Abstract
Introduction
Reduced bone mineral density (BMD) and disruption of normal bony architecture are the characteristics of osteopenia and osteoporosis and in patients undergoing total hip arthroplasty (THA) may cause failure of trabecular ingrowth. The purpose of this study is to evaluate the impact of reduced BMD on outcomes following primary elective THA.
Methods
A retrospective chart review of 650 elective THAs with a DEXA scan in their electronic health record (EHR) from 2011 to 2020 was conducted at an urban, academic center and a regional, health center. Patients were separated into three cohorts based on their t-score and the World Health Organizations definitions: normal (t-score ≥ − 1), osteopenia (t-score < − 1.0 and > − 2.5), and osteoporosis (t-score ≤ − 2.5). Demographic and outcome data were assessed. Subsidence was assessed for patients with non-cemented THAs. Regression models were used to account for demographic differences.
Results
650 elective THAs, of which only 11 were cemented, were included in the study. Patients with osteopenia and osteoporosis were significantly older than those without (p = 0.002 and p < 0.0001, respectively) and had a lower BMI (p < 0.0001 and p < 0.0001, respectively). PFx was significantly greater in patients with osteoporosis when compared to those with normal BMD (6.5% vs. 1.0%; p = 0.04). No such difference was found between osteoporotic and osteopenic patients. The revision rate was significantly higher for osteoporotic patients than osteopenic patients (7.5% vs. 1.5%; p = 0.04). No such difference was found between the other comparison groups.
Conclusion
Patients with osteoporosis were older with reduced BMI and had increased PFx after non-cemented elective THA. Understanding this can help surgeons formulate an appropriate preoperative plan for the treatment of patients with osteoporotic bone undergoing elective THA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteopenia and osteoporosis are two of the most prevalent orthopedic disorders affecting patients undergoing THA [1]. These conditions are classified as having reduced bone mineral density (BMD) and disruption of normal bony architecture [2]. Recent estimates state that approximately 10 million people over the age of 50 in the United States have osteoporosis of the hip, while an additional 33.6 million people over age 50 in the United States have osteopenia of the hip [3]. These numbers are expected to continue to rise as the American population over age 65 is expected to increase to 20% of the overall population by 2030 [4]. Furthermore, a study by Bernatz et al. determined the rate of preoperative osteoporosis in lower extremity arthroplasty to be as high as 33% [5]. Given osteoporotic patients’ increased susceptibility to orthopedic injury at baseline, surgeons are constantly faced with dilemmas regarding optimal treatment strategies for patients with reduced BMD to avoid adverse outcomes [4].
As THA techniques continue to change and advance, there is an increasing need to assess the impact of these changes on patients with reduced BMD. While cemented implant design has historically been used in osteoporotic patients undergoing THA, cementless techniques have become increasingly popular in recent years [6]. However, uncemented THA poses unique challenges compared to cemented THA. Osseointegration of uncemented THA requires more initial stability and bone in/on growth to produce optimal surgical outcomes when compared to cemented implants [7]. Studies have shown that reduced BMD, especially in the hip, was a risk factor for delayed translational stability [8, 9]. Given osteoporotic and osteopenic patients’ diminished ability to reform bone compared to patients with normal BMD, and the need for increased initial implant stability in uncemented THA, variance in techniques can affect post-surgical outcomes [10].
Despite increasing rates of uncemented THA and the high prevalence of reduced BMD in THA patients, there is a paucity of literature examining the effect of reduced BMD on THA perioperative outcomes. These outcomes include subsidence, periprosthetic fracture (PFx), reoperation, and revision. One such study by Kligman and Kirsh found that the presence of osteoporosis did not significantly impact survival rate of cementless THA [11]. However, this study was done with a small sample size of 22 osteoporotic patients and the performed surgeries date back from 1991 to 1996.
As a result, this study seeks to determine the impact of reduced BMD on surgical outcomes in a larger sample size of patients from the most recent decade who underwent primary elective THA in order to obtain relevant analysis that pertains to the current period. It was hypothesized that reduced BMD would be associated with increased likelihood of adverse post-operative outcomes following THA.
Materials and methods
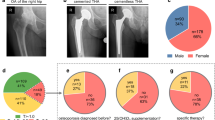
A multi-site retrospective study was conducted at an urban, academic institution and a regional health center to investigate patients who had undergone primary elective uncemented or cemented THA, identified by their universal device identifier (UDI). Given the implementation of the electronic medical record (EMR) at the academic institution in 2011, 2011–2020 was chosen as the designated time frame to ensure reliable data. Using the electronic data warehouse (EDW), it was determined that over 30,000 THA cases were performed in total at the aforementioned institutions in the desired time period, with approximately 1% of cases involving cemented femoral stems. 650 THA cases with a record of a DEXA scan done for any reason within 1 year of the surgery date were found. Of these 650 patients, 267 came from the regional health center and 383 came from urban academic center. Additionally, only 11 patients had a cemented femoral THA.
Using the World Health Organization’s (WHO) definition of normal BMD, each patient was sorted into one of the following three cohorts based on their DEXA scan t-scores: normal (t-score ≥ − 1), osteopenia (− 2.5 < t-score < − 1.0), and osteoporosis (t-score ≤ − 2.5) [12]. Of the 650 patients, 226 were found to have normal BMD, 331 were found to have osteopenia, and 93 were found to have osteoporosis.
A retrospective chart review was conducted to collect baseline demographics, including sex, race, age, American Society of Anesthesiologists (ASA) score, body mass index (BMI), and smoking status. Primary outcomes data were also collected, including length of stay (LOS), discharge destination (home, skilled nursing facility [SNF], or Rehab), incidence of post-operative periprosthetic fracture (PFx), reoperation, revision, and femoral stem subsidence. In the present study, revision was considered removal and replacement of one or more implant components, whereas reoperation was defined as an additional procedure at the initial surgical site that did not require component removal or exchange. It should be noted that subsidence was only assessed for patients with non-cemented THAs.
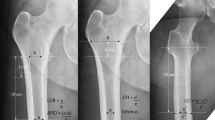
Two authors measured subsidence using standardized methods [13, 14]. Immediate post-operative radiographs were used to manually measure perpendicular distance from the shoulder of the femoral stem to the superior-most point of the greater trochanter using a standardized linear radiographic marker with regularly spaced distance intervals to determine magnification. The same manner of measurement was used with radiographs taken from the most recent follow-up, and the difference was recorded as subsidence.
Statistical analysis
The mean age, ASA score, BMI, and corresponding standard deviations were calculated for each group. Next, the mean LOS, femoral stem subsidence, and corresponding standard deviations were calculated for each group. Demographic characteristics and perioperative outcomes of osteopenia and osteoporosis groups were compared individually with those of the normal BMD groups. To compare values between two groups, statistical differences between continuous variables were calculated using independent two-sample t tests. Resulting p values were compared to an alpha value of 0.05 to test for statistical significance. The demographic characteristics and perioperative outcomes of the osteopenia and osteoporosis groups were compared to each other in a similar manner.
Analysis of demographic data showed that there were significant differences in age and BMI between the three cohorts. Multilinear and logistic regression analyses controlling for these demographic characteristics, along with race, were performed on outcomes data to account for these differences and yield a more accurate analysis. Due to the significant difference found in average ASA score and sex composition between the osteopenia and normal BMD cohorts, these two demographic characteristics were additionally controlled for in the regression analyses only dealing with the osteopenia and normal BMD patients. Unstandardized beta coefficients calculated from the regression models in order to determine effect strength. In addition, corresponding 95% confidence intervals were calculated. It should be noted that when performing data analysis on the subsidence data, all patients who underwent cemented THA were excluded.
All statistical analysis was done using Microsoft Excel software (Microsoft Corporation, Richmond, WA) and IBM SPSS Statistics (IBM Corporation, Armonk, NY).
Results
Patient demographic characteristics
Across all three cohorts race did not differ with 185 patients (81.8%) identifying as white, 25 patients (11.1%) identifying as black, and 16 patients (7.1%) identifying as other in the normal BMD group, 294 patients (88.8%) identifying as white, 12 patients (3.6%) identifying as black, and 25 patients (7.6%) identifying as other with osteopenia, and 81 patients (87.1%) identifying as white, 1 patient (1.1%) identifying as black, and 11 patients (11.8%) identifying as other with osteoporosis.
Full patient demographic data are shown in Tables 1, 2 and 3. Several demographic differences were identified between patient cohorts. Female-to-male composition significantly differed between the osteopenia (22 males, 6.6%; 309 females, 93.4%) and normal BMD (28 males, 12.4%; 198 females, 87.6%) cohorts (p = 0.03). No such difference was found between patients with normal BMD and osteoporosis (p = 0.22). Compared to patients with normal BMD (65.7 years ± 8.9), patients with osteopenia and osteoporosis were on average older (68.2 years ± 7.9, p = 0.002; 69.6 years ± 8.7 p ≤ 0.001. In addition, patients in the osteopenia and osteoporosis cohorts had on average lower BMI (26.5 kg/m2 ± 4.8, p < 0.0001; 25.3 kg/m2 ± 4.7, p < 0.0001) compared to patients in the normal BMD cohort (29.2 kg/m2 ± 6.1). Patients with osteoporosis were also found to have a lower BMI than patients with osteopenia (p = 0.009).
Outcomes
Outcome-related data are summarized in Tables 4, 5 and 6. LOS was 1.8 ± 1.2 for patients with normal BMD, 1.8 ± 1.3 for patients with osteopenia, and 2.3 ± 1.7 for patients with osteoporosis. Upon controlling for demographic differences, when compared to patients with normal BMD and osteopenia, patients with osteoporosis had longer LOS (p = 0.02, \(\beta\) = 0.47, 95% CI [− 0.11, 0.83]; p = 0.0009, \(\beta\) = 0.5, 95% CI [0.18, 0.82]).
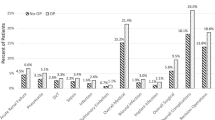
PFx occurred in two patients (1.0%) with normal BMD, seven patients (2.1%) with osteopenia, and six patients (5.0%) with osteoporosis. Average femoral stem subsidence in patients with normal BMD was 2.1 mm ± 2.2, 2.0 mm ± 2.2 for patients with osteopenia, and 2.5 mm ± 2.4 for patients with osteoporosis. After controlling for race, age, sex, ASA, and BMI, when compared to patients with normal BMD, patients with osteopenia and osteoporosis did not demonstrate statistically significantly higher rates of subsidence (p = 0.66, \(\beta\) = − 0.1, 95% CI [− 0.45, 0.66] and p = 0.14, \(\beta\) = 0.28, 95% CI [− 0.33, 0.89], respectively). Additionally, patients with osteoporosis did not demonstrate higher rates of subsidence than patients with osteopenia (p = 0.07, \(\beta\) = 0.5, 95% CI [− 0.05, 1.06]). Incidence of PFx was significantly higher in patients with osteoporosis compared to patients with normal BMD (p = 0.04, \(\beta\) = 0.06, 95% CI [− 0.02, 0.10]), but no such difference was found when comparing incidence of PFx in osteoporotic and osteopenic patients (p = 0.11, \(\beta\) = 0.04, 95% CI [− 0.002, 0.08]) or when comparing incidence of PFx in osteopenic and normal BMD patients (p = 0.22, \(\beta\) = 0.02, 95% CI [− 0.01, 0.04]).
6 patients with normal BMD underwent revision. Of these six patients, two underwent revision due to PFx, both requiring exchange of the femoral component. Additionally, seven patients with osteoporosis underwent revision. Of these seven patients, four underwent revision due to PFx with three patients requiring exchange of the femoral component and one patient requiring exchange of acetabular component. 5 patients with osteopenia underwent revision. Of these five patients, four underwent revision due to incidence of PFx with one patient requiring acetabular and femoral component exchange and three patients requiring femoral component exchange alone. There was no statistically significant difference found in revision rate between the osteopenic cohort and normal BMD cohort (p = 0.37, \(\beta\) = − 0.005, 95% CI [− 0.02, 0.04]) or between the osteoporotic cohort and normal BMD cohort (p = 0.10, \(\beta\) = 0.05, 95% CI [− 0.003, 0.10]). However, revision rate between the osteoporotic cohort and osteopenic cohort was found to be statistically significantly different (p = 0.04, \(\beta\) = 0.06, 95% CI [− 0.02, 0.1]). Furthermore, no significant difference was found in any other outcomes measure between the osteopenia and normal BMD cohort.
Discussion
Given the commonality of risk factors such as advanced age and sedentary lifestyle that predispose patients to osteopenia, osteoporosis, and the need for THA, it is very common for patients undergoing elective THA to have reduced BMD and be at increased risk for adverse post-operative outcomes [15]. While this issue is partially mitigated by cemented THA, the inability to form proper bone can often create significant discrepancies in surgical outcomes between patients with reduced BMD and without reduced BMD when undergoing cementless THA [16]. Despite this, cementless THA has grown in popularity due to ease in surgical procedure and fear of adverse effects associated with the cementing process during surgery [9]. As surgical practices and techniques for THA continue to develop and change, it is necessary to explore the effects of these practices on frequently presenting subgroups of THA patients. The present study compared THA results of patients with reduced BMD to patients without reduced BMD and found longer LOS and greater rates of PFx in patients with reduced BMD undergoing uncemented THA, as well as higher rates of revision in osteoporotic patients.
The present study determined that reduced BMD is associated with longer LOS following THA compared to patients with normal BMD. As the number of outpatient THAs and THAs performed at ambulatory centers continues to rise, it has become increasingly important to identify the factors that contribute to variable post-op LOS [17]. This has become especially evident since the removal of THA from the inpatient only list, which allows the procedure to be performed outpatient [18]. Differences in LOS present potential to adversely affect patients’ post-operative course. Recent research has indicated that shorter post-operative hospital LOS is associated with decreased risk for readmission but can result in significant cost savings for payers and hospitals alike [19, 20]. Therefore, patients with reduced BMD may be more likely to be subject to a more complicated and costly post-operative course.
In addition to lengthened LOS, results from the present study also found reduced BMD to be associated with higher rates of PFx. PFx is currently the third leading cause for revision surgery following THA [21]. Revision due to PFx can be a complicated surgery and involves surgeon assessment of implant loosening, bone loss and quality, and type of fracture [22]. Patients with osteoporosis commonly present with poorer bone quality as well as periprosthetic bone loss, which can further complicate the procedure [23]. As a result, these patients may be subject to complex and expensive revision procedures involving use of modular tapered fluted stem, impaction bone grafting, or megaprosthesis to compensate for the bone loss and poor bone quality [24].
Interestingly, while revision rate trended toward statistical significance in osteoporosis patients versus normal BMD patients, it did not reach the threshold for significance, but was significant in osteoporosis versus osteopenia patients. The increased revision rate in osteoporosis versus osteopenic patients can likely be explained by the poorer bone quality in osteoporotic patients. A study by Lee et al. [23] determined reduced BMD to be an independent risk factor for increased revision rates following primary THA. These results were further supported by a study by Aro et al. [8] which found reduced BMD to adversely affect stability and osteointegration of THA implants. While reduced BMD has been shown to increase post-operative complications via increased periprosthetic bone loss, certain factors such as older age and female sex have been cited as independent risk factors for periprosthetic bone loss [23]. Given the slight variance in age and sex between groups in the present study, it is possible that these demographic differences contributed to some degree to differences in outcomes. However, the p values and corresponding confidence intervals support the significance of the impact of reduced BMD on revision rate. Additionally, patients with osteoporosis trended toward a greater degree of subsidence compared to both osteopenic and normal BMD patients. However, this difference was not found to be statistically significant.
The findings from the present study pose numerous implications for surgeons and providers. Interestingly, a large portion of preoperative osteoporosis and osteopenia goes undiagnosed. A study by Delsmann et al. [25] examined 268 elderly patients awaiting THA and found that 73% of the patients who were diagnosed with osteoporosis were not diagnosed prior to surgery. Another study by Maier et al. [9] found that only 4% of orthopedic surgeons test for BMD prior to surgery, but 26% of physicians state they would change their treatment strategy to include implantation of a cemented prosthesis if the patient had a preoperative T score between − 1.5 and − 2, and 40% of surgeons would adjust their treatment strategy to include implantation of a cemented prosthesis in patients with preoperative T scores between − 2 and − 2.5. The same study found that only approximately 6% of surgeons routinely perform DEXA scans on patients prior to undergoing primary THA, a value only slightly higher than the 2% reported in this study [9]. These findings in conjunction with the results of the present study support the need for increased emphasis to be placed on the identification of reduced BMD prior to surgery to allow surgeons to maximize treatment of osteoporotic and osteopenic patients.
In addition to being underdiagnosed, patients with reduced BMD awaiting THA are undertreated. A study by Bernatz et al. [26] found that 25% of TJA patients meet the criteria to receive osteoporosis medication, but only 5% actually received medication. However, research into THA outcomes in patients receiving osteoporosis medication is less definitive. A study by Khatod et al. [27] found that bisphosphonate use was associated with not only lower rates of revision after THA, but also higher rates of periprosthetic fracture. Given the inconclusive relationship between bisphosphonate use and THA outcomes, extra onus is placed on surgeons identifying reduced BMD preoperatively and tailoring peri- and post-operative strategies to optimize surgical outcomes.
The present study is not without its limitations. The retrospective nature of the study provides an opportunity for selection bias to have been introduced, which we attempted to minimize by systematically controlling for demographic and confounding variables. Furthermore, rate of cemented femoral stem usage in the present study was 1.7%, whereas nationally reported average annual rates of cemented femoral stem usage are roughly 14%. A smaller margin between cemented femoral stem rates would improve the context of the findings. In addition, outcomes were only reported within 1 year of surgery. Examining outcomes at longer term intervals would provide further perspective into differences in post-surgical outcomes between groups. Moreover, patients who underwent a DEXA scan within 1 year of surgery were included in the study. As a result, patients who were diagnosed with osteoporosis in the year prior to surgery were more likely to have had modified treatment plans such as osteoporosis medical treatment, or use of cemented femoral stems, causing them to potentially meet exclusion criteria from the present study. In addition, the results of this study should be interpreted while keeping in mind that the overall strength effect for many of the comparisons was relatively small. Lastly, variance in degree of osteoporosis was not used in assessing outcomes. Osteoporosis presents with different levels of severity and analyzing outcomes at different T scores could provide information that further supports the study’s findings. Despite these limitations, the findings from the study remain valid in demonstrating that reduced BMD is associated with decreased post-operative outcomes including increased risk of PFx and longer LOS.
In conclusion, reduced BMD is an underdiagnosed condition that can lead to increased likelihood of experiencing adverse surgical outcomes in patients undergoing uncemented THA. Surgeons should consider increased screening of high-risk patients to identify reduced BMD preoperatively and form an operative plan that best suits the patient. Future studies should be directed toward discovering the effects of reduced BMD on longer term post-operative outcomes in patients undergoing THA or examining patients diagnosed with reduced BMD exclusively post-operatively.
Data availability
The datasets generated and analyzed during the study are not publicly available, but may be available from the corresponding author upon reasonable request.
References
Domingues VR, de Campos GC, Plapler PG, de Rezende MU (2015) Prevalence of osteoporosis in patients awaiting total hip arthroplasty. Acta Ortop Bras. https://doi.org/10.1590/1413-78522015230100981
Porter JL, Varacallo M (2022) Osteoporosis. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Office of the Surgeon General (US) (2004) The Frequency of Bone Disease Bone Health and Osteoporosis: A Report of the Surgeon General. Rockville
Varacallo M, Seaman TJ, Jandu JS, Pizzutillo P (2022) Osteopenia. In: StatPearls. StatPearls Publishing, Treasure Island (FL)
Bernatz JT, Brooks AE, Nguyen BP, Shin ED, Binkley NC, Anderson PA et al (2020) Prevalence and treatment of osteoporosis prior to elective shoulder arthroplasty. JAAOS. https://doi.org/10.5435/JAAOSGlobal-D-20-00204
Rhyu KH, Lee SM, Chun YS, Kim K, Cho YJ, Yoo MC (2012) Does osteoporosis increase early subsidence of cementless double-tapered femoral stem in hip arthroplasty? J Arthroplasty. https://doi.org/10.1016/j.arth.2011.10.026
Karuppal R (2016) Biological fixation of total hip arthroplasty: facts and factors. J Orthop. https://doi.org/10.1016/j.jor.2016.06.002
Aro HT, Alm JJ, Moritz N, Mäkinen TJ, Lankinen P (2012) Low BMD affects initial stability and delays stem osseointegration in cementless total hip arthroplasty in women: a 2-year RSA study of 39 patients. Acta Orthop 83:107–114. https://doi.org/10.3109/17453674.2012.678798
Maier GS, Kolbow K, Lazovic D, Maus U (2016) The importance of bone mineral density in hip arthroplasty: results of a survey asking orthopaedic surgeons about their opinions and attitudes concerning osteoporosis and hip arthroplasty. Adv Orthop 2016:8079354. https://doi.org/10.1155/2016/8079354
Demontiero O, Vidal C, Duque G (2012) Aging and bone loss: new insights for the clinician. Ther Adv Musculoskelet Dis. https://doi.org/10.1177/1759720X11430858
Kligman M, Kirsh G (2000) Hydroxyapatite-coated total hip arthroplasty in osteoporotic patients. Bull Hosp Jt Dis 59(3):136–139
Akkawi I, Zmerly H (2018) Osteoporosis: current concepts. Joints. https://doi.org/10.1055/s-0038-1660790
Clair AJ, Gabor JA, Patel KS, Friedlander S, Deshmukh AJ, Schwarzkopf R (2020) Subsidence following revision total hip arthroplasty using modular and monolithic components. J Arthroplasty. https://doi.org/10.1016/j.arth.2020.03.008
Ries C, Boese CK, Dietrich F, Miehlke W, Heisel C (2019) Femoral stem subsidence in cementless total hip arthroplasty: a retrospective single-centre study. Int Orthop. https://doi.org/10.1007/s00264-018-4020-x
Karaguzel G, Holick MF (2010) Diagnosis and treatment of osteopenia. Rev Endocr Metab Disord 11:237–251. https://doi.org/10.1007/s11154-010-9154-0
Yang C, Han X, Wang J, Yuan Z, Wang T, Zhao M et al (2019) Cemented versus uncemented femoral component total hip arthroplasty in elderly patients with primary osteoporosis: retrospective analysis with 5-year follow-up. J Int Med Res. https://doi.org/10.1177/0300060518825428
DeMik DE, Carender CN, Kohler JG, An Q, Brown TS, Bedard NA (2022) Recent increases in outpatient total hip arthroplasty have not increased early complications. J Arthroplasty 37:325-329.e1. https://doi.org/10.1016/j.arth.2021.11.003
Sheehy AM, Caponi B, Gangireddy S, Hamedani AG, Pothof JJ, Siegal E et al (2014) Observation and inpatient status: clinical impact of the 2-midnight rule. J Hosp Med 9:203–209. https://doi.org/10.1002/jhm.2163
DeMik DE, Carender CN, Glass NA, Callaghan JJ, Bedard NA (2021) Home discharge has increased after total hip arthroplasty, however rates vary between large databases. J Arthroplasty. https://doi.org/10.1016/j.arth.2020.08.039
Burn E, Edwards CJ, Murray DW, Silman A, Cooper C, Arden NK et al (2018) Trends and determinants of length of stay and hospital reimbursement following knee and hip replacement: evidence from linked primary care and NHS hospital records from 1997 to 2014. BMJ Open. https://doi.org/10.1136/bmjopen-2017-019146
Giaretta S, Momoli A, Porcelli G, Micheloni GM (2019) Diagnosis and management of periprosthetic femoral fractures after hip arthroplasty. Injury 50(Suppl 2):S29-33. https://doi.org/10.1016/j.injury.2019.01.053
Pavone V, de Cristo C, di Stefano A, Costarella L, Testa G, Sessa G (2019) Periprosthetic femoral fractures after total hip arthroplasty: an algorithm of treatment. Injury 50(Suppl 2):S45-51. https://doi.org/10.1016/j.injury.2019.01.044
Lee S-W, Kim W-Y, Song J-H, Kim J-H, Lee H-H (2021) Factors affecting periprosthetic bone loss after hip arthroplasty. Hip Pelvis 33:53–61. https://doi.org/10.5371/hp.2021.33.2.53
Sheth NP, Nelson CL, Paprosky WG (2013) Femoral bone loss in revision total hip arthroplasty: evaluation and management. J Am Acad Orthop Surg 21:601–612. https://doi.org/10.5435/JAAOS-21-10-601
Delsmann MM, Strahl A, Mühlenfeld M, Jandl NM, Beil FT, Ries C et al (2021) High prevalence and undertreatment of osteoporosis in elderly patients undergoing total hip arthroplasty. Osteop Int. https://doi.org/10.1007/s00198-021-05881-y
Bernatz JT, Brooks AE, Squire MW, Illgen RI, Binkley NC, Anderson PA (2019) Osteoporosis is common and undertreated prior to total joint arthroplasty. J Arthroplasty. https://doi.org/10.1016/j.arth.2019.03.044
Khatod M, Inacio MCS, Dell RM, Bini SA, Paxton EW, Namba RS (2015) Association of bisphosphonate use and risk of revision after THA: outcomes from a US Total Joint Replacement Registry. Clin Orthop Relat Res. https://doi.org/10.1007/s11999-015-4263-4
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fiedler, B., Patel, V., Lygrisse, K.A. et al. The effect of reduced bone mineral density on elective total hip arthroplasty outcomes. Arch Orthop Trauma Surg 143, 5993–5999 (2023). https://doi.org/10.1007/s00402-023-04830-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-023-04830-0