Abstract
Objective
Our objective was to evaluate the efficacy and safety of Batroxobin on blood loss during spinal operations.
Methods
After obtaining approval from the ethics committee at the hospital along with informed written consent, we performed a double-blind, randomized, placebo-controlled study with 100 patients who were randomized equally into 2 groups (Batroxobin and placebo). Patients received either 2 ku IV 15 min before surgery and followed 1 ku IM of Batroxobin following surgery, or an equivalent volume of placebo (normal saline). Cost of Batroxobin treatment is amounted to 84.75 euros. The primary outcomes were intraoperative, 24 h postoperative, and total perioperative blood loss. Secondary outcomes were hemoglobin (Hb), red blood cell count (RBC), the volume of blood/fluid transfusion intraoperatively, and 24 h postoperatively. Safety evaluation parameters were the incidence of venous thrombosis in the lower extremities, active partial thromboplastin time, prothrombin time, thrombin time, and fibrinogen. The data were analyzed using the Statistical Package for the Social Science Version 12.0. The results were presented as mean ± SEM. The Mann–Whitney test and Independent Student t test, when appropriate, were used to compare the 2 groups, and differences were considered significant if the P value was <0.05.
Results
88 patients were included in the analysis while 12 patients were withdrawn from the study due to extended surgical duration, change of surgical procedure, or after the patients’ request. The total perioperative blood loss was approximately 31 % lower in patients given Batroxobin versus placebo (700.5 ± 45.81 vs 485.7 ± 30.01 mL, P = 0.001). The Batroxobin group had significantly less intraoperative blood loss (326.1 ± 24.16) compared to the placebo group (556.0 ± 43.58), but there was no difference in the amount of blood/fluid transfused, postoperatively Hb, or RBC between the two groups. After the operation, coagulation parameters were not significantly different between the 2 groups at the days 1 or 3 postoperatively. No adverse events related to the use of Batroxobin were recorded. There were no cases of superficial wound infection. None of the subjects died during the study.
Conclusions
In this study, prophylactic use of Batroxobin provided an effective and cheap method for reducing blood loss without coagulopathy during or after operations. The use of Batroxobin for patients undergoing one-level PLIF surgery safely and effectively reduced the total amount of perioperative blood loss.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surgical procedures lead inevitably to bleeding. Blood loss during spinal operations can be excessive; this may exceed the patient’s total blood volume, especially in spinal fusion [23] and deformity correction [13]. Decompression, instrumentation, correction, and fusion procedures may require considerable time and large wound surfaces. Dealing with branches of segmental blood vessels, cancellous bone, with its rich blood supply, and the internal vertebral venous plexuses are often associated with significant perioperative blood loss. In the end, complicated anatomical relationships, bone proliferation, and osseous variations may obscure the surgical goal by either increasing the risk of bleeding or requiring additional operative time.
Large blood loss can lead to multiple deleterious consequences, such as multiple blood component transfusions with their inherent risks (blood-borne disease transmission, increased incidence of wound infections, haemolytic/nonhemolytic transfusion reactions, and monetary expense), fluid shifts with concomitant pulmonary and/or cerebral edema, coagulation disorders, and the development of a shock-like state [8, 16, 17, 19]. Therefore, multiple suggestions have been proposed to reduce blood loss in spinal operations, including surgical technique, operative time, number of vertebral levels fused, anesthetics, mean arterial blood pressure, and hemostatic agents.
Batroxobin(s) are hemostatic agents refined and purified from Bothrops atrox moojeni venom, and act as a thrombin-like enzyme in the mechanism of blood coagulation [5, 20]. In contrast to thrombin, Batroxobin is only involved in the conversion of fibrinogen and does not affect other coagulation factors or cells [5]. Therefore, its hemostatic effect occurs only at the point of injury, avoiding large-scale intravascular coagulation [5]. However, few studies have reported the safety and efficacy of Batroxobin in spinal surgery. Therefore, our aim in this study was to evaluate the effectiveness of Batroxobin objectively in reducing blood loss in patients undergoing spine fusion surgery. Additionally, we observed the appearance or absence of perioperative complications associated with the use of Batroxobin.
Materials and methods
Trial design
This trial was a prospective, randomized, double-blind, placebo-controlled study that enrolled 100 patients from June 2011 to December 2013. Inclusion criteria were patients undergoing one-level posterior lumbar interbody fusion (PLIF) surgery for degenerative lumbar disease. Ages ranged from 18 to 70 years. The protocol and amendments were approved by the ethics committee at the hospital, and written informed consent was provided by all patients before any study-related procedures were performed. Sample size and power: the main outcome measure was perioperative blood loss, a total of 26 patients (13 patients in each group) are needed to achieve 80 % power at two-sided 5 % significance level. Patients were not included if they had significant chronic diseases, coagulopathy, or liver and/or kidney dysfunction. Also, patients were excluded if the duration of their surgery was extended past 5 h or if a cerebrospinal fluid fistula occurred during the operation. Patients were randomized into two equal groups by means of a sealed envelope method (on the basis of a block-randomized computer-generated list), and the randomization code was kept unknown to any of the investigators until the study was complete. The drug or placebo solution was drawn into a syringe by a nurse not participating in the study and was delivered to the investigator who was unaware of the content. The saline and Batroxobin solutions appeared transparent and completely identical at the time the syringes were given to the investigator. Thus, the patients, the anesthesiologist, the surgeon, and the study observer were all blinded with respect to the study group. Patients received either 2 ku IV in 15 min before the surgical incision is made and followed 1 ku IM at the time of wound closure of Batroxobin or an equivalent volume of placebo (normal saline). The price for Batroxobin medication is 28.25 euros per ku, overall cost of Batroxobin medication for each patient is 84.75 euros.
Anesthetic technique
All patients received the same general anesthesia. Induction of anesthesia was carried out using intravenous drugs (propofol 3–4 mg/kg, fentanyl 1 µg/kg, and atracurium 0.5 mg/kg, as muscle relaxant) with tracheal intubation and assisted nitrogen dioxide and oxygen ventilation at a ratio of 2:1. After intubation, anesthesia was maintained with inhalation of 1 MAC sevoflurane in a mixture of air and 50 % oxygen. Atracurium and fentanyl were given during the procedure when required. Patients were monitored as per our standard procedure, and the mean arterial blood pressure was maintained between 60 and 70 mm Hg during surgery. Blood was transfused during surgery and post operation if hemoglobin levels dropped (below 9 g/L or hematocrit 27 %) with a decreasing trend. No coagulant was used postoperatively.
Study objectives
The patient’s data sheet recorded the demographic data, patient’s weight, past medical history, comorbid conditions, current medications, current surgical diagnosis, operation to be performed, and preoperative laboratory workup, including complete blood count, clotting profile, renal, and liver function tests. It also included accurate calculation of blood loss during and after surgery. The amount of intraoperative blood loss was accurately calculated by measuring blood collected by drain and suction canisters, weighing the swabs, and subtracting all irrigation fluids used during surgery. After surgery, the amount of drainage collected through self-suction devices was accurately calculated and recorded. The amount of fluid, blood, packed red blood cells, and its substitutes which were transfused during and after surgery were also documented.
The primary outcomes were intraoperative, 24 h postoperative, and total perioperative blood loss (determined from combining the intraoperative and postoperative blood loss). Secondary outcomes were hemoglobin (Hb), red blood cell count (RBC), the volume of blood transfusion and fluid transfusion intraoperatively and 24 h postoperatively. Safety evaluation parameters were the incidence of venous thrombosis of the lower extremities, active partial thromboplastin time (APTT), prothrombin time (FT), thrombin time (TT), platelet count (PLT), and fibrinogen (FIB) after the first 24 h and 3rd day postoperatively. We examined any complications occurring within the first 12 weeks after surgery.
For statistical analyses, we used the Fisher exact, χ 2, Mann–Whitney test and t tests, when appropriate, using Statistical Package for the Social Science Version 12.0. The blood loss and volume of transfusion intraoperatively and 24 h postoperatively were analyzed for intergroup comparison. In addition, 24-h and 3-day laboratory results were specifically analyzed for intergroup differences. The results were presented as mean ± SEM. P < 0.05 was considered statistically significant.
Results
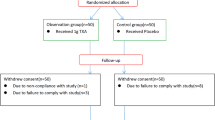
From June 2011 to December 2013, 100 patients were randomized. A total of 88 patients were available for analysis (Table 1); 12 patients (4 in Batroxobin group, 8 in placebo group) were excluded from the study. There were 46 patients in the Batroxobin group and 42 patients in the placebo group (Table 1). Baseline characteristics (age, sex, height, weight, HB, RBC, WBC, APTT, TT, ALP, FT, and FIB) were similar between treatment groups (Table 2).
Primary outcome measures
The Batroxobin group had significantly less intraoperative blood loss (326.1 ± 24.16 ml) compared to the placebo group (556.0 ± 43.58 ml) (Table 2), although there were no differences in the amount of postoperative blood loss during the first 24 h between the Batroxobin (166.8 ± 13.50 ml) and placebo groups (159.7 ± 14.38 ml) (Table 2). The Batroxobin group (485.7 ± 30.01 ml) continued to have significantly less total blood loss (intraoperative plus postoperative blood loss) than the placebo group (700.5 ± 45.81 ml).
Secondary outcome measures
The mean amount of intraoperative transfused autologous blood was 28.04 ± 13.89 ml in the Batroxobin group compared with 50.24 ± 24.47 ml in the control group (Table 2), which was not statistically significant (P = 0.6224). The volume of blood transfusion used during first 24 h postoperatively was similar (8.696 ± 8.696 vs 31.19 ± 26.16 ml, P = 0.5150).
For the volume of fluid transfusion, a significant difference between the Batroxobin and placebo group during operation or the first 24 h postoperative was not observed (Table 2). There were no significant differences between the 2 groups in Hb or RBC on days 1 or 3 postoperatively (Table 3).
Safety evaluation parameters
After the operation, coagulation parameters were not significantly different between the 2 groups at the days 1 or 3 postoperatively (Table 3). There were no intraoperative complications in either of the 2 groups. None of the patients experienced seizures, myocardial infarction (MI), deep venous thrombosis (DVT), or pulmonary embolus during their postoperative period. No adverse events related to the use of Batroxobin were recorded. No cases of superficial wound infection were recorded. No patients died during the study.
Discussion
The main finding of this study was that the application of Batroxobin significantly reduced intraoperative blood loss and total blood loss in one-level PLIF surgery. Patients treated with Batroxobin also had higher, although not statistically significant, Hb and RBC levels on 1st and 3rd day postoperatively. The Batroxobin group had lower volume of blood transfusion during their intraoperative and 24 h postoperative procedures than patients receiving the placebo treatment without statistically significant. The use of Batroxobin did not affect the parameters of coagulation, including APTT, PT, TT, FIB and PLT at day 1 or 3 postoperatively. In our study, no complications associated with the administration of Batroxobin (e.g. medication/allergic reaction, MI, DVT, stroke, renal failure, or pulmonary embolism) were observed during the follow-up.
During spinal fusion surgery, decompression, instrumentation, correction and fusion procedures require considerable time and large wound surfaces, which can lead to enormous blood loss and transfusion requirement. Furthermore, there are various disadvantages for hemostasis that either increase the risk of bleeding or require additional operative time in spinal surgery, such as particular spinal anatomical structure, spongy vertebrae with its rich blood supply, and the fragile venous plexus wall that cannot self-contract after injury. In addition, the use of effective hemostasis methods (local pressure, blood vessels ligation, and hot coagulation) are restricted, so surgeons rely more on local coagulation than on traditional measures. Thus, a safe and effective intervention to reduce the number of blood loss to these patients during and immediately following surgery is required.
During local coagulation, hemostasis is achieved with the formation of blood clots, which are lodged in the injury site by a fibrin network. Fibrin network formation is triggered by thrombin as fibrinogen is converted to fibrin. Initially this formation is not stable, but coagulation factor XIII activated by thrombin stimulates the polymerization of fibrin which forms cross-linking networks [5, 22]. Batroxobin, a thrombin-like enzyme with fibrinogen clotting activity, is refined and purified from the Bothrops atrox venom. Alone, Batroxobin is not toxic and does not affect cells or other blood coagulation factors [5, 18]. Batroxobin only affect split-off fibrinopeptide A chains which in turn promotes blood fibrin monomer conversion and induces the formation of component FX particles on the surface of phospholipid under the condition that the injured vascular points release platelets factor III [20]. In You et al. [26], both the results of the fibrinogen clotting assay and the whole blood clotting assay showed that the addition of Batroxobin can accelerate fibrinogen formation. The results of the mouse liver model showed the fibrin formed by Batroxobin promoted large amounts of blood clots formed through the entrapment of erythrocytes, and the amount of total blood loss significantly decreased. Consequently, cross-linking fibrin is formed by Batroxobin, and its hemostatic effect occurs only at the point of injury and does not affect other coagulation factors and cells, avoiding large-scale intravascular coagulation [5]. Sugai et al. [21] determined that the half-life of Batroxobin in the plasma was 6 h in human. Intraoperative administration of Batroxobin can ensure the effective plasma concentrations during operation and within 6 h postoperatively in our study, meanwhile, avoided hypercoagulable activity after spinal surgery. The properties of Batroxobin may provide therapeutic advantages.
Xu et al. [24] found that Batroxobin could markedly reduce the blood loss and transfusion requirements in adolescent idiopathic scoliosis surgery. The blood loss in the Batroxobin group decreased by 35.3 % compared with Placebo group. Additionally, the amount of allogeneic blood transfusion of group Batroxobin was comparably reduced by 57.6 % compared to group Placebo. Overall drainage of the Batroxobin group decreased by 23.0 % compared with the placebo group. The FFP of the Batroxobin group was reduced by 63.4 % as compared with the placebo group. Liu et al. found that Batroxobin could reduce the bleeding (1138 ± 121–806 ± 84 ml), transfusion volume (3.0 ± 0.5–1.7 ± 0.4 U) and drainage volume (172 ± 28–98 ± 16 ml) 24 h after surgery in spinal operation (include cervical, tumor, and fracture) with enhanced coagulation function. Zeng et al. [27] performed meta-analyses to evaluate the effect of Batroxobin agents on perioperative hemorrhage in thoracic surgery, and concluded there are lots of disadvantages in trials that were included in meta-analyses. First of all, methodological qualities of all trials were poor, and several bias factors existed. None of these trials reported sample size calculation and specified allocation concealment. Secondly, the meta-analyses only included two trials that had reported intraoperative blood loss. In addition, in the only two trials, data are a bit unclear. Thirdly, there were potential for measurement bias because blinding outcome assessment was not applied in these trials. Therefore, there is not enough evidence to draw a positive conclusion of hemostatic effect of Batroxobin in thoracic surgery; although mean differences between Batroxobin and no Batroxobin groups were statistically significant. Spinal surgery has its own characteristics, for example, there are various disadvantages for hemostasis that increase the risk of bleeding in spinal surgery (as mentioned above). In addition, anticoagulant (Heparin and Protamine Sulfate) [4, 15] must be used in thoracic surgery, but anticoagulant is not necessary in spinal surgery. Before any conclusions can be drawn it is necessary to look closely at the hemostatic effect of Batroxobin in spinal surgery. So, we performed a double-blind, randomized, placebo-controlled study to evaluate the effect of Batroxobin on perioperative hemorrhage in spinal surgery.
Our results are similar to those found by previous studies. Intraoperative administration of Batroxobin minimized the total blood loss to 485.7 ± 30.01 ml decreasing by 30.5 % compared to the control group 700.5 ± 45.81 ml under the given conditions of this study. Further analysis found that blood loss during operation on the Batroxobin group (326.1 ± 24.16 ml) decreased by 41.4 % compared to the control group (556.0 ± 43.58), but the blood loss during the first 24 h after operation did not show any significant difference. Therefore, Batroxobin may decrease the amount of total blood loss mainly by reducing the intraoperative bleeding. In contrast to previous reports in our study, the volume of autologous blood transfusion in the Batroxobin group was lower than the placebo group during operation and the first 24 h postoperative. This difference was not found to be significant. Also, we were unable to find significant differences between the 2 groups with regards to Hb and RBC levels on days 1 or 3 postoperatively.
Tranexamic acid (TXA), a synthetic antifibrinolytic agent, is one of the most common hemostatics used to deal with hemorrhage by retarding fibrinolysis and blood clot degradation [12]. TXA has been successfully applied to treat major hemorrhaging in different types of operations, including spinal surgery [1, 2, 6, 7, 11, 28]. Elwatidy et al. [12] reported a significant reduction in blood loss in patients who were administered TXA during spinal surgery. In that study, blood loss during surgery was reduced by 48 % with the use of TXA. The amount of blood and fluids collected from wound drains was 55 % less in patients who received TXA, and the total amount of blood loss was less by 49 % in the TXA group. Consequently, the amount of blood transfusion used to treat patients in the TXA group was 80 % less than in the placebo group. There were also significant differences in the postoperative Hb and HCT values of patients in both groups. There were no hemodynamic disturbances, apparent thromboembolic complications, or other drug complications associated with its use. Yagi et al. [25] reported, during posterior spinal fusion for the treatment of idiopathic scoliosis in adolescents, that the TXA group had significantly less intraoperative blood loss (613 ± 195 mL) than the control group (1079 ± 421 mL; P < 0.001) as well as postoperative blood loss (155 ± 86 and 263 ± 105 mL, respectively; P < 0.001). The TXA group received significantly less blood during the surgical procedure than the control group (258 ± 246 and 377 ± 200 mL, respectively; P < 0.001). There were no major intraoperative complications for any of the treatment groups. Whereas, Baldus et al. [3] reported no advantage of TXA administration for blood loss during lumbar pedicle subtraction osteotomy (PSO) surgery (10 patients, 1838 ± 1096 mL vs 10 patients 1502 ± 1241 mL). The differences between these studies involve (1) the dose of TXA and (2) the type of surgery. As reported in a meta-analysis, TXA could mitigate hemorrhage from 25.5 to 49.2 % compared to the placebo group [2]. According to the aforementioned data, the reduction of blood loss due to treatment with Batroxobin in our study approximated to that of TXA. This might indicate that the hemostatic efficacy of these two styptics is similar.
Anticoagulant (Heparin and Protamine Sulfate) must be used in thoracic surgery, but the utility and safety of anticoagulant following spinal surgery is controversial. Some study showed [10, 14] that most commonly performed spine surgeries done through a posterior approach are associated with a very low risk of VTE. Meanwhile, anticoagulant [9] is accompanied by a definable risk of serious wound and bleeding complications. Anticoagulant may be used only for long and complex spinal surgeries, such as anterior–posterior (circumferential) spinal surgery, and in patients with known thromboembolic risk factors, such as multiple trauma, malignancy, paralysis, spinal cord injury, malignancy, or hypercoagulable state. So, using anticoagulant should be considered carefully and on an individual case-by-case basis in spinal surgery. In our study, routine one-level PLIF surgery did not cause thromboembolic risk. In addition, Batroxobin is only involved in the conversion of fibrinogen and does not cause blood hypercoagulation. Thus, no anticoagulant was used postoperatively during study.
Currently, the research on the effect of hemostatics during spinal operations is mainly concentrated in scoliosis surgery. Other studies focus on the cervical, tumor, fracture, and lumbar PSO surgery. The results of these studies indicate that the average intraoperative hemorrhage about more than 1500 ml and hemostatic drugs can significantly reduce the amount of bleeding and blood transfusion. In all of the reports mentioned earlier, patients were treated by different types or different levels of the surgery. The strength of our study is the consistency of our patients’ pathology and treatment. This study enrolled a series of patients with lumbar degenerative disease having one-level PLIF surgery performed by three experienced surgeons at single institution. Batroxobin decreased the perioperative bleeding by 30.5 % (approximately 215 ml) compared to the control group (700.5 ± 45.81 ml), but the difference did not lead to the difference on blood transfusion, Hb, and RBC in our study. One-level PLIF surgery alone was chosen in our study due to the following: Firstly, one-level PLIF surgery is the most common spinal fusion surgery; secondly, performing a single type of surgical procedure increases the comparability between groups; lastly, the perioperative bleeding is moderate during one-level PLIF surgery leading to a more stable drug concentration in the bloodstream that are required for curative effect analysis.
Conclusions
In this study, the use of Batroxobin for patients undergoing one-level PLIF surgery was found to be both effective and safe. It significantly reduced the total amount of perioperative blood loss without increased incidence of coagulopathy. Meanwhile, no DVT or side effects were detected. Due to the limited number of patients in this study, our results need further validation on a larger number of patients. We are currently planning a prospective, randomized, double-blind, multicenter study to determine the safety and efficacy of Batroxobin used during major spinal surgery. This multicenter prospective comparative analysis will provide additional information about the efficacy and safety of Batroxobin and other hemostatics.
References
Alshryda S, Sarda P, Sukeik M, Nargol A, Blenkinsopp J, Mason JM (2011) Tranexamic acid in total knee replacement: a systematic review and meta-analysis. J Bone Joint Surg Br 93(12):1577–1585
Alshryda S, Sukeik M, Sarda P, Blenkinsopp J, Haddad FS, Mason JM (2014) A systematic review and meta-analysis of the topical administration of tranexamic acid in total hip and knee replacement. Bone Joint J 96-B(8):1005–1015
Baldus CR, Bridwell KH, Lenke LG, Okubadejo GO (2010) Can we safely reduce blood loss during lumbar pedicle subtraction osteotomy procedures using tranexamic acid or aprotinin? A comparative study with controls. Spine 35(2):235–239
Besser MW, Ortmann E, Klein AA (2015) Haemostatic management of cardiac surgical haemorrhage. Anaesthesia 70(Suppl 1):87-e31
Braud S, Bon C, Wisner A (2000) Snake venom proteins acting on hemostasis. Biochimie 82(9–10):851–859
Brown JR, Birkmeyer NJ, O’Connor GT (2007) Meta-analysis comparing the effectiveness and adverse outcomes of antifibrinolytic agents in cardiac surgery. Circulation 115(22):2801–2813
Crescenti A, Borghi G, Bignami E et al (2011) Intraoperative use of tranexamic acid to reduce transfusion rate in patients undergoing radical retropubic prostatectomy: double blind, randomised, placebo controlled trial. BMJ 343:d5701
Despotis GJ, Avidan MS, Hogue CW Jr (2001) Mechanisms and attenuation of hemostatic activation during extracorporeal circulation. Ann Thorac Surg 72(5):S1821–S1831
Devlin JW, Tyburski JG, Moed B (2001) Implementation and evaluation of guidelines for use of enoxaparin as deep vein thrombosis prophylaxis after major trauma. Pharmacotherapy 21(6):740–747
Ee PL, Kempen PM (2006) Elective surgery days after myocardial infarction: clinical and ethical considerations. J Clin Anesth 18(5):363–366
Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D (2010) Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine 35(9 Suppl):S47–S56
Hardy JF, Belisle S (1997) Natural and synthetic antifibrinolytics: inert, poisonous or therapeutic agents? Can J Anaesth J Can d’anesthesie 44(9):913–917
Hassan N, Halanski M, Wincek J et al (2011) Blood management in pediatric spinal deformity surgery: review of a 2-year experience. Transfusion 51(10):2133–2141
Ho WK, Baccala M, Thom J, Eikelboom JW (2005) High prevalence of abnormal preoperative coagulation tests in patients with adolescent idiopathic scoliosis. J Thromb Haemost JTH 3(5):1094–1095
Machovec KA, Jooste EH, Walczak RJ et al (2014) A change in anticoagulation monitoring improves safety, reduces transfusion, and reduces costs in infants on cardiopulmonary bypass. Paediatr Anaesth. doi:10.1111/pan.12591 [Epub ahead of print]
Mitchell JP, Schuller D, Calandrino FS, Schuster DP (1992) Improved outcome based on fluid management in critically ill patients requiring pulmonary artery catheterization. Am Rev Respir Dis 145(5):990–998
Murray DJ, Gress K, Weinstein SL (1992) Coagulopathy after reinfusion of autologous scavenged red blood cells. Anesth Analg 75(1):125–129
Pirkle H (1998) Thrombin-like enzymes from snake venoms: an updated inventory. Scientific and Standardization Committee’s Registry of exogenous hemostatic factors. Thromb Haemost 79(3):675–683
Silliman CC, Paterson AJ, Dickey WO et al (1997) The association of biologically active lipids with the development of transfusion-related acute lung injury: a retrospective study. Transfusion 37(7):719–726
Stocker K, Barlow GH (1976) The coagulant enzyme from Bothrops atrox venom (batroxobin). Methods Enzymol 45:214–223
Sugai K, Imamura Y, Uechi S et al (1986) Metabolic fate of batroxobin in human. Yakugaku Zasshi J Pharm Soc Jpn 106(4):335–342
Tanaka KA, Key NS, Levy JH (2009) Blood coagulation: hemostasis and thrombin regulation. Anesth Analg 108(5):1433–1446
Verma K, Errico TJ, Vaz KM, Lonner BS (2010) A prospective, randomized, double-blinded single-site control study comparing blood loss prevention of tranexamic acid (TXA) to epsilon aminocaproic acid (EACA) for corrective spinal surgery. BMC Surg 10:13
Xu C, Wu A, Yue Y (2012) Which is more effective in adolescent idiopathic scoliosis surgery: batroxobin, tranexamic acid or a combination? Arch Orthop Trauma Surg 132(1):25–31
Yagi M, Hasegawa J, Nagoshi N et al (2012) Does the intraoperative tranexamic acid decrease operative blood loss during posterior spinal fusion for treatment of adolescent idiopathic scoliosis? Spine 37(21):E1336–E1342
You KE, Koo MA, Lee DH et al (2014) The effective control of a bleeding injury using a medical adhesive containing batroxobin. Biomed Mater 9(2):025002
Zeng Z, Xiao P, Chen J, Wei Y (2009) Are batroxobin agents effective for perioperative hemorrhage in thoracic surgery? A systematic review of randomized controlled trials. Blood Coagul Fibrinolysis Int J Haemost Thromb 20(2):101–107
Zhang H, Chen J, Chen F, Que W (2012) The effect of tranexamic acid on blood loss and use of blood products in total knee arthroplasty: a meta-analysis. Knee Surg Sports Traumatol Arthrosc 20(9):1742–1752
Conflict of interest
The authors have no personal, financial, or institutional interest in any of the drugs, materials, or devices described in this article.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Hu, HM., Chen, L., Frary, C.E. et al. The beneficial effect of Batroxobin on blood loss reduction in spinal fusion surgery: a prospective, randomized, double-blind, placebo-controlled study. Arch Orthop Trauma Surg 135, 491–497 (2015). https://doi.org/10.1007/s00402-015-2183-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-015-2183-0