), forward primer: 5′CATCGGCAAGAAAGAAGGAC 3′ and reverse primer: 5′CACCAAATGTTCCCAAATCC 3′; Catalase (Ta: 53 °C), forward primer: 5′GAATCTCCGCACTTCTCCAG 3′ and reverse primer: 5′AGGCCAGTCCTGACAAAATG 3′; LXR-α (Ta: 62 °C), forward primer: 5′GAGAGGCTGCAGCACACATA 3′ and reverse primer: 5′ACAGTCATTCGTGCACATCC 3′. All primers were designed with the online primer designing tool Primer3.
Statistical analysis
Statistical analysis was performed with software sigma stat version 3.5 for data analysis for significant differences. Mean/standard deviation was calculated for all normally distributed continuous variables. The Student's t test was applied for comparison of catecholamines levels and gene expression in patients and controls. Mann–Whitney test was applied for gene expression comparison for the perilesional site of patients vs. normal healthy controls. ANOVA test was applied to compare the mean of continuous data. Categorical/qualitative variables were analysed using the Chi-square test between individual groups.
Results
Level of catecholamines in blood samples and skin biopsies
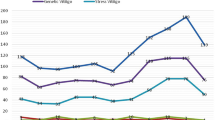
NE, E and DA got separated with a retention time of 3.76, 4.09 and 4.81 min, respectively (a small shift observed in the elution profile of samples in comparison to standards). Levels of NE, E and DA observed in patients plasma samples were 766.08 ± 101.61, 193.46 ± 71.45 and 66.06 ± 16.41 pg/ml, respectively, while in controls were 725.31 ± 93.45, 198.90 ± 111.37 and 78.27 ± 6.01 pg/ml, respectively. The difference in these levels was not statistically significant among patients and controls. In perilesional skin biopsies, levels of NE, E and DA in patients were 3.73 ± 0.91, 13.37 ± 4.57 and 3.84 ± 1.40 pg/mg of the sample, respectively, while in controls were 2.15 ± 0.99, 6.43 ± 3.36 and 1.53 ± 1.22 pg/mg of the sample, respectively. A significant increase was observed in NE (P = 0.035), E (P = 0.024) as well as DA (P = 0.006) in perilesional skin biopsies of patients in comparison to controls (Fig. 1).
Comparison for catecholamines (E, NE and DA) level in patients vs. controls after analyzing quantitatively with external standard method via HPLC; 1.1, Bar-graph of results for plasma samples; 1.2, Bar-graph of results for skin biopsies. Statistical significance is shown by *P < 0.05; 1.3, Catecholamines elution profiles via HPLC analysis: A known pure standards for E, NE and DA; B Plasma samples of patients; C Plasma samples of controls. D Tissue extracts prepared from skin biopsies of patients; E Tissue extracts prepared from skin biopsies of controls. Peaks of Nor-Epinephrine (NE), Epinephrine (E), and Dopamine (DA) are indicated
Catalase upregulation and oxidative stress presence
The mRNA expression level of the Catalase gene was found to be upregulated at the perilesional site of patients in comparison to the normal healthy donor (fold change 2.99 ± 1.76, P = 0.037) and at the perilesional site in comparison to the non-affected site of the same patients (fold change 2.76 ± 0.89, P ≤ 0.001) as well as in blood samples of patients in comparison to the normal healthy donor (fold change 6.23 ± 10.86, P = 0.002) (Fig. 2).
Transcriptional expression pattern of Catalase. A Graphical representation of real-time PCR-based analysis for fold change in the expression level of Catalase in vitiligo patients and controls. B Agarose gel electrophoresis representation for Catalase expression level in blood samples of controls (CB) and patients (PB). C Agarose gel electrophoresis representation for Catalase expression in skin biopsies of controls (C) as well as normal (PN) and perilesional site of same patients (PPL). Each bar represents the mean of relative gene expression level. Statistical significance is shown by *P < 0.05, **P < 0.001
Altered catecholamines metabolism
Transcriptional expression of GTPCH1 was observed to be increased significantly at the perilesional site of patients in comparison to controls (fold change 9.14 ± 3.18, P = 0.004) as well as at the perilesional site in comparison to the non-affected site of the same patient (fold change 4.95 ± 1.96, P ≤ 0.001). The mRNA expression level of COMT was also found significantly increased at the perilesional site of patients in comparison to normal healthy controls (fold change 3.40 ± 2.24, P = 0.046) but observed to be downregulated significantly at the perilesional site in comparison to the non-affected site of the same patient (fold change 0.82 ± 0.12, P = 0.019) (Fig. 3).
Transcriptional expression pattern for GTPCH-1 and COMT in vitiligo patients and controls. Graphical representation of real-time PCR-based analysis for fold change in gene expression level: A for patients vs. healthy controls; B perilesional site vs. normal site of the same patient. C Agarose gel electrophoresis representation for genes expression level for skin biopsies of normal (PN) and perilesional site of same patients (PPL) as well as healthy controls (C). Each bar represents the mean of relative gene expression level. Statistical significance is shown by *P < 0.05, **P < 0.001
Inflammatory response at the perilesional site in vitiligo patients
LXR-α, an inflammatory response regulating gene was found upregulated significantly (P = 0.006) at the perilesional site of patients in comparison to the non-affected site of the same patient with a fold change of 3.75 ± 1.28 (Fig. 4).
Transcriptional expression pattern for LXR-alpha in vitiligo patients: A Graphical representation of real-time PCR-based analysis for fold change in expression level at perilesional site vs. normal site of the same patient. B Agarose gel electrophoresis representation for expression level in skin biopsies of normal (PN) and perilesional site of the same patient (PPL). Each bar represents the mean of relative gene expression level. Statistical significance is shown by *P < 0.05, **P < 0.001
Increased expression level of immunoregulatory genes CTLA4 and PTPN22
We found mRNA expression levels of both CTLA4 and PTPN22 to be increased significantly at the perilesional site of patients in comparison to controls with fold change 6.82 ± 1.52 (P = 0.010) and 4.24 ± 2.80 (P = 0.026), respectively. In addition, both were found increased at perilesional site in comparison to normal site of same patient with fold change 2.39 ± 0.88 (P = 0.003) and 3.04 ± 0.81 (P ≤ 0.001), respectively (Fig. 5).
Transcriptional expression pattern for CTLA-4 and PTPN-22 in vitiligo patients and controls. Graphical representation of real-time PCR-based analysis for fold change in gene expression level: A for patients vs. healthy controls; B perilesional site vs. normal site of same patients. C Agarose gel electrophoresis representation for genes expression level for skin biopsies of normal (PN) and perilesional site of same patients (PPL) as well as healthy controls (C). Each bar represents the mean of relative gene expression level. Statistical significance is shown by *P < 0.05, **P < 0.001
Discussion
Oxidative stress has been postulated to play an important role in vitiligo pathogenesis. In our study, the levels of catecholamines (E, NE and DA) were found significantly upregulated in skin biopsies from the perilesional site of patients in comparison to controls. In blood samples, no significant difference was observed for catecholamines level. However, in previous studies, upregulated NE level in the blood has been reported as precipitating factor for non-segmental vitiligo [11]. Though we also observed an upregulated level of NE in blood samples but this was not statistically significant.
In addition to this, expression of Catalase was found to be elevated in the patient's blood as well as perilesional skin biopsies in comparison to normal healthy controls. Expression of Catalase was found more at the perilesional site as compared to the biopsies from normal sites of the same patient. This may be to dissipate the increased oxidative stress at the lesional site due to toxic metabolites of accumulated catecholamines. Molecular epidemiologic findings suggest that the CAT − 89A > T variant genotypes were associated with a significant decrease in Catalase enzyme activity and a genetic predisposition for vitiligo in Chinese people [12].
To explore it further, we found upregulated expressions of catecholamines’ metabolism regulatory genes COMT and GTPCH1 at the perilesional site of patients in comparison to normal healthy controls. Though on comparing with same patient's normal site, COMT expression was observed downregulated at the perilesional site, while GTPCH1 was still found upregulated. Increased expression of catecholamines metabolism regulatory genes COMT and GTPCH1 at the perilesional site of patients in comparison to normal healthy controls observed in the present study is probably associated with the increased level of catecholamines. As GTPCH1 plays role in maintaining biosynthesis of catecholamines, while COMT facilitates catecholamines degradation via methylation. Thus, upregulated GTPCH1 expression level supports more biosynthesis and more secretion of catecholamines, while COMT upregulation may be to normalize the catecholamines concentration by inducing the degradation of catecholamines. The observed lower level of COMT expression at the perilesional site of a patient in comparison to the normal site probably contributes to the accumulation of catecholamines at the perilesional site. An appropriate cause behind this lowered level of COMT expression may be an inflammatory response that has been reported earlier to downregulate COMT expression [4]. Induced inflammatory response at the perilesional site probably inhibits the upregulation of COMT expression level which further leads to reduced catabolism the increased level of catecholamines and reduced neutralization of its toxic effects. Along with this, the inflammatory response has also been known to induce GTPCH1 expression [5].
We observed an upregulated LXR-α expression at the perilesional site than the normal site of patients that support the presence of more inflammatory response at the perilesional site. Previous studies have reported increased inflammatory response in vitiligo patients’ serum that may be a consequence of induced immune response in patients [13, 14]. To confirm the presence of an active immune response, upregulated levels of CTLA4 and PTPN22 were observed at the perilesional site of patients in comparison to normal healthy controls as well as the normal site of the same patient. Elevated expression levels support the presence of induced immune response accordingly. Increased CTLA4 expression has been correlated with higher severity in other inflammatory diseases [9]. An inverse association has been found between PTPN22 expression level and the number of pre-Tregs and Tregs cells [15]. Treg cells are known to have a role in maintaining inflammatory response as depletion in Treg cells can upregulate proinflammatory cytokines [16]. An aberration in the suppressive function of regulatory T cells (Tregs) has been reported in vitiligo patients [17, 18], while an increase in Treg cells has been observed to prevent the development of vitiligo [19]. Thus, an increased PTPN22 expression in vitiligo patients as observed in our study supports the possible role of PTPN22 in inducing inflammatory response via Treg cell inhibition. Furthermore, an increased inflammatory response has been reported to induce catecholamines secretion [2]. Thus, observed upregulated catecholamines levels in skin biopsies may be in response to inflammatory cytokines. Alteration in catecholamines metabolism due to improper degradation via COMT can lead to accumulation of their by-products which are toxic to melanocytes and can cause oxidative stress development. Furthermore, genetically predisposed weak antioxidant system in vitiligo patients may be an additional factor responsible for melanocyte damage [20]. Upregulation of GTPCH1 expression itself may be in response to oxidative stress as maintenance of tetrahydrobiopterin (BH4) homeostasis is critical for the preservation of cellular redox balance, while de novo synthesis of BH4 is under the strict control of the rate-limiting enzyme GTPCH1 [7, 21]. Moreover, BH4 has been reported to have reactive oxygen species scavenging activity [22]. Thus, increased immune response as evidenced by increased CTLA4 and PTPN22 expression, elevated catecholamine levels supported by increased GTPCH1 and decreased COMT expression and increased Catalase levels observed in our study point towards increased oxidative stress at the perilesional sites in active vitiligo patients.
Conclusion
Accumulation of catecholamines and their by-products can lead to oxidative stress development due to improper metabolism. An inflammatory response is a possible cause of induced catecholamine secretion. Possibly catecholamines and inflammatory cytokines are two immediate factors to initiate a cascade effect for cell death after causing oxidative stress as well as alteration of differential gene expression system of melanocytes leading to the deterioration of cell physiology and cell death ultimately.
Availability of data and materials
The data sets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Rashighi M, Harris JE (2017) Vitiligo pathogenesis and emerging treatments. Dermatol Clin 35(2):257–265. https://doi.org/10.1016/j.det.2016.11.014
Fan J, Zhang B, Shu HF, Zhang XY, Wang X, Kuang F et al (2009) Interleukin-6 increases intracellular Ca2+ concentration and induces catecholamine secretion in rat carotid body glomus cells. J Neurosci Res 87(12):2757–2762. https://doi.org/10.1002/jnr.22107
Schallreuter KU, Wood JM, Pittelkow MR, Gutlich M, Lemke KR, Rodl W et al (1994) Regulation of melanin biosynthesis in the human epidermis by tetrahydrobiopterin. Science 263(5152):1444–1446
Tchivileva IE, Nackley AG, Qian L, Wentworth S, Conrad M, Diatchenko LB (2009) Characterization of NF-kB-mediated inhibition of catechol-O-methyltransferase. Mol Pain 5:13. https://doi.org/10.1186/1744-8069-5-13
Katusic ZS, Stelter A, Milstien S (1998) Cytokines stimulate GTP cyclohydrolase I gene expression in cultured human umbilical vein endothelial cells. Arterioscler Thromb Vasc Biol 18(1):27–32. https://doi.org/10.1161/01.atv.18.1.27
Li K, Li C, Gao L, Yang L, Li M, Liu L et al (2009) A functional single-nucleotide polymorphism in the catechol-O-methyltransferase gene alter vitiligo risk in a Chinese population. Arch Dermatol Res 301(9):681–687. https://doi.org/10.1007/s00403-008-0920-8
Thöny B, Auerbach G, Blau N (2000) Tetrahydrobiopterin biosynthesis, regeneration and functions. Biochem J 347(Pt 1):1–16
Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM et al (1994) CTLA-4 can function as a negative regulator of T cell activation. Immunity 1(5):405–413. https://doi.org/10.1016/1074-7613(94)90071-x
Steiner K, Moosig F, Csernok E, Selleng K, Gross WL, Fleischer B et al (2001) Increased expression of CTLA-4 (CD152) by T and B lymphocytes in Wegener’s granulomatosis. Clin Exp Immunol 126(1):143–150. https://doi.org/10.1046/j.1365-2249.2001.01575.x
Rieck M, Arechiga A, Onengut-Gumuscu S, Greenbaum C, Concannon P, Buckner JH (2007) Genetic variation in PTPN22 corresponds to altered function of T and B lymphocytes. J Immunol 179(7):4704–4710. https://doi.org/10.4049/jimmunol.179.7.4704
Gauthier Y, Cario Andre M, Taïeb A (2003) A critical appraisal of vitiligo etiologic theories. Is melanocyte loss a melanocytorrhagy? Pigment Cell Res 16(4):322–332. https://doi.org/10.1034/j.1600-0749.2003.00070.x
Liu L, Li C, Gao J, Li K, Zhang R, Wang G et al (2010) Promoter variant in the catalase gene is associated with vitiligo in Chinese people. J Investig Dermatol 130(11):2647–2653. https://doi.org/10.1038/jid.2010.192
Singh S, Singh U, Pandey SS (2012) Serum concentration of IL-6, IL-2, TNF-α, and IFNγ in Vitiligo patients. Indian J Dermatol 57(1):12–14. https://doi.org/10.4103/0019-5154.92668
Sushama S, Dixit N, Gautam RK, Arora P, Khurana A, Anubhuti A (2019) Cytokine profile (IL-2, IL-6, IL-17, IL-22, and TNF-α) in vitiligo-New insight into pathogenesis of disease. J Cosmet Dermatol 18(1):337–341. https://doi.org/10.1111/jocd.12517
Maine CJ, Hamilton-Williams EE, Cheung J, Stanford SM, Bottini N, Wicker LS et al (2012) PTPN22 alters the development of regulatory T cells in the thymus. J Immunol 188(11):5267–5275. https://doi.org/10.4049/jimmunol.1200150
Christensen AD, Skov S, Kvist PH, Haase C (2015) Depletion of regulatory T cells in a hapten-induced inflammation model results in prolonged and increased inflammation driven by T cells. Clin Exp Immunol 179(3):485–499. https://doi.org/10.1111/cei.12466
Klarquist J, Denman CJ, Hernandez C, Wainwright DA, Strickland FM, Overbeck A et al (2010) Reduced skin homing by functional Treg in vitiligo. Pigment Cell Melanoma Res 23(2):276–286. https://doi.org/10.1111/j.1755-148X.2010.00688.x
Lin M, Zhang BX, Shen N, Dong XJ, Zhang C, Qi XY et al (2014) Regulatory T cells from active non-segmental vitiligo exhibit lower suppressive ability on CD8+CLA+ T cells. Eur J Dermatol 24(6):676–682. https://doi.org/10.1684/ejd.2014.2436
Chatterjee S, Eby JM, Al-Khami AA, Soloshchenko M, Kang HK, Kaur N et al (2014) A quantitative increase in regulatory T cells controls development of vitiligo. J Investig Dermatol 134(5):1285–1294. https://doi.org/10.1038/jid.2013.540
Namazi MR (2007) Neurogenic dysregulation, oxidative stress, autoimmunity, and melanocytorrhagy in vitiligo: can they be interconnected? Pigment Cell Res 20(5):360–363. https://doi.org/10.1111/j.1600-0749.2007.00408.x
Schulz E, Jansen T, Wenzel P, Daiber A, Münzel T (2008) Nitric oxide, tetrahydrobiopterin, oxidative stress, and endothelial dysfunction in hypertension. Antioxid Redox Signal 10(6):1115–1126. https://doi.org/10.1089/ars.2007.1989
Nakamura K, Bindokas VP, Kowlessur D, Elas M, Milstien S, Marks JD et al (2001) Tetrahydrobiopterin scavenges superoxide in dopaminergic neurons. J Biol Chem 276(37):34402–34407. https://doi.org/10.1074/jbc.M103766200
Funding
None.
Author information
Authors and Affiliations
Contributions
Study concept and design: ST, AB, DP. Analysis and interpretation of data: ST, VT, AB. Drafting of the manuscript: ST, VT. Critical revision of the manuscript for important intellectual content: ST, VT, AB, DP. Statistical analysis: ST, VT. Study supervision: AB, DP.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Institutional ethical committee approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Ethics Committee.
Consent to participate
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Tanwar, S., Thakur, V., Bhatia, A. et al. Catecholamines’ accumulation and their disturbed metabolism at perilesional site: a possible cause of vitiligo progression. Arch Dermatol Res 315, 173–180 (2023). https://doi.org/10.1007/s00403-022-02333-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-022-02333-3