Abstract
Atopic eczema is often worsened by stress. While acute stress is associated with increased turnover of serotonin (5-hydroxytryptamine; 5-HT), chronic stress causes a decrease. In chronic stress, there is a decrease of the 5-HT1A receptor (R)- and an increase in the 5-HT2AR-responsiveness to 5-HT. In the present study, the impact of chronic mild stress on the expression of 5-HT1A and 5-HT2A receptors and serotonin transporter protein (SERT) was investigated in eczematous skin and brain of atopic-like NC/Nga mice. Twenty-four NC/Nga mice were subjected to chronic mild stress for 12 weeks, and eczema was induced by applying a mite antigen (Dermatophagoides pteronyssinus) on the ears for the last 4 weeks. The mice were divided into three groups, eight per group, stressed eczematous (SE), non-stressed eczematous (NSE) and stressed control (SC). The biopsies were analysed by immunohistochemistry, using a streptavidin–biotin technique. There was an increased number of 5-HT containing dermal mast cell-like mononuclear cells in the skin of mice with eczema (SE and NSE, respectively) compared with the SC, and a tendency to more 5-HT-positive cells in the SE compared with the NSE group. Increased 5-HT1AR immunoreactivity (IR) in the skin and hippocampus of the eczematous groups compared to the control group was seen, but no difference between the SE and NSE groups. The epidermal immunoreactivity for 5-HT2AR was highest in the SE and NSE compared to the SC group, and was also higher in the SE compared to NSE. 5-HT2AR expression was also seen on nerve bundles, the number and intensity of such bundles being decreased in the SE compared to the NSE group. In the CA1 area of the hippocampus, there was an increase in the quantity of cells immunoreactive for 5-HT2AR in the SE versus the NSE group and also in the SE versus the SC group. SERT-IR was found also on nerve bundles with a decreased number in the SE compared to the NSE and SC group. There is a modulation of the expression of serotonergic markers in the eczematous skin and brain of the atopic-like mouse during chronic mild stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atopic eczema is a chronic inflammatory skin disease that can be worsened by stress. There are different murine models that may be used to study the pathogenesis of atopic eczema, the most common being the NC/Nga strain [12, 19]. The stress worsening of atopic eczema has been shown in this mouse strain [1].
There is a bidirectional contact between the skin and the neuroendocrine system. The ways in which the skin and brain may communicate are principally two, either endocrine or neural. Ligands and receptors for stress hormones and neuromediators are expressed in the skin [28]. There is a hypothalamic–pituitary–adrenal (HPA) axis in the skin and afferent neural fibers may interact with the skin through axonal reflexes.
A stress response involves activation of both the HPA axis and the autonomic nervous system, both of which interact with the immune system, activating skin mast cells, macrophages, keratinocytes and T lymphocytes. Thus, stress may cause skin inflammation, which per se may result in mood disorders including anxiety and depression [32].
Serotonin (5-hydroxytryptamine; 5-HT) is an important neuromediator involved in skin–brain communication, and localized both in the brain and periphery. 5-HT has many effects in a variety of organs, being involved in blood pressure regulation, stress response, appetite, sleep and memory etc., and also exerts modulatory effects on cell firing and/or biochemical responses at the cellular level. There is a close relationship between plasma cortisol concentrations and 5-HT. Acute stress is associated with an increase in the turnover of 5-HT, whereas chronic stress, with a sustained increase in plasma cortisol, causes a reduction in serotonin turnover and release [16]. On the other hand, in stress conditions, brain 5-HT was shown to modulate synthesis of adrenocorticotropic hormone (ACTH) and cortisol [9, 10].
There is a serotonergic system in the skin of different species, which is activated during inflammation [24, 29]. In humans, the most abundant peripheral 5-HT source is blood platelets that can transport, store and release 5-HT. Additionally, in mice and dogs, dermal mast cells contain 5-HT [11]. In the nervous system, and perhaps in non-neuronal cells, the magnitude and duration of serotonergic responses is greatly influenced by the serotonin transporter protein (SERT).
The effect of 5-HT is mediated by 7 different families of receptors (R) with 14 different subtypes being characterized [5], with further diversity generated by alternative splicing and RNA editing. Among the best characterized of these 5-HTRs are the 5-HT1A and 5-HT2A receptors. 5-HT1AR has a stabilizing and differentiating effect on brain cells and an anti-inflammatory effect in mice with allergic contact eczema [20]. 5-HT2AR on the other hand increases cell proliferation and apoptosis [3]. Ketanserine, a 5-HT2AR antagonist, has an anti-inflammatory effect in murine allergic contact eczema [2]. Of these receptors 5-HT1A shows a decreased responsiveness during chronic mild stress [15, 31], while the 5-HT2AR affinity for 5-HT is increased by chronic stress [25].
In the present study, we investigated how the expression of 5-HT, 5-HT1AR, 5-HT2AR and SERT varies in the skin and brain in NC/Nga mice, depending on whether mice with eczema were subjected to chronic mild stress. Our findings demonstrate that there is a modulation of the expression of serotonergic markers in the eczematous skin and brains of these mice during chronic mild stress.
Materials and methods
Animals
In total, 24 6-week-old female NC/Nga mice (Charles River Laboratories, Germany) were used. The experiments were approved by the local Animal Ethics Committee. Animals were left to acclimate for 1 week prior to experiments after delivery from the vendor.
Chronic stress and immunization
The chronic mild stress procedure used has been described by Lanfumey et al. [15] and was used in our previous study [18]. Briefly, mice were kept in a Scantainer box type 50-SCNT-Z11 (Scanbur AS, Köge, Denmark), in a conventional facility, and fed with pellets (R70, Granngården, Malmö, Sweden) and tap water. Different stressors were used on a weekly basis, such as reversed light/dark cycle, one period of confinement to small cages for 12 h, two periods of placement with foreign mice for 2 h, one period of continuous overnight illumination, one overnight period of wet soil, one period for 12 h of cage-tilting (30°) and one period, 3 h, of food and water deprivation.
Mice were divided into three groups (eight mice per group). One group was stressed and sensitized (stressed eczematous, SE). The mice in this group were subjected to chronic mild stress for 12 weeks, and eczema was induced from week 9, by painting their ears with a mite antigen, Dermatophagoides pteronyssinus (Allergon, Ängelholm, Sweden), at a concentration of 10 mg/ml, which had been dissolved in phosphate-buffered saline (PBS) and 0.5% Tween 20. A non-sensitized control group was similarly stressed (stressed control, SC), and had their ears painted using the solvent. A second sensitized group was relieved from stress (non-stressed eczematous, NSE), being kept in a regular cage and the mice in this group were also painted on their ears with the mite antigen from week 9. These mice were maintained on a 12 h light/dark cycle under controlled temperature between 18 and 22°C and a humidity of 40–60%.
Processing of samples
After 12 weeks, the animals were killed by cervical dislocation and the ear thickness was immediately measured using a calliper (Kroeplin, Schluchtern, Germany), before being preserved together with the brains, for further analysis. Plasma corticosterone was measured using a corticosterone RIA kit (RS 490 11) from IBL (Hamburg, Germany) following the instructions of the manufacturer.
Tissue samples from the ears and brains were subjected to immunohistochemistry in order to analyze changes in serotonergic markers. For this purpose, the samples were fixed in 4% formalin with 0.2% picric acid for 2 h at 4°C. They were then rinsed with 0.1 mol/L phosphate buffer containing 10% sucrose for at least 24 h. Tissues were then embedded in Tissue-tek (Sakura Finetek, Zoeterwoude, The Netherlands) and sectioned (14 μm thick) using a Microm cryostat (Heidelberg, Germany). Cryosections were then mounted on Super Frost Plus glass slides (Menzel-Gläser, Freiburg, Germany) and stored at −70°C until being used for immunohistochemistry employing an immunofluorescence technique.
Immunohistochemistry
A streptavidin-biotin technique was used to detect all antibody-labelled molecules. The primary antibodies used were rabbit polyclonal antibodies against 5-HT (20080; dilution 1:10,000; DiaSorin, Stillwater, MN, USA), 5-HT2AR (24288; 1:300; ImmunoStar, Hudson, WI, USA) and SERT (SERT48; 1:2,000 [4, 21]), as well as a guinea pig antibody against 5-HT1AR (AB5406; 1:7,500; Chemicon, Temecula, CA, USA). Slides were then incubated with secondary biotinylated anti-rabbit (BA-1000) or anti-guinea pig (BA-7000) antibodies (1:2,000; Vector, Burlingame, CA, USA), and finally the fluorochrome Cy2-labelled streptavidin (PA42001; 1:2,000; Amersham Pharmacia Biotech, Uppsala, Sweden) was added for visualization of antibody-target labelling.
When staining for 5-HT, adjacent sections were also stained with a rabbit polyclonal antibody against tryptase (1:20,000), a kind gift from prof. I. Harvima, Tampere, Finland.
We also performed double staining in order to confirm the neuronal characteristics of the 5-HT2AR and SERT-positive nerve-like bundles. For this purpose we used a guinea pig polyclonal antibody against protein gene-product 9.5 (PGP 9.5) (GP14104), at a dilution of 1:1,000 (Neuromics, Minneapolis, MN, USA), the secondary biotinylated anti-guinea pig antibody (from above) and then a streptavidine-conjugated Texas Red (SA-5006; 1;2,000; Vector).
As controls, the primary antibodies were omitted or normal rabbit serum IgG (X 936, Dako, Glostrup, Denmark), or guinea pig serum IgG (006-000-003; Jackson ImmunoResearch, West Grove, PA, USA) were used in the same dilution as the primary antibodies. In addition, we performed preadsorption for 5-HT (serotonin creatine sulfate monohydrate, 85030; Sigma-Aldrich, Stockholm, Sweden), 5-HT1AR (AG349, Chemicon), 5-HT2AR (24333, Immunostar) and SERT (K596 peptide- the target for the SERT antisera). In the case of 5-HT, we utilized serotonin creatine sulfate at a concentration of 10−3 mol/L, the 5-HT1AR and 5-HT2AR peptides were used at a concentration of 5 μg/ml, respectively, whereas the SERT peptide was used at 1 μM. All the above mentioned controls resulted in substantially decreased or abolished signals.
Finally, the sections were mounted with Kaiser’s glycerol gelatine (Merck, Darmstadt, Germany) before being covered with glass slips.
Microscopy
Labelled sections were analyzed using a fluorescence Zeiss Axioskop 2 MOT microscope (Carl Zeiss, Stockholm, Sweden). Slides were coded prior to analysis to permit blind evaluation by the investigator (AR).
The numbers of 5-HT-positive mononuclear cells were counted in four representative fields per ear section and the mean of four sections per mouse was calculated.
Epidermal area of expression was estimated as: 0–25% (1), 26–50% (2), and higher than 50% (3). Fluorescence intensity was scored as: none = 0, low = 1, moderate = 2 and high = 3. The number of immunoreactive nerve bundles were calculated per section, the absolute number being given, and the intensity of staining being semiquantified as none = 0, low = 1, moderate = 2 and high = 3.
For the brain, a semiquantitative technique was used. The cells in the prefrontal cortex immunoreactive for the different markers were evaluated as: none = 0, low = 1, moderate = 2 and high = 3, and their intensity as above. In the hippocampus, we focused on CA1 and CA3 regions and used 1 = 1–3, 2 = 4–6 and 3 = 7 or more positive cells in the respective area. The intensity was scored as above.
Statistical analysis
For comparison of the number of 5-HT-positive cells between the groups, Student’s t-test was used. For the semiquantitative data, the chi-square test and/or Fisher’s exact test were used. A p value of <0.05 was regarded as significant.
Results
General findings
The diameter of the ears was larger in the SE group, 0.49 ± 0.11 (mean ± SD) mm, compared to the NSE group, 0.36 ± 0.13 (p < 0.01) and the SC group, 0.23 ± 0.00 (p < 0.001).
Corticosterone levels
The SE group showed lower (p < 0.05) corticosterone values 496.4 ± 221.5 ng/ml, compared to other groups, 738.6 ± 232.1 in the NSE and 805.2 ± 144.4 ng/ml in the SC.
Skin
Table 1 summarizes the data.
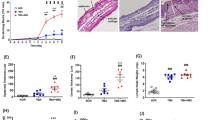
There was an increase (p < 0.01) in the number of 5-HT-containing dermal mononuclear cells (Fig. 1a, b) in the mice with induced eczema (SE 295 ± 46 cells/section and NSE 259 ± 58) compared with the control group (SC 164 ± 23). There was a trend (p = 0.09) toward more 5-HT-containing cells in the SE group, compared with the NSE. In the SC group, the 5-HT-positive cells were smaller and less granulated. In the SE group, these cells were larger, often degranulating, widely distributed and located closer to the epithelium compared with both the NSE and SC groups. When staining adjacent sections for tryptase a similar expression was obtained indicating that these 5-HT-positive cells are mast cells.
5-HT1AR-immunoreactivity (IR) was seen in the skin sections (Table 1; Fig. 2a), with an increase in the eczematous groups (p < 0.01 for SE and p < 0.05 for NSE) compared to the control, and with no difference between the SE and NSE groups.
5-HT2AR-IR was found in nerves, Langerhans cell-like cells and in the apical epithelium (Fig. 3a, shown for SE). In both eczema groups, the dendrites of the Langerhans cell-like cells were longer than in the SC group. The 5-HT2AR epidermal area immunoreactivity was most evident in the SE (2.4 ± 0.5) and NSE (2.1 ± 0.4) groups compared to the SC (1.3 ± 0.5) group, with a difference (p < 0.01) between the SE and NSE groups, and between the SE and SC (p < 0.001) groups, whereas the intensity was highest in the SE (2.8 ± 0.4) and NSE (2.6 ± 0.6) groups compared to the SC (1.4 ± 0.5) group. A difference in intensity levels was detected (p < 0.05) between the SE and NSE, and also between SE and SC (p < 0.001).
5-HT2AR expression was also seen on nerve bundles (Fig. 4a). This was confirmed by double staining with PGP 9.5 (Fig. 4a–c). The number of 5-HT2AR-positive nerve bundles was decreased (p < 0.01) in the SE (6.3 ± 3.1 bundles per section) compared to the NSE (11.3 ± 3.6), and SC (8.8 ± 3.4) groups (Fig. 5). In addition, the fluorescence intensity of the nerve bundles for 5-HT2AR was lower (p < 0.01) in the SE (1.8 ± 0.5) as compared with the NSE (2.6 ± 0.7) and SC (2.3 ± 0.4) groups (Fig. 6).
SERT-IR, in addition to the epidermis, was also found in nerve bundles (Fig. 7) with a decreased number in the SE (5.9 ± 2.4) compared to the NSE (11.9 ± 3.1; p < 0.001) and SC (9.5 ± 3.2; p < 0.05) groups. There was no difference in bundle intensity between the NSE (2.4 ± 0.6), SE (2.1 ± 0.4) and the SC (2.2 ± 0.6) groups.
Brain
Prefrontal cortex
Expression of all the serotonergic markers was low and no significant changes were found between the groups (data not shown).
Hippocampus
There was no difference in neither 5-HT- nor SERT-IR in CA1 and CA3 areas between the groups (data not shown). Immunoreactivity was generally low and restricted to fibers.
There was a hippocampal (CA1 and CA3) expression of 5-HT1AR. There was an increase (p < 0.05) in the SE compared to the SC group, however, with no difference between the SE and NSE, regarding fluorescence intensity in CA1. In the CA3, a higher (p < 0.05) immunoreactive cell number in the eczematous groups compared to SC, and an increase (p < 0.05) in fluorescence intensity in the NSE in contrast to SC, were obtained (Table 1; Fig. 2b).
In the hippocampal CA1 area, we noted an increase in the quantity of cells immunoreactive for 5-HT2AR (Fig. 3b, c) in the SE (1.5 ± 0.5) compared to the NSE (1.0 ± 0.0; p < 0.05), and SC (1.0 ± 0.0; p < 0.05) groups, whereas the fluorescence intensity was 1.6 ± 0.5 in SE, 1.1 ± 0.4 in NSE and 1.3 ± 0.5 in SC, with a tendency (p = 0.07) toward an increase in SE compared to NSE (Fig. 8). In the CA3 area, no differences could be found between the SE and NSE.
Discussion
The highest degree of eczema in our study was found in the group exposed to chronic mild stress. This finding is consistent with other studies showing that chronic stress may increase an eczematous reaction [1, 6, 22].
It is interesting that the SE group had the lowest level of corticosterone, while both the NSE and SC groups had higher levels. The low level in the SE group indicates a chronic HPA suppression when both stress and inflammation are combined. In atopic eczema patients, a blunt HPA axis responsiveness to stress has been described, resulting in a failure to mount a sufficient cortisol response [7].
In the present study, we observed a decreased neuronal expression of 5-HT2AR and SERT in the skin of an atopic-like mouse strain exposed to chronic mild stress. At the same time, we detected increased hippocampal expression of 5-HT2AR, in the CA1 area. These findings are interesting since the CA1 area has been associated with chronic stress [17, 27]. The discrepancy with an increased hippocampal signal for 5-HT2AR but a decreased skin nerve signal for this marker, in the SE group, might be due to a downregulation of this receptor in the peripheral neurons and a central upregulation in the hippocampus. In addition, the increased 5-HT2AR expression may suggest a lower level of the ligand influenced by both stress and eczema. This may in turn be reflected on the activity of the HPA system and the subsequent lower levels of corticosterone in the SE mice.
Both 5-HT1 and 5-HT2 receptors exist on peripheral nerve endings. It has been recently reported that 5-HT1/5-HT2 receptors may be involved in scratching in mice [14]. Prior electron microscopy studies demonstrated that 5-HT2AR receptors are expressed on peripheral sensory axons in the rat skin [8]. In the present study, we confirm by immunohistochemistry that these receptors also exist on sensory nerve fibres extending far out into the mouse epidermis. 5-HT2ARs have also been reported to contribute to mechanical hyperalgesia in a rat model of neuropathic pain [23]. Both nociceptive and A-δ nerve cell bodies are also known to express 5-HT2AR in rat dorsal root ganglia [30].
We also detected an increase of 5-HT2AR immunoreactivity in the ear epithelium of the SE compared to the NSE, and SC mice. The mild chronic stress appears to have resulted in a worsening of the inflammatory status of the skin exposed to the mite antigen, and also induced a stronger scratching behavior. The explanation could either be that the stress reduced the degree to which the mice were able to cope with the itch stimuli, causing a greater scratching behavior. Alternatively, stress responsive pathways in the brain may indirectly, via neural or humoral pathways, increase the inflammatory response through an impact on inflammatory cells, such as keratinocytes, which have been shown to express 5-HT2AR and 5-HT1AR [24, 29]. Further studies are needed to distinguish between these possibilities.
Serotonin transporter protein was shown to be present in nerve bundles in the mouse ears, where we observed a downregulation in the eczema group exposed to stress compared to non-stressed eczema group. This is interesting, since SERT modulating effects have been suggested to be mediated through 5-HT2AR [26].
It has been suggested that a 5-HT1AR agonist may be of use in the clinical management of stress-associated aggravation of atopic eczema in humans [13]. However, in the present study, we could not find any difference in 5-HT1AR expression between the eczematous groups, SE and NSE, neither in the skin nor brain. It was more an upregulation dependent on the inflammation, which could be seen in the skin and in the brain.
5-HT containing mast cell-like cells were found to be more numerous in the skin of the SE and NSE groups compared to the SC, suggesting that a good part of this effect is caused by the eczema procedure. However, there was also a tendency for more 5-HT-positive cells in the SE than in the NSE group suggesting a possible added effect of stress. The cells were also larger, more often degranulating and located closer to the basement membrane, consistent with a more vigorous inflammation. In this context we note a lack of 5-HT1A receptors on the mast cells, in contrast to human and dog findings [11, 24]. The increase in the number of mast cell-like cells and their degranulation with secretion of 5-HT in the skin might contribute to a decreased peripheral neuronal 5-HT2AR expression.
In conclusion, we observed a modulation of expression of studied serotonergic markers in the eczematous skin and brains of the atopic-like mouse, during chronic mild stress. Further studies are needed to investigate the mechanistic basis for these observations, which may be relevant for pathogenic mechanisms as well as potential therapies for atopic eczema worsened by stress.
References
Amano H, Negishi I, Akiyama H, Ishikawa O (2008) Psychological stress can trigger atopic dermatitis in NC/Nga mice: An inhibitory effect of corticotropin-releasing factor. Neuropsychopharmacology 33:566–573
Ameisen J-C, Meade R, Askenase PW (1989) A new interpretation of the involvement of serotonin in delayed-type hypersensitivity. Serotonin-2 receptor antagonists inhibit contact sensitivity by an effect on T cells. J Immunol 142:3171–3179
Azmitia EC (2001) Modern views on an ancient chemical: serotonin effects on cell proliferation, maturation, and apoptosis. Brain Res Bull 56:413–424
Bauman AL, Apparsundaram S, Ramamoorthy S, Wadzinski BE, Vaughan RA, Blakely RD (2000) Cocaine and antidepressant-sensitive biogenic amine transporters exist in regulated complexes with protein phosphatase 2A. J Neurosci 20:7571–7578
Bockaert J, Claeysen S, Bécamel C, Dumuis A, Marin P (2006) Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation. Cell Tissue Res 326:553–572
Bowers SL, Bilbo SD, Dhabhar FS, Nelson RJ (2008) Stressor-specific alterations in corticosterone and immune responses in mice. Brain Behav Immun 22:105–113
Buske-Kirschbaum A, Ebrecht M, Hellhammer DH (2010) Blunted HPA axis responsiveness to stress in atopic patients is associated with the acuity and severeness of allergic inflammation. Brain Behav Immun 24:1347–1353
Carlton SM, Coggeshall RE (1997) Immunohistochemical localization of 5-HT2A receptors in peripheral sensory axons in rat glabrous skin. Brain Res 763:271–275
Chaouloff F (1993) Physiopharmacological interactions between stress hormones and central serotonergic systems. Brain Res Brain Res Rev 18:1–32
Dinan TG (1996) Serotonin: current understanding and the way forward. Int Clin Psychopharmacol 11(Suppl 1):19–21
Fröberg GK, Lindberg R, Ritter M, Nordlind K (2009) Expression of serotonin and its 5-HT1A receptor in canine cutaneous mast cell tumours. J Comp Pathol 141:89–97
Jin H, He R, Oyoshi M, Geha RS (2009) Animal models of atopic dermatitis. J Invest Dermatol 129:31–40
Kawana S, Kato Y, Omi T (2010) Efficacy of a 5-HT1a receptor agonist in atopic dermatitis. Clin Exp Dermatol 35:835–840
Kim DK, Kim HJ, Kim H et al (2008) Involvement of serotonin receptors 5-HT1 and 5-HT2 in 12(S)-HPETE-induced scratching in mice. Eur J Pharmacol 579:390–394
Lanfumey L, Pardon M-C, Laaris N et al (1999) 5-HT1A autoreceptor desensitization by chronic ultramild stress in mice. NeuroReport 10:3369–3374
Leonard BE, Myint A (2009) The psychoneuroimmunology of depression. Hum Psychopharmacol 24:165–175
Lucassen PJ, Vollmann-Honsdorf GK, Gleisberg M, Czéh B, De Kloet RE (2001) Chronic psychosocial stress differentially affects apoptosis in hippocampal subregions and cortex of the adult tree shrew. Eur J Neurosci 14:161–166
Lönndahl L, Lonne-Rahm SB, Nordlind K, Theodorsson E, El-Nour H (2010) Decreased innervation of eczematous skin in NC/Nga atopic mice during chronic mild stress. Immunopharmacol Immunotoxicol 32:147–152
Matsuda H, Watanabe N, Geba GP et al (1997) Development of atopic dermatitis-like skin lesion with IgE hyperproduction in NC/Nga mice. Int Immunol 9:461–466
McAloon MH, ChandraSekar A, Lin YJ, Hwang GC, Sharpe RJ (1995) Buspirone inhibits contact hypersensitivity in the mouse. Int Archs Allergy Appl Immunol 107:437–438
Miner LH, Schroeter S, Blakely RD, Sesack SR (2000) Ultrastructural localization of the serotonin transporter in superficial and deep layers of the rat prelimbic prefrontal cortex and its spatial relationship to dopamine terminals. J Comp Neurol 427:220–234
Nakano Y (2004) Stress-induced modulation of skin immune function: two types of antigen-presenting cells in the epidermis are differentially regulated by chronic stress. Br J Dermatol 151:50–64
Nitanda A, Yasunami N, Tokumo K, Fujii H, Hirai T, Nishio H (2005) Contribution of the peripheral 5-HT2A receptor to mechanical hyperalgesia in a rat model of neuropathic pain. Neurochem Int 47:394–400
Nordlind K, Azmitia EC, Slominski A (2008) The skin as a mirror of the soul: exploring the possible role of serotonin. Exp Dermatol 17:301–311
Ossowska G, Nowa G, Kata R, Klenk-Majewska B, Danilczuk Z, Zebrowska-Lupina I (2001) Brain monoamine receptors in a chronic unpredictable stress model in rats. J Neural Transm 108:311–319
Pellegrino TC, Bayer BM (2002) Role of central 5-HT (2) receptors in fluoxetine-induced decreases in T lymphocyte activity. Brain Behav Immun 16:87–103
Ritchie LJ, De Butte M, Pappas BA (2004) Chronic mild stress exacerbates the effect of permanent bilateral common carotid artery occlusion on CA1 neurons. Brain Res 1014:228–235
Slominski A, Wortsman J, Luger T, Paus R, Solomon S (2000) Corticotropin releasing hormone and propiomelanocortin involvement in the cutaneous response to stress. Physiol Rev 80:979–1020
Slominski A, Wortsman J, Tobin DJ (2005) The cutaneous serotonergic/melatonergic system: securing a place under the sun. Faseb J 19:176–194
Van Steenwinckel J, Noghero A, Thibault K, Brisorgueil M-J, Fischer J, Conrath M (2009) The 5-HT2A receptor is mainly expressed in nociceptive sensory neurons in rat lumbar dorsal root ganglia. Neuroscience 161:838–846
Wang S, Zhang Z, Guo Y, Teng G, Chen B (2009) Decreased expression of serotonin 1A receptor in the dentate gyrus in association with chronic mild stress: a rat model of post-stroke depression. Psychiatry Res 170:245–251
Zachariae R (2009) Psychoneuroimmunology: a bio-psycho-social approach to health and disease. Scand J Psychol 50:645–651
Acknowledgments
This study was supported by a grant from the Welander/Finsen foundation. Dr. Aram Rasul is supported by the Ministry of Higher Education in Kurdistan, Iraq. The technical assistance of Anna-Lena Kastman and Eva Lindqvist is gratefully acknowledged as is the efforts of Qiao Han in SERT antibody production. We are thankful to Prof. Lars Olson, Department of Neuroscience, Karolinska Institutet, for valuable comments during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rasul, A., El-Nour, H., Blakely, R.D. et al. Effect of chronic mild stress on serotonergic markers in the skin and brain of the NC/Nga atopic-like mouse strain. Arch Dermatol Res 303, 625–633 (2011). https://doi.org/10.1007/s00403-011-1138-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00403-011-1138-8