Abstract
Introduction
Few studies evaluated clinical benefits of pre-operative templating in total hip arthroplasty (THA). We investigated whether mismatch between planned and real implant sizes and medio-lateral offsets compromises THA outcomes.
Materials and methods
We reviewed records of 184 primary THAs with pre-operative CT scans used for templating. Acetabular offset (AO), femoral offset (FO) and global offset (GO) were measured on pre-operative CT scans, during acetate templating, and post-operative antero-posterior radiographs. Multivariable analyses were performed to determine if Forgotten Joint Score (FJS) and Oxford Hip Score (OHS) at > 2 years were associated with differences between post-operative and planned parameters.
Results
The FJS and OHS were not influenced by mismatch of component sizes nor of FO and GO. The FJS was better when the post-operative AO was greater than planned (p = 0.050). The FJS differed among arthritic types (p = 0.015). Multivariable analyses confirmed that older patients had better OHS (beta − 0.16; p = 0.033) and FJS (beta 0.74; p = 0.002), medialized hips had worse FJS (beta − 20.1; p = 0.041) and hips with greater AO than planned had better FJS (beta 1.71; p = 0.024)
Conclusions
Implanting a component of different size than planned did not compromise THA outcomes, but medialized hips had worse scores, and conservative acetabular reaming improved scores.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pre-operative templating has justifiably become a standard step in total hip arthroplasty (THA), as it helps surgeons predict implant sizes (intramedullary step) and optimize component positioning and orientation (extramedullary step) [1,2,3]. It allows surgeons to restore native (pre-arthritic) hip architecture, including limb length, femoral and acetabular offsets, to obtain adequate joint stability and muscle tensions [1, 4,5,6,7,8,9]. Both intra- and extra-medullary steps are critical for optimal functional outcomes, reducing complications and improving implant survival [10,11,12,13,14].
Several strategies and technologies have been developed to improve intra-operative implant positioning [15], including systems based on plain radiographs, computed tomography (CT), or navigation [16]. Although pre-operative templating on digital radiographs has proved to be more accurate than on printed radiographs [17,18,19], there is no current evidence of its superiority for predicting implant size and restoring native (pre-arthritic) offsets [10, 20,21,22,23,24,25]. By contrast, three-dimensional (3D) CT templating has been shown to reduce errors related to anteversion, magnification, and pelvic orientation, and thereby enables accurate planning for most morphotypes [15, 26,27,28,29,30,31].
The importance of pre-operative planning has been emphasized by many authors [1,2,3, 13, 15, 28,29,30,31,32,33,34], yet very few have studied the benefits of templating regarding clinical outcomes [13, 29, 34]. In a previous study (Part I) [35], the authors described a classification system to distinguish five types of architectural hip deformities, based on femoral head translation patterns, and advised surgeons to adapt their templating strategy accordingly. In a second study (Part II) [36], they described and evaluated a simplified technique for 3D templating using pre-operative CT scans.
The purpose of the current study was to investigate whether the clinical outcomes of THA were different among types of architectural hip deformities or affected by mismatch between planned and real reconstructions, in terms of component sizes and medio-lateral offsets.
Material and methods
The authors retrospectively reviewed the records of all consecutive patients who received primary THA, by the senior author (MB) between March 2011 and December 2014. All patients were operated through a posterior approach and received the Corail™ hip stem (DePuy, Leeds, UK) and the Pinnacle™ acetabular cup (DePuy, Leeds, UK). A total of 193 hips from 186 patients met the study criteria, but 2 hips from 2 patients were excluded because of artefacts or distortions on their pre-operative CT scans due to technical errors during image acquisition. The cohort comprised 100 men and 84 women, aged 64 ± 8 years (range 35–78), with a body mass index (BMI) of 26.1 ± 4.9 kg/m2 (range 17–59). All patients had provided written informed consent prior to surgery, to use their images and data for the purposes of research and publication.
Classification of architectural hip deformities
Since 2010, routine pre-operative radiographic hip assessment includes frontal and lateral plain radiographs centered on the hip, full weight-bearing pelvic radiographs centered on the pubic symphysis with a film focus distance of 115 cm, as well as CT scans of the hip joint [37]. As described in the authors’ previous study (Part I) [35], arthritic translation of the femoral head center was minimal (centered < 3 mm) in 116 hips (60.7%), lateral in 51 hips (26.7%), proximo-lateral in 11 hips (5.8%), medial in 8 hips (4.2%) and proximal in 5 hips (2.6%).
Planning rationale
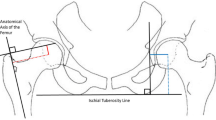
Pre-operative templating was performed using acetate templates of the femoral and acetabular components at 100% magnification, super-imposed on the printed adjusted coronal plane (Part II) [36] which was taken parallel to the neck axis and to the femoral shaft axis. The templating strategy aimed to restore the native (pre-arthritic) joint architecture in terms of femoral offset and limb length (Fig. 1), using the most suitable stem type (standard offset, high offset or coxa vara) whilst taking into account the varus-valgus alignment [38], and positioning the cup at the level of the true acetabular floor to optimize primary fixation [39, 40]. The neck cut level, measured from the lesser trochanter, was calculated and adjusted in cases with pre-operative limb length discrepancy. The templating strategy would therefore prevent limb shortening and correct pathologic lateralization and/or proximalization, but deliberately avoids correction of pathologic medialization, as this could exacerbate muscle tensions and lead to post-operative pain or stiffness. Given that the Corail™ stem is an uncemented compaction-broaching prosthesis, the optimal size depends on the density of the cancellous bone, and therefore the pre-operative choice of the stem size was only used as a guide with the final size being based on the best intra-operative fit [10]. It is worth noting that the fixation concept of the non-anatomic stem used in this series [41] influences also the accuracy of the planning. Murphy et al. [38] demonstrated that the varus-valgus orientation of the Corail stem relative to the diaphyseal axis depends on the shape of the proximal femur. Since stem templating is generally aligned with the femoral diaphyseal axis, any discrepancy of final stem alignment could thereby alter the post-operative offsets.
The pre-operative templating strategy: (1) each hip is classified as either centered, medialized, lateralized, proximalized, or proximo-lateralized; (2) the native (pre-arthritic) centers of the femoral head and acetabulum are located and marked; (3) the template of the optimal acetabular cup (diameter defined using the transverse CT slice) is positioned slightly superior and medial to the center of the native acetabulum, to account for reaming; (4) the femoral stem is positioned such that the templated head center is raised to match the templated cup center cranio-caudally, but maintaining the pathologic (pre-operative) femoral head center medio-laterally, except for medialized hips, where the templated head center is shifted to match the templated cup center both cranio-caudally and medio-laterally; (5) this templating strategy therefore prevents limb shortening by adjusting femoral head height to the level of acetabular reaming required, and corrects pathologic lateralization and/or proximalization, but does not correct pathologic medialization to avoid exacerbating muscle tensions
CT and radiographic measurements
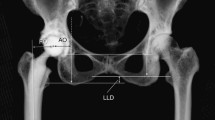
Femoral offset (FO), acetabular offset (AO), and global offset (GO) were measured on pre-operative CT scans, during acetate templating, and on post-operative true anteroposterior radiographs after correction of magnification, such that the diameter of the prosthetic head on the radiograph matches the implanted one, with internal rotation adjusted under fluoroscopic guidance to expose the full length of the neck of the stem (Fig. 2). The neck cut level was also measured in mm millimeters, as a proxy for limb length, from the base of the lesser trochanter on the CT scan. The authors distinguished between hips that were implanted with: (A) no mismatch between planned and implanted implant sizes; (B) mismatch of stem size only; (C) mismatch of cup size only; and (D) mismatch of both stem and cup sizes.
Acetabular and femoral offsets were measured on pre-operative CT scans, during acetate templating, and on post-operative true antero-posterior radiographs. The neck cut level was also measured in mm from the base of the lesser trochanter on the CT scan. AO acetabular offset, FO femoral offset, FC femoral head center, FNA femoral neck angle, tAO templated acetabular offset, tFO templated femoral offset, tNCL templated neck cut level, pAO post-operative acetabular offset, pFO post-operative femoral offset, pNCL post-operative neck cut level
Post-operative evaluation
All patients were mailed paper questionnaires to collect the following patient-reported outcome measures (PROMs):
- 1.
Forgotten Joint Score (FJS) on a scale from 0 (worst) to 100 (best) [42];
- 2.
Oxford Hip Score (OHS) on a scale from 60 (worst) to 12 (best) [43].
Statistics
Shapiro–Wilk tests were used to assess the normality of data distribution. For non-Gaussian quantitative data, differences between groups were evaluated using Wilcoxon rank sum tests (Mann–Whitney U test) and Kruskal–Wallis test. For categorical data, differences between groups were evaluated using Fisher exact tests. To assess the validity of post-operative radiographs compared with CT scans, measurements on true antero-posterior radiographs were repeated for 20 hips using post-operative CT scans at identical follow-up, and demonstrated excellent intra-class correlation coefficients (ICC) for acetabular offset (ICC 0.82; CI 0.59–0.92) femoral offset (ICC 0.82; CI 0.59–0.92) and global offset (ICC 0.78; CI 0.52–0.91). Multivariable linear regressions were performed to determine associations between two scores (FJS and OHS) and ten independent variables:
Gender, age, arthritic type, stem type;
Differences between pre- and post-operative: △AO, △FO;
Differences between planned and post-operative: △AO, △FO, △Stem size, △Cup size.
Statistical analyses were performed using R version 3.3.2 (R Foundation for Statistical Computing, Vienna, Austria). p values < 0.05 were considered statistically significant.
Results
None of the patients were lost-to follow-up. At a mean follow-up of 3.2 ± 0.7 years (range 1.8–4.8), the median post-operative OHS and FJS were 15.0 (range 12.0–55.6) and 81.3 (range 0–100), respectively (Table 1). The size of the implanted components matched exactly what was planned for: (A) both stem and cup in 80 hips (42%); (B) for the cup, but not the stem in 47 hips (25%); (C) for the stem but not the cup in 37 hips (19%); and (D) for neither component in 27 hips (14%). The mismatch between planned and implanted component sizes did not significantly influence the post-operative the OHS nor the FJS (Fig. 3). The offset reconstructions were within ± 3 mm of the planned AO in 139 hips (72.8%), of the planned FO in 78 hips (41%), and of the planned GO in 70 hips (36.5%). The mismatch between post-operative and planned FO and GO did not influence post-operative OHS or FJS (Fig. 4). The mismatch between post-operative and planned AO did not influence post-operative OHS, but seems to be associated with post-operative FJS, as patients with increased AO reported greater FJS compared to patients with decreased AO (p = 0.050).
Forgotten Joint Score (FJS) and Oxford Hip Score (OHS) as they relate to planned versus actual implant sizes: Group A, no implant sizes mismatch; Group B, only stem size mismatch; Group C, only cup size mismatch; Group D, stem and cup sizes mismatches. The plots illustrate median values (bold lines), interquartile ranges (white boxes), 95% confidence interval (whiskers) and outliers (dots)
Forgotten Joint Score (FJS) and Oxford Hip Score (OHS) related to acetabular offset (AO), femoral offset (FO) and global offset (GO) differences between post-operative and planned hip architecture. The plots illustrate median values (bold lines), interquartile ranges (white boxes), 95% confidence interval (whiskers) and outliers (dots). p values indicated only where statistically significant differences were found
Types of architectural hip deformities
The post-operative FJS was significantly different among the types of architectural hip deformities (p = 0.015), ranging from a median of 44.8 (range 18.8–93.8) for medialized hips, to a median of 95.8 (range 64.6–100.0) for proximalized hips (Table 2). It is worth noting that the pre-operative AO was maintained in medialized hips, whereas it was significantly reduced in other types of hip deformities (p < 0.001).
Linear regression analyses
Multivariable regressions revealed that OHS was only independently associated with patient age (better scores in older patients; beta − 0.16; CI − 0.31 to − 0.01; p = 0.033) (Table 3). Multivariable regressions revealed that FJS was independently associated with medialized hip deformity (worse scores in medialized hips; beta − 20.1; CI − 39.34 to − 0.87; p = 0.041), patient age (better scores in older patients; beta 0.74; CI 0.28–1.20; p = 0.002) and differences between post-operative and planned AO (better scores when AO increased compared to planning; beta 1.71; CI 0.22–3.19; p = 0.024) (Table 4).
Discussion
The purpose of the present study was to investigate whether the clinical outcomes of THA were affected by mismatch between planned and real reconstruction, in terms of component sizes and medio-lateral offsets. Several significant findings emerged from this study. First, it demonstrated that implanting a component one or more sizes different to the pre-operative plan did not compromise clinical scores. Second, it revealed that patients with medialized arthritis have worse scores. Third, it suggested that conservative reaming of the acetabulum, limiting cup medialization, is associated with better scores.
In this study, patients implanted with the planned cup and stem sizes did not report significantly better clinical scores compared those in which implant sizes deviated by one increment from what was planned. Despite pre-operative templating allowing surgeons to anticipate certain technical features of an operation, and the likely intra-operative positioning of the components, the ‘intra-operative feeling’ during femoral broaching and acetabular reaming remains of fundamental importance. This is particularly relevant to femoral preparation as intra-medullary parameters cannot always be predicted, notably for the Corail® stem, which relies on a compaction-broaching technique. The optimal implant size is therefore determined intra-operatively based on the primary stability of the broach. Using such techniques, the density of the cancellous bone, which cannot be appreciated on pre-operative images, has an important influence on the optimal stem size. It is therefore debatable as to whether the current study’s conclusions can be extrapolated to stems whose stability is based on cortical contact [41]. Nevertheless, our results suggest that, at least with compaction-broaching stems, surgeons should not only rely on their pre-operative planning for implant sizing, but also on intra-operative feedback.
Our study reveals that patients with medialized hips were independently associated with a worse FJS, compared to patients with centered hips. This difference can be highlighted only with the very sensitive FJS, but not with the more conventional OHS, and cannot be compared meaningfully with data from the literature as this score is only of recent use [42]. In medialized hips, despite a median pre-operative medialization of the center of rotation by 5.5 mm (up to 9.7 mm) (Part I) [35], the authors deliberately did not lateralize the cups, and the pre-operative acetabular offset remained unchanged. These findings are in agreement with Baghdadi et al. [44], who reported better implant survival when the native center of rotation was restored in medialized hips. In the latter series, the acetabular offset was increased by 11 ± 6 mm (lateralization of the cup) whilst the femoral offset was reduced by 4 ± 9 mm.
In this study cohort, acetabular planning was of a conventional nature, aligning the cup with the true acetabular floor [39, 40]. The multivariable analysis demonstrated that the FJS was better when the acetabular cup was lateralized compared to its planned position (p = 0.002), which suggests that conservative acetabular preparation, preserving the acetabular floor depth, improves functional outcomes [39, 40] (Fig. 4). This finding, independent of the type of hip deformities, contradicts studies that emphasize the benefits of cup medialization, especially in terms of moment arm and abduction motion [45,46,47,48]. Meermans et al. [40] showed that reaming to the acetabular floor leads to cranial displacement of the centre of rotation, and thereby alters kinematics [49,50,51,52] and increases acetabular stresses, which may lead to implant loosening [53]. Therefore, it has been recommended for patients with adequate pre-operative acetabular coverage, to obtain stable primary fixation of the cup via conservative reaming [30, 31, 39, 40].
Whilst there is a general consensus that THA should restore native (pre-arthritic) hip architecture as closely as possible, restoration of offsets must be adapted to the type of hip deformities. Our findings advocate restoration of the native (pre-arthritic) acetabular offset for better functional outcomes. This suggests ‘conservative reaming’ [40] in centered and proximalized hips, but ‘controlled medialization’ in lateralized and proximo-lateralized hips. The latter could be achieved by meticulous removal of medial acetabular osteophytes without systematic alignment to the true floor. In these four types of hip deformities, restoration of the native (pre-arthritic) femoral offset led to satisfactory outcomes. Theoretically, we also intended to restore the native (pre-arthritic) global offset which means a reduction of the pre-operative global offset in lateralized and proximo-lateralized hips due to the correction of the arthritic remodeling. Other studies have reported better clinical outcomes with restoration of the native (pre-arthritic) global offset [26, 29, 33, 34] and warn against decreasing it, as this could alter gait mechanics [29], reduce abductor muscle strength [34], exacerbate polyethylene (PE) wear, and cause limping, impingement, or dislocation [33, 54]. In medialized hips, the cup should compensate for arthritic medialization and therefore be proportionally lateralized, leading sometimes to a significant reduction in the femoral offset to avoid any over-tensioning of the abductors [13, 45, 55].
This study had some inherent strengths and weaknesses. In terms of strengths, firstly, the pre-operative templating was based on CT, which eliminated magnification bias and allowed templating in the true plane of the femoral neck, with resultant accuracy in offset calculation. Furthermore, it was a consecutive series of patients, with a single type of implant and bearing couple used, with sub-types of this implant chosen to address different types of architectural hip deformities. Finally, the use of both OHS and FJS is of particular relevance due to the sensitivity of these PROMs in detecting subtle differences. However, some limitations of this study should also be noted. First, the findings do not address the core question of whether matching the planned reconstruction improves the outcomes of THA, as outcomes depend on both the ability to match the pre-operative plan (surgical technique) and on its clinical relevance (planning strategy). Second, the pre-operative CT measurements were compared to post-operative plain radiograph measurements, albeit magnification was precisely corrected. Although post-operative CT scans could not have been used for the global series considering the additional cost and exposure to radiation, the authors retrieved a sufficient number of post-operative CT scans to demonstrate the validity of post-operative radiographs. Third, the small number of medialized, proximalized, and proximo-lateralized hips weakens the subgroup analyses.
Conclusion
This study demonstrates that implanting a component of a size different to the pre-operative templating did not compromise clinical scores and therefore suggests that surgeons, first and foremost, should respect their intra-operative findings when it comes to the ultimate implant size selection. Furthermore, the study reveals that patients with medialized hips have worse clinical scores and suggests that conservative reaming of the acetabulum might lead to improved functional outcomes in THA.
References
Della Valle AG, Padgett DE, Salvati EA (2005) Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg 13(7):455–462
Shemesh SS, Robinson J, Keswani A, Bronson MJ, Moucha CS, Chen D (2017) The Accuracy of digital templating for primary total hip arthroplasty: is there a difference between direct anterior and posterior approaches? J Arthroplasty 32(6):1884–1889. https://doi.org/10.1016/j.arth.2016.12.032
Shin JK, Son SM, Kim TW, Shin WC, Lee JS, Suh KT (2016) Accuracy and reliability of preoperative on-screen templating using digital radiographs for total hip arthroplasty. Hip Pelvis 28(4):201–207. https://doi.org/10.5371/hp.2016.28.4.201
Schmalzried TP (2005) Preoperative templating and biomechanics in total hip arthroplasty. Orthopedics 28(8 Suppl):s849–s851
Gonzalez Della Valle A, Slullitel G, Piccaluga F, Salvati EA (2005) The precision and usefulness of preoperative planning for cemented and hybrid primary total hip arthroplasty. J Arthroplasty 20(1):51–58. https://doi.org/10.1016/j.arth.2004.04.016
Jolles BM, Zangger P, Leyvraz PF (2002) Factors predisposing to dislocation after primary total hip arthroplasty: a multivariate analysis. J Arthroplasty 17(3):282–288
Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stockl B (2005) Reducing the risk of dislocation after total hip arthroplasty: the effect of orientation of the acetabular component. J Bone Jt Surg Br 87(6):762–769. https://doi.org/10.1302/0301-620X.87B6.14745
Fottner A, Woiczinski M, Kistler M, Schroder C, Schmidutz TF, Jansson V, Schmidutz F (2017) Influence of undersized cementless hip stems on primary stability and strain distribution. Arch Orthop Trauma Surg 137(10):1435–1441. https://doi.org/10.1007/s00402-017-2784-x
Rudiger HA, Guillemin M, Latypova A, Terrier A (2017) Effect of changes of femoral offset on abductor and joint reaction forces in total hip arthroplasty. Arch Orthop Trauma Surg 137(11):1579–1585. https://doi.org/10.1007/s00402-017-2788-6
Petretta R, Strelzow J, Ohly NE, Misur P, Masri BA (2015) Acetate templating on digital images is more accurate than computer-based templating for total hip arthroplasty. Clin Orthop Relat Res 473(12):3752–3759. https://doi.org/10.1007/s11999-015-4321-y
Asayama I, Chamnongkich S, Simpson KJ, Kinsey TL, Mahoney OM (2005) Reconstructed hip joint position and abductor muscle strength after total hip arthroplasty. J Arthroplasty 20(4):414–420. https://doi.org/10.1016/j.arth.2004.01.016
Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH (2001) Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res 388:125–134
Liebs TR, Nasser L, Herzberg W, Ruther W, Hassenpflug J (2014) The influence of femoral offset on health-related quality of life after total hip replacement. Bone Jt J 96-B(1):36–42. https://doi.org/10.1302/0301-620X.96B1.31530
Cassidy KA, Noticewala MS, Macaulay W, Lee JH, Geller JA (2012) Effect of femoral offset on pain and function after total hip arthroplasty. J Arthroplasty 27(10):1863–1869. https://doi.org/10.1016/j.arth.2012.05.001
Hassani H, Cherix S, Ek ET, Rudiger HA (2014) Comparisons of preoperative three-dimensional planning and surgical reconstruction in primary cementless total hip arthroplasty. J Arthroplasty 29(6):1273–1277. https://doi.org/10.1016/j.arth.2013.12.033
Dastane M, Dorr LD, Tarwala R, Wan Z (2011) Hip offset in total hip arthroplasty: quantitative measurement with navigation. Clin Orthop Relat Res 469(2):429–436. https://doi.org/10.1007/s11999-010-1554-7
Kumar PG, Kirmani SJ, Humberg H, Kavarthapu V, Li P (2009) Reproducibility and accuracy of templating uncemented THA with digital radiographic and digital TraumaCad templating software. Orthopedics 32(11):815. https://doi.org/10.3928/01477447-20090922-08
Bertz A, Indrekvam K, Ahmed M, Englund E, Sayed-Noor AS (2012) Validity and reliability of preoperative templating in total hip arthroplasty using a digital templating system. Skeletal Radiol 41(10):1245–1249. https://doi.org/10.1007/s00256-012-1431-4
Bono JV (2004) Digital templating in total hip arthroplasty. J Bone Jt Surg Am 86-A(Suppl 2):118–122
Crooijmans HJ, Laumen AM, van Pul C, van Mourik JB (2009) A new digital preoperative planning method for total hip arthroplasties. Clin Orthop Relat Res 467(4):909–916. https://doi.org/10.1007/s11999-008-0486-y
Gamble P, de Beer J, Petruccelli D, Winemaker M (2010) The accuracy of digital templating in uncemented total hip arthroplasty. J Arthroplasty 25(4):529–532. https://doi.org/10.1016/j.arth.2009.04.011
Della Valle AG, Comba F, Taveras N, Salvati EA (2008) The utility and precision of analogue and digital preoperative planning for total hip arthroplasty. Int Orthop 32(3):289–294. https://doi.org/10.1007/s00264-006-0317-2
Iorio R, Siegel J, Specht LM, Tilzey JF, Hartman A, Healy WL (2009) A comparison of acetate vs digital templating for preoperative planning of total hip arthroplasty: is digital templating accurate and safe? J Arthroplasty 24(2):175–179. https://doi.org/10.1016/j.arth.2007.11.019
Kosashvili Y, Shasha N, Olschewski E, Safir O, White L, Gross A, Backstein D (2009) Digital versus conventional templating techniques in preoperative planning for total hip arthroplasty. Can J Surg 52(1):6–11
The B, Diercks RL, van Ooijen PM, van Horn JR (2005) Comparison of analog and digital preoperative planning in total hip and knee arthroplasties. A prospective study of 173 hips and 65 total knees. Acta Orthop 76(1):78–84. https://doi.org/10.1080/00016470510030364
Flecher X, Ollivier M, Argenson JN (2016) Lower limb length and offset in total hip arthroplasty. Orthop Traumatol Surg Res 102(1 Suppl):S9–20. https://doi.org/10.1016/j.otsr.2015.11.001
Ollivier M, Parratte S, Lecoz L, Flecher X, Argenson JN (2013) Relation between lower extremity alignment and proximal femur anatomy. Parameters during total hip arthroplasty. Orthop Traumatol Surg Res 99(5):493–500. https://doi.org/10.1016/j.otsr.2013.02.006
Pasquier G, Ducharne G, Ali ES, Giraud F, Mouttet A, Durante E (2010) Total hip arthroplasty offset measurement: is CT scan the most accurate option? Orthop Traumatol Surg Res 96(4):367–375. https://doi.org/10.1016/j.otsr.2010.02.006
Sariali E, Klouche S, Mouttet A, Pascal-Moussellard H (2014) The effect of femoral offset modification on gait after total hip arthroplasty. Acta Orthop 85(2):123–127. https://doi.org/10.3109/17453674.2014.889980
Sariali E, Mauprivez R, Khiami F, Pascal-Mousselard H, Catonne Y (2012) Accuracy of the preoperative planning for cementless total hip arthroplasty. A randomised comparison between three-dimensional computerised planning and conventional templating. Orthop Traumatol Surg Res 98(2):151–158. https://doi.org/10.1016/j.otsr.2011.09.023
Sariali E, Mouttet A, Pasquier G, Durante E, Catone Y (2009) Accuracy of reconstruction of the hip using computerised three-dimensional pre-operative planning and a cementless modular neck. J Bone Jt Surg Br 91(3):333–340. https://doi.org/10.1302/0301-620X.91B3.21390
Inoue D, Kabata T, Maeda T, Kajino Y, Fujita K, Hasegawa K, Yamamoto T, Tsuchiya H (2015) Value of computed tomography-based three-dimensional surgical preoperative planning software in total hip arthroplasty with developmental dysplasia of the hip. J Orthop Sci 20(2):340–346. https://doi.org/10.1007/s00776-014-0683-3
Liu YP, Hao YD (2014) Restoration of femoral offset, rotation centers, limbs length equality of Chinese total hip arthroplasty patients. Pak J Med Sci 30(1):116–121. https://doi.org/10.12669/pjms.301.3635
Mahmood SS, Mukka SS, Crnalic S, Wretenberg P, Sayed-Noor AS (2016) Association between changes in global femoral offset after total hip arthroplasty and function, quality of life, and abductor muscle strength. A prospective cohort study of 222 patients. Acta Orthop 87(1):36–41. https://doi.org/10.3109/17453674.2015.1091955
Kase M, O'Loughlin PF, Ait-Si-Selmi T, Pagenstert G, Langlois J, Bothorel H, Bonnin MP (2019) Pre-operative templating in THA. Part I: a classification of architectural hip deformities. Arch Orthop Trauma Surg 140:129–137. https://doi.org/10.1007/s00402-019-03298-1
Kobayashi H, Cech A, Kase M, Pagenstert G, Carrillon G, O'Loughlin PF, Bothorel H, Ait-Si-Selmi T, Bonnin MP (2019) Pre-operative templating in THA. Part II: a CT-based strategy to correct architectural hip deformities. Arch Orthop Trauma Surg 140:129–137. https://doi.org/10.1007/s00402-019-03298-1
Bonnin MP, Neto CC, Aitsiselmi T, Murphy CG, Bossard N, Roche S (2015) Increased incidence of femoral fractures in small femurs and women undergoing uncemented total hip arthroplasty—why? Bone Jt J 97-B(6):741–748. https://doi.org/10.1302/0301-620X.97B6.35022
Murphy CG, Bonnin MP, Desbiolles AH, Carrillon Y, Aїt Si Selmi T (2016) Varus will have varus; a radiological study to assess and predict varus stem placement in uncemented femoral stems. Hip Int 26(6):554–560. https://doi.org/10.5301/hipint.5000412
Bonnin MP, Archbold PH, Basiglini L, Fessy MH, Beverland DE (2012) Do we medialise the hip centre of rotation in total hip arthroplasty? Influence of acetabular offset and surgical technique. Hip Int 22(4):371–378. https://doi.org/10.5301/HIP.2012.9350
Meermans G, Doorn JV, Kats JJ (2016) Restoration of the centre of rotation in primary total hip arthroplasty: the influence of acetabular floor depth and reaming technique. Bone Jt J 98-B(12):1597–1603. https://doi.org/10.1302/0301-620X.98B12.BJJ-2016-0345.R1
Khanuja HS, Vakil JJ, Goddard MS, Mont MA (2011) Cementless femoral fixation in total hip arthroplasty. J Bone Jt Surg Am 93(5):500–509. https://doi.org/10.2106/JBJS.J.00774
Behrend H, Giesinger K, Giesinger JM, Kuster MS (2012) The "forgotten joint" as the ultimate goal in joint arthroplasty: validation of a new patient-reported outcome measure. J Arthroplasty 27(3):430–436e431. https://doi.org/10.1016/j.arth.2011.06.035
Delaunay C, Epinette JA, Dawson J, Murray D, Jolles BM (2009) Cross-cultural adaptations of the Oxford-12 HIP score to the French speaking population. Orthop Traumatol Surg Res 95(2):89–99. https://doi.org/10.1016/j.otsr.2009.01.003
Baghdadi YM, Larson AN, Sierra RJ (2013) Restoration of the hip center during THA performed for protrusio acetabuli is associated with better implant survival. Clin Orthop Relat Res 471(10):3251–3259. https://doi.org/10.1007/s11999-013-3072-x
Bonnin MP, Archbold PH, Basiglini L, Selmi TA, Beverland DE (2011) Should the acetabular cup be medialised in total hip arthroplasty. Hip Int 21(4):428–435. https://doi.org/10.5301/HIP.2011.8582
Terrier A, Levrero Florencio F, Rudiger HA (2014) Benefit of cup medialization in total hip arthroplasty is associated with femoral anatomy. Clin Orthop Relat Res 472(10):3159–3165. https://doi.org/10.1007/s11999-014-3787-3
Terrier A, Parvex V, Rudiger HA (2016) Impact of individual anatomy on the benefit of cup medialisation in total hip arthroplasty. Hip Int 26(6):537–542. https://doi.org/10.5301/hipint.5000392
Tezuka T, Inaba Y, Kobayashi N, Ike H, Kubota S, Kawamura M, Saito T (2015) Effects of hip joint center location and femoral offset on abductor muscle strength after total hip arthroplasty. Mod Rheumatol 25(4):630–636. https://doi.org/10.3109/14397595.2014.988863
Delp SL, Maloney W (1993) Effects of hip center location on the moment-generating capacity of the muscles. J Biomech 26(4–5):485–499
Delp SL, Wixson RL, Komattu AV, Kocmond JH (1996) How superior placement of the joint center in hip arthroplasty affects the abductor muscles. Clin Orthop Relat Res 328:137–146
Doehring TC, Rubash HE, Dore DE (1999) Micromotion measurements with hip center and modular neck length alterations. Clin Orthop Relat Res 362:230–239
Doehring TC, Rubash HE, Shelley FJ, Schwendeman LJ, Donaldson TK, Navalgund YA (1996) Effect of superior and superolateral relocations of the hip center on hip joint forces. An experimental and analytical analysis. J Arthroplasty 11(6):693–703
Miles AW, McNamee PB (1989) Strain gauge and photoelastic evaluation of the load transfer in the pelvis in total hip replacement: the effect of the position of the axis of rotation. Proc Inst Mech Eng H 203(2):103–107. https://doi.org/10.1243/PIME_PROC_1989_203_018_01
Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB (2009) Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res 467(11):2895–2900. https://doi.org/10.1007/s11999-009-0845-3
Ji HM, Won SH, Han J, Won YY (2017) Does femoral offset recover and affect the functional outcome of patients with displaced femoral neck fracture following hemiarthroplasty? Injury 48(6):1170–1174. https://doi.org/10.1016/j.injury.2017.03.022
Acknowledgements
The authors are grateful to Mr. Mo Saffarini for his assistance with manuscript preparation and illustrations.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors AC, MK, HK, YC, PFOL, and HB declare that they have no conflict of interest. TASS receives royalties and or consulting fees from DePuy-Synthes, Symbios, and Corin-Tornier. MPB receives royalties and or consulting fees from DePuy-Synthes, Symbios, Corin-Tornier, Wright-Tornier, and Integra.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional review board (IRB) who approved this study in advance (COS- RGDS-2019-05-005-BONNIN-M) and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cech, A., Kase, M., Kobayashi, H. et al. Pre-operative planning in THA. Part III: do implant size prediction and offset restoration influence functional outcomes after THA?. Arch Orthop Trauma Surg 140, 563–573 (2020). https://doi.org/10.1007/s00402-020-03342-5
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-020-03342-5