Abstract
Parkinson’s disease (PD), the most common neurodegenerative movement disorder, is characterized by the progressive loss of nigral dopamine neurons. The deposition of fibrillary aggregated α-synuclein in Lewy bodies (LB), that is considered to play a causative role in the disease, constitutes another key neuropathological hallmark of PD. We have recently described that synapsin III (Syn III), a synaptic phosphoprotein that regulates dopamine release in cooperation with α-synuclein, is present in the α-synuclein insoluble fibrils composing the LB of patients affected by PD. Moreover, we observed that silencing of Syn III gene could prevent α-synuclein fibrillary aggregation in vitro. This evidence suggests that Syn III might be crucially involved in α-synuclein pathological deposition. To test this hypothesis, we studied whether mice knock-out (ko) for Syn III might be protected from α-synuclein aggregation and nigrostriatal neuron degeneration resulting from the unilateral injection of adeno-associated viral vectors (AAV)-mediating human wild-type (wt) α-synuclein overexpression (AAV-hαsyn). We found that Syn III ko mice injected with AAV-hαsyn did not develop fibrillary insoluble α-synuclein aggregates, showed reduced amount of α-synuclein oligomers detected by in situ proximity ligation assay (PLA) and lower levels of Ser129-phosphorylated α-synuclein. Moreover, the nigrostriatal neurons of Syn III ko mice were protected from both synaptic damage and degeneration triggered by the AAV-hαsyn injection. Our observations indicate that Syn III constitutes a crucial mediator of α-synuclein aggregation and toxicity and identify Syn III as a novel therapeutic target for PD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Brain α-synuclein deposition in Lewy bodies (LB) and Lewy neurities (LN) is a key neuropathological hallmark of Parkinson’s disease (PD) [22, 50]. This is the most common neurodegenerative movement disorder and is characterized by the progressive loss of dopaminergic neurons of the substantia nigra. Alpha-synuclein aggregation is thought to be causatively involved in neuronal cell degeneration in PD, although the mechanisms underlying its toxicity need clarification. Several studies indicate that impairment of synaptic functions in PD is related to aggregation of α-synuclein at synaptic sites [2]. At the presynaptic terminals, α-synuclein physiologically interacts with a multiplicity of proteins, such as vesicle-associated membrane protein 2 (VAMP2) [14, 17], synapsin III (Syn III) [31, 59] or the dopamine transporter (DAT) [27] and modulates the turnover of synaptic vesicles (SV) [13, 55]. Alpha-synuclein also regulates SV exocytosis by assisting the Soluble NSF Attachment Protein Receptor (SNARE) complex assembly. This function is mediated through the binding of presynaptic membranes and of the vesicular SNARE protein VAMP2 with its N- and C-terminal domains, respectively [15, 17]. Conversely, we observed that α-synuclein aggregation alters the distribution of SNAREs at striatal dopaminergic terminals [21], supporting that this phenomenon may be associated with changes in the proper distribution of SV. On this line, α-synuclein multimers have been found to induce the clustering of SV resulting in the reduction of their mobility [57]. Moreover, α-synuclein aggregation also compromises DAT trafficking by directly altering its distribution in the striatum [6, 9].
Recently, we have shown that the cooperation between α-synuclein and the synaptic phosphoprotein Syn III is relevant for ensuring dopamine neuron synaptic function [59]. To date, among the different synapsin isoforms, Syn III is selectively involved in the control of striatal dopamine release [24]. In addition, we have shown that Syn III is a crucial α-synuclein interactant and a key component of LB fibrils in the brain of patients affected by PD [31]. These observations, when coupled to the fact that Syn III gene silencing can prevent the formation of α-synuclein inclusions in primary murine dopaminergic neurons [59], support that Syn III may be involved in α-synuclein aggregation and toxicity. We aimed at testing this working hypothesis by evaluating whether the formation of fibrillary insoluble deposits of α-synuclein and nigrostriatal neuron degeneration, induced by the injection of adeno-associated viral vectors (AAV)-mediating human α-synuclein overexpression (AAV-hαsyn), may be hampered in Syn III knock out (ko) mice.
In line with previous observations [16, 25, 32, 36], we found that the inoculation of AAV-hαsyn in the substantia nigra of wild-type (wt) mice resulted in the overexpression and aggregation of human α-synuclein in the nigrostriatal system. These events were associated with partial loss of nigral tyrosine hydroxylase (TH)-positive neurons and fibers as well as of DAT-positive synaptic terminals. At this pathological stage, we also detected specific changes in SV organization and synaptic proteins (Syn III, VAMP2, DAT) in the striatum, although these alterations were not sufficient to induce a significant increase in the number of amphetamine-induced ipsilateral rotations. Conversely, albeit the Syn III ko mice inoculated with AAV-hαsyn exhibited an overexpression of human α-synuclein that was comparable to that observed in wt mice, they showed very mild signs of α-synuclein aggregation. Furthermore, they were also protected from the AAV-hαsyn injection-related synaptic changes and nigrostriatal degeneration.
Materials and methods
Animals
Two-month-old Syn III ko mice on C57BL/6J background, a kind gift of Prof. Fabio Benfenati, Italian Institute of Technology, Genoa, Italy, and C57BL/6J wt mice were used for this study. Mice were bred in our animal house facility at the Department of Molecular and Translational Medicine of University of Brescia, Brescia, Italy. Animals were maintained under a 12-h light–dark cycle at a room temperature (rt) of 22 °C and had ad libitum food and water. All experiments were made in accordance with Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used. All experimental and surgical procedures conformed to the National Research Guide for the Care and Use of Laboratory Animals and were approved by the Animal Research Committees of the University of Brescia (Protocol Permit 719/2015-PR). All achievements were made to minimize animal suffering and to reduce the number of animals used.
Animal surgery
The plasmids for the production of AAV serotype 2/6 inducing the overexpression of either human wt α-synuclein or green fluorescent protein (AAV-GFP), driven by the Syn I promoter and enhanced using a woodchuck hepatitis virus posttranscriptional regulatory element (WPRE), were produced as previously described and acquired by the University of Lund, Sweden, at the titers 4.7 × 1014 and 6.1 × 1014 genome copies/ml, respectively [16, 32]. Two-month-old (25 g) wt and Syn III ko mice were injected in the left substantia nigra with either AAV-GFP or AAV-hαsyn diluted at 5 × 1013 genome copies/ml. Briefly, animals were anesthetized and placed in a stereotactic head frame (Stoelting, IL, USA). After making a midline incision of the scalp, a hole was drilled in the appropriate location for the substantia nigra at the left site of the skull. Two microliters of viral vector were injected at a rate of 0.2 µl/min with a 33-gauge needle on a 10 µL Hamilton syringe at the following coordinates: antero-posterior − 3.60; medio-lateral + 1.15; dorsal–ventral − 3.75 relative to Bregma [37]. The needle was left in place for additional 5 min before being slowly retracted from the brain. Mice were then killed at 8 weeks from the injections for immunohistochemical or biochemical analysis.
Immunohistochemistry (IHC), immunofluorescence, thioflavin-S staining and proximity ligation assay (PLA)
Immunohistochemical studies were performed as previously described. For more details, please see Supplementary methods (Online Resource 1).
Stereological quantification of nigral TH-positive and Nissl-positive neurons and image analysis in the striatum
Please see Supplementary methods (Online Resource 1).
Confocal microscopy
The slides were observed by a LSM 510 Zeiss confocal laser microscope (Carl Zeiss S.p.A., Milan, Italy) with the laser set on λ = 405–488–543 nm and the height of the sections scanning = 1 μm. Images (512 × 512 pixels) were then reconstructed using ZEISS ZEN Imaging Software (Carl Zeiss S.p.A.).
Real-time PCR (rtPCR)
Total RNA was extracted by substantia nigra of wt and Syn III ko mice using an RNA extraction kit (RNeasy Mini Kit, Qiagen, Hilden, GE) according to the manufacturer’s recommendations. Two µg of RNA were retrotranscribed by using QuantiTect Reverse Transcription Kit (Qiagen). Real-time PCR was performed by using SYBR Green Master Mix (Applied Biosystems, Foster City, USA) and the following primer pairs: SNCA for tgttggaggagcagtggtg, SNCA rev gtacccctcctcacccttgc; GAPDH for tcaacagcaactcccactctt, GAPDH rev ccagggtttcttactccttgg. The SNCA primers pair was able to recognize both human and mouse α-synuclein.
The ViiA7 Real-Time PCR system (Life Technologies, Grand Island, NY, U.S.A.) as used for 40 cycles of 95 °C for 15 s and 60 °C for 1 min. mRNA expression was normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression. Each experimental condition was analyzed in triplicate and the resulting data were averaged subjected to statistical analysis.
Western blot (wb) analysis
Fresh frozen tissues from the substantia nigra and striatum were collected from mouse brains after cervical dislocation. Total proteins were extracted with Radiommunoprepitation Assay (RIPA) buffer made up by 50 mM Tris–HCl pH 7.4, 150 mM NaCl, NP-40 1%, sodium deoxycholate 0.1%, sodium dodecyl sulfate (SDS) 0.1%, 1 mM NaF, 1 mM NaVO4 plus complete protease inhibitor mixture (Roche Diagnostics, Mannheim, Germany). Protein concentration in the samples was measured by using the Bio-Rad DCTM protein assay kit (Bio-Rad Laboratories, Milan, Italy). Equal amounts of proteins (30 µg) were run on 10% polyacrylamide gels and transferred onto polyvinylidene fluoride (PVDF) membrane. Densitometric analysis of the bands was performed using ImageJ Software and all bands were normalized to GAPDH levels as a control of equal loading of samples in the total protein extracts. For densitometry analysis of bands, each experimental condition was performed in triplicate and the resulting data were subjected to statistical analysis.
Detergent-insoluble α-synuclein extraction
Alpha-synuclein insoluble aggregates were extracted from substantia nigra fresh tissues, after homogenization in Tris Buffer Saline+ (TBS+) solution composed of 50 mM Tris–HCl pH 7.4, 175 mM NaCl, 5 mM ethylenediaminetetraacetic acid (EDTA), 0.1 mM phenylmethylsulfonyl fluoride (PMSF), 1 mM N-ethyl-maleimide, plus complete protease inhibitor mixture (Roche Diagnostics) and centrifugation at 4 °C at 50,000g for 30 min [54]. Pellets were re-suspended in TBS+ solution plus 1% Triton X-100 and centrifuged at 4 °C at 50,000g for 30 min and then in RIPA buffer and subjected to a further centrifugation. The final pellets were resuspended in urea 8 M plus SDS 5% and loaded on 10% homemade polyacrylamide gel. For densitometry analysis of bands, each experimental condition was performed in triplicate and the resulting data were averaged and subjected to statistical analysis. All bands were normalized to beta-actin levels as a control of equal loading of samples in the UREA/SDS protein extracts.
Transmission electron microscopy (TEM) ultrastructural morphological analysis
Wild-type and Syn III ko mice were anesthetized and perfused as previously described. DAT-positive striatal dopaminergic synapses were visualized according to the methods described by Moss and Bolam [35] with minor modifications. For detailed protocol please see Supplementary methods (Online Resource 1).
Amphetamine-induced rotations test
Mice were placed in a cylinder with a diameter of 11.5 cm and height of 14 cm, in a closed room to avoid any environmental disturbance and allowed to habituate for 5 min before the intraperitoneal injection of 5 mg/kg of d-Amphetamine (Sigma) in line with previously described protocols [12]. Each mouse was monitored for 15 min and scored for full body rotations (360°). The ipsilateral rotations number normalized versus the total number of ipsilateral + contralateral rotations was used to evaluate percentage changes of this parameters with the 100% corresponding to the value of GFP-injected wt mice.
Antibodies
A list of the primary antibodies and of their working concentrations for immunohistochemistry, in situ PLA and wb studies is summarized in Suppl. Table 1 (Online Resource 2). The secondary antibodies used for fluorescence immunohistochemistry were a goat anti-mouse IgG Cy3-conjugated, a goat anti-rabbit Alexa488-conjugated, goat anti-rabbit Alexa405-conjugated and a goat anti-rat IgG 488-conjugated (Jackson ImmunoResearch, Pennsylvania, USA). For bright-field microscopy, we used biotinylated goat anti-rabbit IgG or goat anti-mouse IgG (Vector Laboratories). The secondary antibodies used for wb were goat anti-rabbit IgG-HRP or goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, Dallas, Texas, USA).
Statistical analysis
Statistical differences between groups (wt AAV-GFP, wt AAV-hαsyn, Syn III ko AAV-GFP and Syn III ko AAV-hαsyn) or between the contralateral and injected sides of either wt or Syn III ko mice were assessed using two-way ANOVA followed by Bonferroni’s multiple comparisons test. All data are presented as mean ± standard error of the mean (SEM) and statistical significance was established at P < 0.05. The numbers (n) of animals used for each experimental group in the different experimental studies ranged between 4 and 6. When possible the data were analyzed in triplicate as described above.
Results
Characterization of α-synuclein and GFP expression in AAV-injected mice
The AAV-mediated overexpression of α-synuclein in the nigrostriatal system of mice or rats has been found to induce PD-like alterations such as the formation of cytoplasmic toxic inclusions [36], alterations in dopamine neurotransmission [32] and progressive degeneration of the nigrostriatal system even after only 8 weeks from the virus inoculation [16, 25, 28, 36].
Wild-type and Syn III ko mice that received a unilateral stereotaxic injection of either AAV-hαsyn or AAV-GFP in the substantia nigra were killed at 8 weeks from AAV inoculation to assess α-synuclein aggregation, striatal synaptic alterations and nigral neuron degeneration. In order to be able to evaluate early synaptic striatal alterations with this mouse model, we performed pilot experiments (not shown). These investigations allowed us to establish the optimal AAV-hαsyn titer to be used to achieve striatal synaptic protein changes in the presence of a moderate nigrostriatal neuron degeneration at this time point.
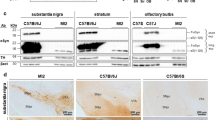
We found that human α-synuclein immunopositivity, detected by using the Syn211 antibody, was abundant in the substantia nigra injected with AAV-hαsyn (Fig. 1a) and in the ipsilateral striatum (Fig. 1b) of both wt and Syn III ko mice. Results from rtPCR experiments confirmed that AAV-hαsyn induced a similar α-synuclein overexpression pattern (about fourfold increase vs AAV-GFP-injected mice) in the substantia nigra of wt and Syn III ko mice (Fig. 1c).
Characterization of α-synuclein expression in the brain of AAV-GFP- and AAV-hαsyn-injected mice. Immunoreactivity for human α-synuclein in the substantia nigra (a) and striatum (b) of wt and Syn III ko mice injected with either AAV-GFP or AAV-hαsyn. Human α-synuclein was overexpressed in the AAV-hαsyn-injected substantia nigra and ipsilateral striatum of wt and Syn III ko mice. No immunoreactivity for human α-synuclein was observed in the same brain areas of AAV-GFP-injected wt mice. c The histogram is showing results from rtPCR experiments evaluating α-synuclein mRNA in the AAV-GFP- and AAV-hαsyn-injected substantia nigra of wt and Syn III ko mice. Data are expressed as fold-increase of mRNA expression with respect to either AAV-GFP-injected wt or AAV-GFP-injected Syn III ko mice. Please note the significant increase of α-synuclein mRNA levels in the AAV-hαsyn-injected mice when compared to the respective AAV-GFP injected littermates (***+ 3.45, P < 0.001 in wt mice, °°°+ 2.79, P < 0.001 in Syn III ko mice, two-way ANOVA + Bonferroni’s multiple comparisons test). n = 4/5 mice for each group. ns non-significant. Scale bars: a, b = 1 mm, higher magnifications = 20 μm
Double fluorescence IHC confirmed that TH-positive neurons of the substantia nigra were efficiently infected by AAV-GFP and AAV-hαsyn, as they exhibited a marked GFP and human α-synuclein immunolabeling, respectively (Fig. 2a). Interestingly, we found that in the AAV-hαsyn injected substatia nigra of wt mice the α-synuclein-positive neurons exhibited an accumulation of Syn III immunoreactivity when compared to the GFP-injected animals [suppl. Figure 1 (Online Resource 3, 1)]. In the AAV-hαsyn-injected wt mice Syn III immunolabeling displayed a widespread distribution in the inner part of the soma with a minor co-localization with α-synuclein in the periphery of the cells, while in the AAV-GFP-injected animals Syn III staining was fainter, although it still involved the cell body [suppl. Fig. 1 (Online Resource 3, 1)]. This peculiar localization profile is in line with previous studies showing that, although Syn III is mainly a presynaptic protein, it can also exhibit an extrasynaptic localization within cell body and processes in neurogenic regions of the adult brain [40, 42].
Characterization of GFP and α-synuclein expression in the substantia nigra and striatum of mice. a Representative photomicrographs showing either GFP or human α-synuclein positivity in TH-positive neurons of the substantia nigra of wt mice injected with AAV-GFP and AAV-hαsyn, respectively. The yellow signal in the merge is indicative of either GFP/TH or human α-synuclein/TH co-localization. (b,c) Western blot analysis confirmed total α-synuclein increase in the AAV-hαsyn-injected substantia nigra and ipsilateral striatum of wt (***+ 1.98, P < 0.001 for the substantia nigra and **+ 1.0, P < 0.01 for the striatum, two-way ANOVA + Bonferroni’s multiple comparisons test) and Syn III ko mice (°°+ 1.70, P < 0.01 for the substantia nigra and °°+ 1.05, P < 0.01 for the striatum, two-way ANOVA + Bonferroni’s multiple comparisons test) when compared to the same brain areas of wt or Syn III ko mice that recieved the AAV-GFP injection. IN: injected side; CL: contralateral side. Consistently, the levels of total α-synuclein in the AAV-hαsyn-injected substantia nigra and ipsilateral striatum were significantly increased when compared to the respective contralateral areas of wt (*+ 0.90, P < 0.05 for the substantia nigra and **+ 1.09, P < 0.01 for the striatum, two-way ANOVA + Bonferroni’s multiple comparisons test), and Syn III ko mice (°+ 0.94, P < 0.05 for the substantia nigra and °°+ 1.12, P < 0.01 for the ipsilateral striatum, two-way ANOVA + Bonferroni’s multiple comparisons test). Please note that GFP-immunopositive bands are reported as a control for the injections. n = 4/5 animals for each group. Scale bar: a = 20 μm
By wb analysis, we found that the levels of total (mouse + human) α-synuclein, detected using SYN-1 antibody, were significantly increased in both the AAV-hαsyn-injected substantia nigra (Fig. 2b) and in the ipsilateral striatum (Fig. 2c) of wt and Syn III ko mice. In addition, we observed that the AAV-hαsyn injection led to a comparable increase of human α-synuclein levels in wt and Syn III ko mice (not shown). Similar results were obtained when we analyzed GFP levels in the substantia nigra and striatum ipsilateral and contralateral to AAV-GFP injection in wt and Syn III ko mice (not shown).
Syn III ko mice did not exhibit TH-positive neuron loss 8 weeks after the injection of AAV-hαsyn
To evaluate the effect of human α-synuclein overexpression in the nigrostriatal system of wt and Syn III ko mice, we first performed stereological cell counts to assess the number of TH-positive neurons in the substantia nigra ipsilateral and contralateral to AAV-hαsyn or AAV-GFP injection. Two-way ANOVA coupled to Bonferroni’s post-comparisons test showed that wt mice displayed a significant decrease in the number of TH-positive neurons at the site of AAV-hαsyn injection when compared to that of AAV-GFP inoculation (Fig. 3a, b). Conversely, Syn III ko mice did not display TH-positive cell loss at the site AAV-hαsyn injection (Fig. 3a, b). No loss of TH-positive cells was observed in the AAV-GFP injected substantia nigra of control or Syn III ko mice (Fig. 3a, b).
The AAV-mediated overexpression of human α-synuclein decreased the number of TH-positive neurons in the substantia nigra of wt, but not of Syn III ko, mice. a Immunolabeling of TH-positive neurons in substantia nigra of wt and Syn III ko mice injected with AAV-GFP or AAV-hαsyn. Please note the reduction of TH-positive cells in the AAV-hαsyn-injected substantia nigra of wt mice (indicated by the rectangle). b The histogram is showing the TH-positive cell numbers in the AAV-GFP or AAV-hαsyn-injected substantia nigra of wt and Syn III ko mice expressed as the % change vs the respective contralateral side. In the wt mice human α-synuclein overexpression induced a statistically significant decrease of TH-positive cells at the site of injection (*** − 34.24%, P < 0.001 vs AAV-GFP-inoculated wt animals; ■■■ − 32.59%, P < 0.001 vs AAV-hαsyn-injected Syn III ko mice, two-way ANOVA + Bonferroni’s post comparisons test). Please note that the overexpression of α-synuclein in Syn III ko mice did not result in the loss of TH-positive neurons of the substantia nigra. c The histogram is showing the Nissl-stained cell number in the AAV-GFP or AAV-hαsyn-injected substantia nigra of wt and Syn III ko mice, expressed as the % change vs the respective contralateral side. The overexpression of α-synuclein in wt mice resulted in a reduction of Nissl-positive cells at the site of injection (** − 31.87%, P < 0.01 vs AAV-GFP inoculated wt animals; ■■ − 34.47%, P < 0.01 vs AAV-hαsyn-injected Syn III ko mice, two-way ANOVA + Bonferroni’s post comparisons test). n = 6 animals for each group. Scale bar: a = 100 μm
These observations were corroborated by counting Nissl-positive cells in the substantia nigra. Indeed we found that the number of Nissl-positive neurons significantly decreased at the site of AAV-hαsyn injection in wt mice but not in Syn III ko mice [Fig, 3c, suppl. Fig. 2a (Online Resource 4, 1)]. This finding substantiates that the decrease of TH-positive cells can be ascribed to nigral neuron degeneration rather than to changes in TH protein expression.
Syn III ko mice were spared from the AAV-hαsyn-induced striatal DAT reduction and redistribution
We evaluated whether the absence of Syn III could block α-synuclein overexpression-related synaptic damage. To date, we previously reported that α-synuclein aggregation alters DAT distribution in vitro and in vivo [6, 9]. Indeed, the DAT is re-distributed in association with aggregated α-synuclein in the brain of PD patients and experimental mouse models [9, 30]. Notably, direct binding and functional coupling of α-synuclein to the DAT has been found to exacerbate dopamine neurotoxicity [27], thus supporting that this event may be crucial for the initiation of nigrostriatal neuron degeneration. We thus aimed to assess whether the α-synuclein overexpression-related DAT reduction and redistribution might be hampered in Syn III ko mice. We found that in the wt mice the AAV-hαsyn injection induced a statistically significant decrease of DAT-immunopositive area in the ipsilateral striatum when compared to that of the contralateral hemisphere (Fig. 4a, b). Conversely, DAT decrease was not visible in the striatum ipsilateral to AAV-hαsyn injection of Syn III ko mice. GFP-overexpression did not affect striatal DAT expression in either wt or Syn III ko mice.
Analysis of DAT-positive terminals in the striatum of AAV-GFP- and AAV-hαsyn-injected wt and Syn III ko mice. a Representative images showing that the overexpression of α-synuclein in the wt mice induced a decrease of DAT-positive area (asterisks) as well as the formation of DAT-positive clumps (black arrows) in the striatum ipsilateral to AAV-hαsyn injection. No loss of DAT-immunoreactivity was observed in the striatum ipsilateral to AAV-hαsyn injection of Syn III ko mice. b Densitometric analysis of DAT-positive area confirmed the presence of a statistically significant decrease of DAT in the striatum ipsilateral to AAV-hαsyn injection of wt mice (* − 37.96%, P < 0.05 vs the contralateral striatum; ** − 46.40%, P < 0.01 vs the striatum ipsilateral to AAV-GFP injection, two-way ANOVA + Bonferroni’s post comparisons test). IN: injected side; CL: contralateral side. c High magnification representative images showing a DAT-positive clump (arrow) as well as a DAT-negative area (asterisk) in the striatum ipsilateral to AAV-hαsyn injection of wt mice and the uniform DAT-immunoreactivity in the striatum of AAV-hαsyn-injected Syn III ko mice. d Image analysis showed a statistically significant increase of the mean area of DAT-positive particles in wt mice injected with AAV-hαsyn (***+ 90.38%, P < 0.001 vs Syn III ko mice with AAV-hαsyn injection; °°° + 98.98%, P < 0.001 vs AAV-GFP-injected wt, two-way ANOVA + Bonferroni’s post comparisons test) that supports the occurrence of dopamine terminal clustering. n = 6 animals for each group. Scale bars: a, c = 20 μm
We then studied DAT distribution. Interestingly, we observed that, beside the general decrease of DAT-immunopositivity (Fig. 4a, c asterisks), the AAV-hαsyn-injected wt mice showed several areas with marked clustering of DAT-positive terminals in the ipsilateral striatum (Fig. 4a, c arrows). Conversely, Syn III ko mice did not show the AAV-hαsyn injection-related formation of DAT-positive clumps in the ipsilateral striatum. DAT clustering in the striatum ipsilateral to AAV-hαsyn injection of wt mice was corroborated by image analysis that showed a significant increase in the average size of DAT-positive particles when compared to Syn III ko mice, that did not show this feature (Fig. 4d). Collectively, these observations fit both with data supporting a reduction of DAT positivity following the overexpression of human α-synuclein in rats [32] and with the redistribution of DAT observed in the striatum of PD patients and experimental mouse models [9, 30].
The dopaminergic innervation in the striatum was then evaluated by analyzing TH-immunolabeling that showed a statistically significant decrease in the area occupied by TH-immunopositive fibers in the striatum ipsilateral to the injection of wt mice that received the AAV-hαsyn inoculation when compared to the AAV-GFP-injected animals or to Syn III ko mice with AAV-hαsyn injection [suppl. Fig. 2b, c (Online Resource 4, 1)].
To evaluate whether DAT redistribution was strictly related to α-synuclein accumulation, we probed the co-localization of DAT with either human α-synuclein or Syn III in the striatal dopaminergic terminals ipsilateral and contralateral to AAV-hαsyn- and AAV-GFP-injection. We found that in wt mice the DAT-positive clumps in the striatum ipsilateral to AAV-hαsyn-injection co-localized with human α-synuclein [suppl. Fig. 3a (Online Resource 5, 1), white arrow]. This is in line with our previous findings showing that DAT redistribution is related to α-synuclein aggregation [6, 9, 21]. Conversely, in spite of the co-localization between human α-synuclein and DAT observed in the striatum ipsilateral to AAV-hαsyn injection in Syn III ko mice, we observed that the DAT and α-synuclein did not co-cluster.
In addition, we found that in the wt mice the redistribution of DAT in the striatum ipsilateral to AAV-hαsyn injection was also accompanied by a redistribution of Syn III [suppl. Fig. 3b (Online Resource 5, 1), asterisks]. This notwithstanding, the proteins did not co-localize [suppl. Fig. 3b (Online Resource 5, 1), asterisks]. The absence of Syn III/DAT co-localization was also corroborated in the striatum contralateral to AAV-hαsyn injection of wt mice, though this area did not show DAT redistribution.
These observations support that human α-synuclein overexpression in the nigrostriatal system of wt mice can affect synaptic function by altering DAT distribution and that this phenomenon is dependent on the presence of Syn III. Nonetheless, the absence of co-localization between DAT and Syn III in the striatum of wt mice does not support the possibility that Syn III can directly mediate DAT/α-synuclein interaction. Therefore, it may be feasible that the lack of DAT redistribution observed in the striatum ipsilateral to AAV-hαsyn injection of Syn III ko mice could be ascribed to an inhibition of α-synuclein aggregation related to the absence of Syn III.
Alpha-synuclein aggregation was prevented in Syn III ko mice
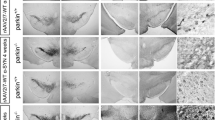
The occurrence of α-synuclein aggregation in the substantia nigra and striatum of AAV-hαsyn-injected wt and Syn III ko mice was at first evaluated by performing thioflavin-S/α-synuclein double labeling. We found a marked presence of thioflavin-S/human α-synuclein-positive inclusions in both the AAV-hαsyn-injected substantia nigra and ipsilateral striatum of wt mice (Fig. 5a, b). Of note, in the striatum thioflavin-S-positivity exceeded human α-synuclein immunopositivity, supporting the occurrence of endogenous mouse α-synuclein aggregation (Fig. 5b, asterisks). However, in the Syn III ko mice, the injection of AAV-hαsyn did not result in the formation of thioflavin-S-positive inclusions in the substantia nigra and ipsilateral striatum, although these areas exhibited a marked positivity for human α-synuclein. The substantia nigra and striatum contralateral to AAV-hαsyn injection of both wt and Syn III ko mice did not show any thioflavin-S signal. These observations indicate that the absence of Syn III could prevent the formation of fibrillary aggregates composed of α-synuclein.
Thioflavin-S/α-synuclein double labeling in the substantia nigra and striatum ipsilateral and contralateral to AAV-hαsyn injection of wt and Syn III ko mice. a Representative images showing that the overexpression of human α-synuclein resulted in the formation of thioflavin-S-positive aggregates only in the AAV-injected substantia nigra of wt mice (arrows), but not in the same brain area of Syn III ko mice and in the contralateral side. b The striatum ipsilateral to AAV-hαsyn injection of Syn III ko mice was devoid of thioflavin-S staining in spite of the abundancy of human α-synuclein immunolabeling. The striatum ipsilateral to AAV-hαsyn-injection of wt mice showed the presence of a marked co-localization between human α-synuclein-immunopositivity and the thioflavin-S signal with several thioflavin-S/α-synuclein-positive neurites (arrows). This notwithstanding, in some areas the thioflavin-S-positivity resulted to exceed human α-synuclein immunopositivity (asterisks). n = 4/5 animals for each group. Scale bars: a, c = 20 μm
To corroborate this finding, we extracted detergent-insoluble α-synuclein from fresh frozen substantia nigra of wt and Syn III ko mice that were injected either with AAV-GFP or AAV-hαsyn (Fig. 6). In particular, we probed the levels of detergent-insoluble α-synuclein aggregates, Syn III and DAT, in the substantia nigra UREA/SDS extracts that were produced by sequential protein extraction [3, 6, 10]. We observed that the UREA/SDS protein fractions from the GFP-injected substantia nigra of both wt and Syn III ko mice did not show human α-synuclein or Syn III positivity (Fig. 6a, b). Conversely, UREA/SDS soluble protein fractions from AAV-hαsyn-injected wt mice resulted positive for human α-synuclein, Syn III and DAT, which were not present in the same brain area of Syn III ko mice. Moreover, we found that detergent insoluble α-synuclein monomers (Fig. 6a, black asterisk) and oligomers (Fig. 6a, red asterisks) increased in the substantia nigra of wt mice that received AAV-hαsyn injection. Conversely, in the blots of AAV-hαsyn-injected Syn III ko mice, we did not observe the presence of high molecular weight α-synuclein oligomers but only of a very faint positive band corresponding to α-syn monomers (black asterisks) and non-specific high molecular weight bands that were also detected in wt mice (Fig. 6a, arrows), supporting that α-synuclein aggregation was hampered in Syn III ko mice. In addition, in line with our recent observations showing the presence of Syn III in LB-composing fibrils purified from the brain of PD patients [31], the positivity for Syn III in UREA/SDS extracts of wt mice indicates that the protein is present within α-synuclein aggregates even in this AAV-based mouse model. Interestingly, when we examined the UREA/SDS protein fractions of wt mice we found the presence of a mild α-synuclein-, Syn III- and DAT-positivity also in the substantia nigra contralateral to AAV-hαsyn-injection (Fig. 6a, b). This feature, that is in line with the above-described wb data, may be related to the diffusion of the AAV-hαsyn vector to the contralateral substantia nigra through interhemispheric connections [45]. The specificity of Syn III detection in the UREA/SDS protein fraction of wt mice was corroborated by the absence of positivity in Syn III ko mice (Fig. 6a, b).
Evaluation of detergent-insoluble α-synuclein and Syn III in the substantia nigra and detection of α-synuclein oligomers by in situ PLA as well as of serine 129-phosphorylated α-synuclein immunolabeling in the striatum ipsilateral and contralateral to AAV-hαsyn injection of wt and Syn III ko mice. a Western blot showed the presence of α-synuclein Syn III and DAT in the UREA/SDS-soluble protein fractions of the AAV-GFP and AAV-hαsyn-injected substantia nigra of wt and Syn III ko mice and in the contralateral areas of the mice overexpressing human α-synuclein. IN: injected side; CL: contralateral side. Red asterisks: α-synuclein oligomers. Black asterisks: α-synuclein monomers. Arrows: non-specific bands. b The histograms are showing results from the densitometric analysis of α-synuclein monomers and oligomers and Syn III-positive bands expressed as optical density (o.d.). Please note that the levels of monomeric α-synuclein in the UREA/SDS protein fraction from the AAV-hαsyn-injected substantia nigra of wt mice were significantly higher than those observed in Syn III ko mice (°°+ 2.14, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test) and in the AAV-GFP-injected substantia nigra of control wt mice (***+ 2.56, P < 0.001, two-way ANOVA + Bonferroni’s post-comparisons test). The Syn III ko mice also showed a very mild and non-significant increase of + 0.42 o.d in detergent-insoluble α-synuclein in the AAV-hαsyn-injected side when compared to the contralateral. At higher molecular weight an increase of α-synuclein oligomers was observed in the AAV-hαsyn-injected wt mice when compared to AAV-GFP injected mice (*+ 1.40, P < 0.05, two-way ANOVA + Bonferroni’s post-comparisons test). Alpha-synuclein oligomers were absent in the Syn III ko mice even when they received AAV-hαsyn injection. The levels of Syn III in the substantia nigra ipsilateral to AAV-hαsyn injection of wt mice were increased when compared to the AAV-GFP injected substantia nigra of the same mouse line (**+ 1.49, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test) or to the contralateral hemisphere (■+ 0.94, P < 0.05, two-way ANOVA + Bonferroni’s post-comparisons test). No Syn III-positive signal was observed in the samples of the Syn III ko mice
We then wanted to probe the formation of α-synuclein oligomers by using the in situ PLA [7, 44, 59]. We found a more diffused and intense PLA signal detecting α-synuclein/α-synuclein interaction in the striatum of wt mice which had received the AAV-hαsyn injection, when compared to Syn III ko mice under the same experimental conditions [suppl. Fig. 4a, c (Online Resource 6, 1)]. The α-synuclein/α-synuclein PLA-positive signal was very low in the contralateral hemisphere of both wt and Syn III ko mice.
Previous studies have shown that the AAV-mediated α-synuclein overexpression in mice results in increased phosphorylation of α-synuclein at serine 129 [58]. To date, almost 90% of α-synuclein in LB is phosphorylated at this site [1]. However, it is still debated whether this specific post-translational modification can enhance or reduce α-synuclein fibrillation with conflicting results deriving from different studies [4, 33, 46, 47]. We thus aimed at analyzing whether α-synuclein phosphorylation might be differently modulated in the wt or Syn III ko mice that received the unilateral injection of AAV-hαsyn [suppl. Fig. 4b (Online Resource 6, 1)]. By immunofluorescence staining, we found that serine 129-phosphorylated α-synuclein was almost undetectable in the striatum ipsilateral to AAV-injection of Syn III ko mice, with no differences with the contralateral site. Conversely, the striatum ipsilateral to the AAV-hαsyn injection of wt mice displayed a marked immunopositivity for serine 129-phosphorylated α-synuclein, although the contralateral site was devoid of signal [suppl. Fig. 4b, d (Online Resource 6, 1)]. These data further support the hypothesis that Syn III is a key regulator of α-synuclein at striatal dopaminergic terminals, as the absence of this protein can impede both α-synuclein phosphorylation and the formation of α-synuclein oligomers.
Absence of Syn III prevented the increase in the number of amphetamine-induced ipsilateral rotations and SV alterations at striatal dopaminergic terminals
We then aimed at evaluating the onset of alterations in dopamine transmission in the AAV-hαsyn-injected mice by counting the number of amphetamine-induced rotations. We found that the mild unilateral degeneration of nigrostriatal neurons observed in the AAV-hαsyn-injected wt mice induced a slight, although not significant, increase in the percentage of ipsilateral amphetamine-induced rotations normalized against the total number of rotations when compared to the same parameter in AAV-GFP injected wt mice or in the Syn III ko animals (Fig. 7a). This observation is in agreement with previous findings showing that a 30% of degeneration in mice with unilateral 6-hydroxydopamine injection correlates with a mild and not significant increase in the number of ipsilateral rotations [12, 23]. In line with the absence of nigrostriatal degeneration in the Syn III ko mice with unilateral AAV-hαsyn injection, we found that these animals did not show differences between the number of ipsilateral rotations normalized against the total number of rotations when compared to their littermates injected with AAV-GFP (Fig. 7a).
Amphetamine-induced rotations and morphological TEM studies. a The amphetamine-induced rotations test showed a slight increase in the ipsilateral rotations against the total number of contralateral + ipsilateral rotations in AAV-hαsyn-injected wt mice when compared to the same parameter of AAV-GFP injected wt mice (+ 30.3% P > 0.05, two-way ANOVA + Bonferroni’s post-comparisons test) or to the Syn III ko animals. b Representative photomicrograph showing the parameters that were analyzed in the TEM images. The red line traces the area of the active zone that delimits the 50 nm distance to borders of the synaptic cleft that are traced in white. For the analysis of SV diameter the borders of SV was delineated (yellow dashed line) and the diameter was traced manually (red line connector). c Transmission electron microscopy images showed the synaptic site of contralateral or ipsilateral striatum of AAV-hαsyn-injected wt or Syn III ko mice. d The number of SV at the active zone significantly decreased in the striatum ipsilateral to AAV-hαsyn injection of wt mice when compared to the contralateral hemisphere (** − 2.65, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test). In the striatum ipsilateral to AAV-hαsyn injection of Syn III ko mice the number of SV significantly increased when compared to the same brain area of wt mice (°°°+ 6.15, P < 0.001, two-way ANOVA + Bonferroni’s post-comparisons test) or to the respective contralateral side (■■+ 2.48, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test). e The analysis of SV size at whole terminals showed a statistically significant increase in the diameter of SV in the ipsilateral striatum of AAV-hαsyn-injected wt mice when compared to the contralateral hemisphere (***+ 8.32, P < 0.001, two-way ANOVA + Bonferroni’s post-comparisons test) or to the same brain area of Syn III ko mice (°°°+ 9.56, P < 0.001, two-way ANOVA + Bonferroni’s post-comparisons test). Scale bar: c = 500 nm
We then analyzed the DAT-immunopositive synaptic terminals of the striatum ipsilateral and contralateral to AAV-hαsyn-injection in wt and Syn III ko mice using morphological TEM. We wanted to estimate whether α-synuclein overexpression and aggregation was able to induce synaptic derangement despite we could not detect significant changes in amphetamine-induced rotations. In particular, we counted the number of SV at the presynaptic active zone and assessed the mean diameter of SV in the whole SV pools to estimate the organization of terminals. Interestingly, we found a statistically significant decrease in the number of SV at the active zone of dopaminergic terminals in the striatum ipsilateral to AAV-hαsyn injection of wt mice, when compared to the contralateral side (Fig. 7c, d). This was accompanied by a statistically significant increase in the mean diameter of SV (Fig. 7e). Conversely, in the striatum ipsilateral to AAV-hαsyn-injection of Syn III ko mice, we observed a statistically significant increase in the number of SV at the active zone (Fig. 7d) without changes in their mean diameter, when compared to the respective contralateral area (Fig. 7c, e). As previously described, stimulus pulse-evoked dopamine release in the striatum is doubled in the Syn III ko mice when compared to wt animals [24]. This notwithstanding, we did not observe changes in the number of SV at the active zone between the contralateral striata of wt and Syn III ko mice.
Syn III deficiency hindered the human α-synuclein overexpression-related redistribution of VAMP2 in the striatum
We previously observed that PD patients and mice transgenic for C-terminally truncated human α-synuclein show a marked redistribution of SNARE proteins and of Syn III in the striatum [21, 59]. Like Syn III, VAMP2 directly interacts with α-synuclein [17, 31, 59]. We thus wanted to probe whether: (1) Syn III is redistributed in the striatum in association with α-synuclein and (2) the absence of Syn III could hamper VAMP2 redistribution or affect its interaction with α-synuclein.
We found that, in wt mice, the overexpression of α-synuclein induced the formation of Syn III-positive clumps in the striatum ipsilateral to AAV-hαsyn-injection, when compared to AAV-GFP-injected animals (Fig. 8a). Similarly, VAMP2 was also accumulated in the striatum ipsilateral to AAV-hαsyn injection of wt mice. The analysis of the mean area of VAMP2-positive particles in the striatum showed that these were significantly increased ipsilaterally to AAV-hαsyn injection in wt mice when compared to AAV-GFP-inoculated wt mice or to AAV-hαsyn-injected Syn III ko mice (Fig. 8c). Conversely, in the Syn III ko mice the immunolabeling of VAMP2 was similar in the striatum ipsilateral to AAV-hαsyn and AAV-GFP injection.
Syn III and VAMP2 distribution as well as α-synuclein/Syn III and α-synuclein/VAMP2 in situ PLA in the striatum ipsilateral to AAV-hαsyn or AAV-GFP injection of wt and Syn III ko mice. a Representative images showing that Syn III and VAMP2-positive clumps were detected in the striatum ipsilateral to AAV-hαsyn injection of wt mice, but not in that of Syn III ko mice, where the distribution of these proteins was similar to that observed in AAV-GFP-injected animals. b Accumulation of α-synuclein/Syn III- and α-synuclein/VAMP2-positive PLA clumps in the striatum ipsilateral to AAV-hαsyn-injection of wt mice. Please note that no change in the distribution of VAMP2 was observed in the human α-synuclein overexpressing Syn III ko mice. The absence of α-synuclein/Syn III PLA signal in the Syn III ko mice is indicative of the specificity of the in situ PLA. c Please note the statistically significant increase of the size of VAMP2-positive clusters in the striatum ipsilateral to AAV-hαsyn injection of wt mice when compared to the same brain area of Syn III ko mice (°°+ 93.7%, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test) or to the AAV-GFP injected mice (***+ 114.4%, P < 0.001 vs wt mice; ■■■+ 120.0%, P < 0.001 vs Syn III ko mice, two-way ANOVA + Bonferroni’s post-comparisons test). d The mean area of α-synuclein/VAMP2 PLA-positive signal was increased in the striatum ipsilateral to AAV-hαsyn injection of wt mice when compared to the AAV-GFP-injected wt (**+ 207.3%, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test) or Syn III ko mice (■+ 191.5%, P < 0.05, two-way ANOVA + Bonferroni’s post-comparisons test) or to the same area of AAV-hαsyn-injected Syn III ko mice (°°+ 182.4%, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test). e The histogram shows that in the wt mice the mean area of Syn III/α-synuclein PLA-positive signal was increased in the striatum ipsilateral to AAV-hαsyn injection when compared that ipsilateral to AAV-GFP-injection (**+ 88.8%, P < 0.01, two-way ANOVA + Bonferroni’s post-comparisons test). n = 4 animals for each group. Scale bars: a, b = 40 μm
We then performed in situ PLA studies to estimate the distribution of α-synuclein/Syn III and α-synuclein/VAMP2 complexes in the striatum ipsilateral to AAV-hαsyn or AAV-GFP injection in wt and Syn III ko mice (Fig. 8b, d). In wt mice, a marked accumulation of clusters containing α-synuclein/Syn III and α-synuclein/VAMP2 complexes was observed in the striatum ipsilateral to AAV-hαsyn injection. These clusters were not visible in the same area of wt mice injected with AAV-GFP. Conversely, in Syn III ko mice, the distribution of VAMP2/α-synuclein complexes was not perturbed by AAV-hαsyn overexpression (Fig. 8b, d).
These results support a redistribution of the synaptic proteins Syn III and VAMP2 occurring in association with α-synuclein aggregate deposition that appears in line with the above-described redistribution of DAT-positive terminals. Since we did not observe co-localization between DAT and Syn III, to further confirm that Syn III redistribution in association with α-synuclein was concomitant to a displacement of dopaminergic terminals in the striatum ipsilateral to AAV-hαsyn injection of wt mice, we probed α-synuclein/Syn III PLA in co-localization with vesicular monoamine transporter 2 (VMAT-2). Interestingly, we found that α-synuclein/Syn III PLA-positive clusters were co-redistributed with VMAT-2 in the AAV-hαsyn-injected wt mice [suppl. Fig. 5 (Online Resource 7, 1)], while the human α-synuclein overexpressing Syn III ko mice did not show alterations. This observation supports that Syn III functions as an accessory mediator for the α-synuclein aggregation-dependent redistribution of synaptic terminals/proteins.
Discussion
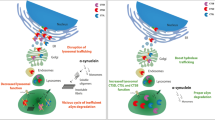
The results of this study show that Syn III deficiency prevents α-synuclein aggregation, nigral neuron degeneration and the onset of synaptic changes in an AAV-hαsyn mouse model of PD. These alterations were not related to differences in the AAV-mediated expression of human α-synuclein between wt and Syn III ko mice. Indeed, in spite of the comparable amount of human α-synuclein, driven by the AAV-hαsyn injections in wt and Syn III ko animals, we found that the Syn III ko mice exhibited a reduction of α-synuclein aggregation. This was supported by the decrease of UREA/SDS detergent-insoluble, as well as phosphorylated α-synuclein levels, lack of thioflavin-S-positive α-synuclein aggregates, and mild α-synuclein oligomers formation detected in this mouse line following AAV-hαsyn injection. In addition, at 8 weeks from the inoculation of AAV-hαsyn, Syn III ko mice did not show degeneration or signs of synaptic alterations. Conversely, in wt mice, human α-synuclein overexpression in the substantia nigra resulted in a significant reduction of TH-positive neurons at the site of injection, as well as in the reduction of DAT-positive terminals and TH-positive fibers in the ipsilateral striatum that was accompanied by DAT redistribution.
To date, since in the present study we did not analyze mice killed at time points longer than 8 weeks after AAV injections, we cannot definitely exclude that the absence of degeneration observed in the Syn III ko mice might be related to a delay in the loss of nigrostriatal neurons.
It has to be mentioned that the reduction of TH-positive cells observed in the substantia nigra of our AAV-hαsyn-injected wt mice is lower than that observed in other studies on AAV-hαsyn mouse models [36]. This can be ascribed to the fact that our plasmid did not contain the cytomegalovirus immediate-early (CMVie)-enhancer that can increase the expression of transduced proteins and the related neurodegeneration. In addition, we used a diverse AAV serotype that might have differently influenced the amount of α-synuclein.
A large body of evidence supports that α-synuclein regulates SV turnover [15] and neurotransmitter release by interacting with a variety of SV proteins as well as SV membranes. However, α-synuclein aggregation impairs these regulatory effects on neuronal function. In particular, it compromises pre-synaptic structures and induces conformational changes that correlate with the onset of synaptic degeneration in the early phases of PD [8, 39, 56]. Therefore, synaptic changes have been proposed as the primum movens for the occurrence of PD symptoms [5, 18, 53]. On this line, we found that the loss of TH-positive neurons observed in the AAV-hαsyn-injected substantia nigra of wt mice, when compared to the same brain area of AAV-GFP-injected wt mice (− 34.24%), was paralleled by a slighter decrease (− 22.1%) of TH-immunopositive fibers and a more marked reduction of striatal DAT-positive terminals (− 46.40%) in the same experimental groups. This observation may indicate that, at the stage of pathology that we analyzed in our AAV-hαsyn-based mouse model, the overexpression of α-synuclein had more severe effects on striatal synaptic terminals than on nigral cell bodies and dopaminergic fibers. Alternatively, the diverse rates of TH and DAT loss may be related to the fact that TH and DAT may be differently subjected to compensatory regulation.
Moreover, we also observed a marked DAT, Syn III, VAMP2 and VMAT2 clustering in the striatum ipsilateral to AAV-hαsyn injection of wt mice. Interestingly, although being in close proximity to areas that also showed Syn III accumulation, DAT-positive clusters were found to co-localize only with human α-synuclein, thus supporting the absence of a direct interaction between DAT and Syn III. This notwithstanding, both DAT and Syn III were found to be present within the detergent-insoluble UREA/SDS protein fractions of the wt mice that received the AAV-hαsyn injections, a finding hinting that α-synuclein aggregation may determine a differential sequestration of the diverse members of its synaptic interactome. The co-localization observed between α-synuclein and DAT is in line with previous studies by our group showing that the DAT is co-redistributed with α-synuclein in the post-mortem caudate putamen of patients affected by sporadic PD and in the striatum of mice transgenic for C-terminally truncated α-synuclein [9, 30, 31]. Interestingly, this type of synaptic protein redistribution following α-synuclein overexpression has never been observed in AAV-based rat models of PD [30, 38], despite the rate of nigrostriatal degeneration was similar to that described in our study in mice. This may indicate that mice and rats could present diverse nigrostriatal vulnerability thresholds to human α-synuclein overexpression. In addition, our findings support that the redistribution of α-synuclein/DAT complexes, that we observed in human brains of PD patients at advanced stages of disease [30], can be reproduced in the AAV-hαsyn mouse model even at initial stages of degeneration. In agreement with our previous observations [21, 59], the in situ PLA that we performed in this study supports that also Syn III and VAMP2 are co-redistributed with α-synuclein. In particular, Syn III/α-synuclein PLA-positive clusters appeared to co-redistribute with the dopaminergic SV marker VMAT2. Notably, Syn III pivotally modulates striatal dopamine release [24, 59], and VAMP2 regulates synaptic vesicle release [52], while VMAT2 and DAT functions are essential for the proper maintenance of dopamine turnover and release at synaptic terminals [29]. Therefore, it is likely that the α-synuclein aggregation-related changes in VAMP2, DAT, VMAT2 and Syn III distribution and/or expression, together with the degeneration of TH-positive neurons, might have induced a reduction in dopamine release in the striatum ipsilateral to AAV-hαsyn injection in wt mice, supporting the idea that Syn III is necessary for α-synuclein aggregation-related synaptic damage. Further studies aimed at defining the exact role of Syn III in association with α-synuclein in the modulation of nigrostriatal dopamine neuron function and SV turnover are currently ongoing by our group.
In line with the above hypothesis, we observed a slight but not significant increase in the number of amphetamine-induced ipsilateral rotations in the AAV-hαsyn injected wt mice that easily correlates with the fact that they only showed a partial unilateral nigrostriatal degeneration. In addition, we found that the injection of AAV-hαsyn altered the size and the distribution of SV at dopaminergic striatal terminals in wt mice when compared to their contralateral side or to Syn III ko mice. Evidence supporting that native α-synuclein promotes the formation of SNARE complex, thus increasing the number of docked vesicles at the active zone [15, 17, 34], hints the occurrence of an impairment of SV turnover in the presence of aggregated and/or oligomeric α-synuclein, that may lose its original regulatory action especially upon VAMP2, a protein that is crucial for SV fusion [51]. Indeed, our results suggest that the aggregation of α-synuclein in wt mice induced VAMP2 sequestration, as supported by the clustering of α-synuclein/VAMP2 PLA-positive signal observed in the striatum ipsilateral to AAV-hαsyn injection of wt mice. This observation, when coupled to the decrease in the number of SV at the active zones of striatal dopaminergic synapses, supports that the α-synuclein aggregation-mediated VAMP2 derangement might have impaired the proper docking of SV at the pre-synaptic membranes. In addition, VAMP2 alterations may have also contributed to increase the size of SV through dysregulations in clathrin-cage adaptor proteins [43]. Conversely, in the Syn III ko mice we did not observe alterations in the size of SV, but they showed a statistically significant increase in the number of SV at the active zone in the striatum ipsilateral to AAV-hαsyn injection, although in the contralateral side this parameter was comparable to the same area of wt mice. This finding suggests that, in agreement with the results of previous studies [17, 20, 34], the elevated levels of non-aggregated α-synuclein might have promoted SV increase at the pre-synaptic membrane of Syn III ko mice.
Although we did not find differences in the organization of SV within the dopaminergic striatal terminals of the hemispheres contralateral to AAV-hαsyn injection of wt and Syn III ko mice, these latter have been found to exhibit a marked increase in electrical stimulation-evoked dopamine release when compared to wt mice [24]. It may thus be feasible that the increased striatal dopamine release occurring in the Syn III ko mice has also contributed to impeding α-synuclein aggregation by reducing dopamine load at synaptic terminals. Indeed, dopamine, and in particular its toxic metabolites, such as 3,4-dihydroxyphenylacetaldehyde (DOPAL), have been found to foster α-synuclein aggregation [41, 48].
The mechanisms through which Syn III mediates α-synuclein clumping still need to be elucidated. The direct binding of Syn III and α-synuclein, detected by in situ PLA, and the presence of Syn III in detergent-insoluble α-synuclein-positive protein fractions support that Syn III interacts with aggregated α-synuclein. This is in line with our previous findings showing the presence of Syn III within α-synuclein fibrils from LB-enriched protein extracts as well as of α-synuclein/Syn III PLA-positive neuropathological deposits in the post-mortem brain of sporadic PD patients [31]. Furthermore, a marked correlation exists between the levels of α-synuclein and Syn III measured in the caudate/putamen of the same subjects [31] that did not exhibit parallel neuropathological alterations of Syn I and Syn II [31]. Therefore, Syn III seems to be the only synapsin affecting α-synuclein aggregation via a direct protein–protein interaction. This notwithstanding, since we observed that other protein members of α-synuclein synaptic interactome, such as VAMP-2, are modulated in response to α-synuclein aggregation in wt mice, we cannot definitely conclude that Syn III is the only synaptic protein contributing to this process, although it seems to play a major role.
Recent evidence supports that oligomeric α-synuclein selectively lowers Syn I and II, thus worsening memory deficits in an Alzheimer disease mouse model [26]. However, it has to be taken into consideration that Syn I and Syn II mis-localize with VMAT2 in the striatum [11], while Syn III is the only synapsin isoform that can regulate striatal dopamine release [24] in conjunction with α-synuclein [59]. Differently from Syn I and Syn II, Syn III is also present in extra-synaptic locations in neurogenic regions of the adult mouse brain, thus displaying a unique regulatory profile that is different from that of the other two synapsins [40, 42]. The fact that the substantia nigra, where we found numerous cell soma positive for Syn III, is one of the neurogenic regions of the adult mouse brain [19, 49], suggests that additional studies are needed to clarify how and why the overexpression of human α-synuclein can induce an increase of Syn III within the neurons of this area.
These findings, when coupled to the present results, indicate that Syn III constitutes a crucial mediator of α-synuclein aggregation and toxicity in PD. Our observations support that Syn III is pivotally involved in α-synuclein-mediated nigrostriatal damage and that the modulation of Syn III, or of its interaction with α-synuclein, can represent a potentially effective therapeutic strategy to cure this disabling neurodegenerative disorder.
References
Anderson JP, Walker DE, Goldstein JM, de Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ (2006) Phosphorylation of Ser-129 is the dominant pathological modification of alpha-synuclein in familial and sporadic Lewy body disease. J Biol Chem 281:29739–29752. https://doi.org/10.1074/jbc.M600933200
Anichtchik O, Calo L, Spillantini MG (2013) Synaptic dysfunction in synucleinopathies. CNS Neurol Disord Drug Targets 12:1094–1100
Baiguera C, Alghisi M, Pinna A, Bellucci A, De Luca MA, Frau L, Morelli M, Ingrassia R, Benarese M, Porrini V, Pellitteri M, Bertini G, Fabene PF, Sigala S, Spillantini MG, Liou HC, Spano PF, Pizzi M (2012) Late-onset Parkinsonism in NFkappaB/c-Rel-deficient mice. Brain J Neurol 135:2750–2765. https://doi.org/10.1093/brain/aws193
Basso E, Antas P, Marijanovic Z, Goncalves S, Tenreiro S, Outeiro TF (2013) PLK2 modulates alpha-synuclein aggregation in yeast and mammalian cells. Mol Neurobiol 48:854–862. https://doi.org/10.1007/s12035-013-8473-z
Bellucci A, Antonini A, Pizzi M, Spano P (2017) The end is the beginning: Parkinson’s disease in the light of brain imaging. Front Aging Neurosci 9:330. https://doi.org/10.3389/fnagi.2017.00330
Bellucci A, Collo G, Sarnico I, Battistin L, Missale C, Spano P (2008) Alpha-synuclein aggregation and cell death triggered by energy deprivation and dopamine overload are counteracted by D2/D3 receptor activation. J Neurochem 106:560–577. https://doi.org/10.1111/j.1471-4159.2008.05406.x
Bellucci A, Fiorentini C, Zaltieri M, Missale C, Spano P (2014) The “in situ” proximity ligation assay to probe protein-protein interactions in intact tissues. Methods Mol Biol 1174:397–405. https://doi.org/10.1007/978-1-4939-0944-5_27
Bellucci A, Mercuri NB, Venneri A, Faustini G, Longhena F, Pizzi M, Missale C, Spano P (2016) Review: Parkinson’s disease: from synaptic loss to connectome dysfunction. Neuropathol Appl Neurobiol 42:77–94. https://doi.org/10.1111/nan.12297
Bellucci A, Navarria L, Falarti E, Zaltieri M, Bono F, Collo G, Spillantini MG, Missale C, Spano P (2011) Redistribution of DAT/alpha-synuclein complexes visualized by “in situ” proximity ligation assay in transgenic mice modelling early Parkinson’s disease. PLoS One 6:e27959. https://doi.org/10.1371/journal.pone.0027959
Bellucci A, Navarria L, Zaltieri M, Falarti E, Bodei S, Sigala S, Battistin L, Spillantini M, Missale C, Spano P (2011) Induction of the unfolded protein response by alpha-synuclein in experimental models of Parkinson’s disease. J Neurochem 116:588–605. https://doi.org/10.1111/j.1471-4159.2010.07143.x
Bogen IL, Boulland JL, Mariussen E, Wright MS, Fonnum F, Kao HT, Walaas SI (2006) Absence of synapsin I and II is accompanied by decreases in vesicular transport of specific neurotransmitters. J Neurochem 96:1458–1466. https://doi.org/10.1111/j.1471-4159.2005.03636.x
Boix J, Padel T, Paul G (2015) A partial lesion model of Parkinson’s disease in mice–characterization of a 6-OHDA-induced medial forebrain bundle lesion. Behav Brain Res 284:196–206. https://doi.org/10.1016/j.bbr.2015.01.053
Burre J (2015) The Synaptic Function of alpha-Synuclein. J Parkinson’s Dis 5:699–713. https://doi.org/10.3233/JPD-150642
Burre J, Sharma M, Sudhof TC (2014) alpha-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation. Proc Natl Acad Sci USA 111:E4274–E4283. https://doi.org/10.1073/pnas.1416598111
Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Sudhof TC (2010) Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro. Science 329:1663–1667. https://doi.org/10.1126/science.1195227
Decressac M, Mattsson B, Lundblad M, Weikop P, Bjorklund A (2012) Progressive neurodegenerative and behavioural changes induced by AAV-mediated overexpression of alpha-synuclein in midbrain dopamine neurons. Neurobiol Dis 45:939–953. https://doi.org/10.1016/j.nbd.2011.12.013
Diao J, Burre J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Sudhof TC, Brunger AT (2013) Native alpha-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2. Elife 2:e00592. https://doi.org/10.7554/eLife.00592
Engelender S, Isacson O (2017) The threshold theory for Parkinson’s disease. Trends Neurosci 40:4–14. https://doi.org/10.1016/j.tins.2016.10.008
Farzanehfar P, Horne MK, Aumann TD (2017) Can valproic acid regulate neurogenesis from Nestin+ cells in the adult midbrain? Neurochem Res 42:2127–2134. https://doi.org/10.1007/s11064-017-2259-z
Fusco G, Pape T, Stephens AD, Mahou P, Costa AR, Kaminski CF, Kaminski Schierle GS, Vendruscolo M, Veglia G, Dobson CM, De Simone A (2016) Structural basis of synaptic vesicle assembly promoted by alpha-synuclein. Nat Commun 7:12563. https://doi.org/10.1038/ncomms12563
Garcia-Reitbock P, Anichtchik O, Bellucci A, Iovino M, Ballini C, Fineberg E, Ghetti B, Della Corte L, Spano P, Tofaris GK, Goedert M, Spillantini MG (2010) SNARE protein redistribution and synaptic failure in a transgenic mouse model of Parkinson’s disease. Brain J Neurol 133:2032–2044. https://doi.org/10.1093/brain/awq132
Goedert M, Spillantini MG, Del Tredici K, Braak H (2013) 100 years of Lewy pathology. Nat Rev Neurol 9:13–24. https://doi.org/10.1038/nrneurol.2012.242
Grealish S, Mattsson B, Draxler P, Bjorklund A (2010) Characterisation of behavioural and neurodegenerative changes induced by intranigral 6-hydroxydopamine lesions in a mouse model of Parkinson’s disease. Eur J Neurosci 31:2266–2278. https://doi.org/10.1111/j.1460-9568.2010.07265.x
Kile BM, Guillot TS, Venton BJ, Wetsel WC, Augustine GJ, Wightman RM (2010) Synapsins differentially control dopamine and serotonin release. J Neurosci 30:9762–9770. https://doi.org/10.1523/JNEUROSCI.2071-09.2010
Kirik D, Rosenblad C, Burger C, Lundberg C, Johansen TE, Muzyczka N, Mandel RJ, Bjorklund A (2002) Parkinson-like neurodegeneration induced by targeted overexpression of alpha-synuclein in the nigrostriatal system. J Neurosci 22:2780–2791
Larson ME, Greimel SJ, Amar F, LaCroix M, Boyle G, Sherman MA, Schley H, Miel C, Schneider JA, Kayed R, Benfenati F, Lee MK, Bennett DA, Lesne SE (2017) Selective lowering of synapsins induced by oligomeric alpha-synuclein exacerbates memory deficits. Proc Natl Acad Sci USA 114:E4648–E4657. https://doi.org/10.1073/pnas.1704698114
Lee FJ, Liu F, Pristupa ZB, Niznik HB (2001) Direct binding and functional coupling of alpha-synuclein to the dopamine transporters accelerate dopamine-induced apoptosis. FASEB J 15:916–926
Lo Bianco C, Ridet JL, Schneider BL, Deglon N, Aebischer P (2002) alpha-Synucleinopathy and selective dopaminergic neuron loss in a rat lentiviral-based model of Parkinson’s disease. Proc Natl Acad Sci USA 99:10813–10818. https://doi.org/10.1073/pnas.152339799
Lohr KM, Masoud ST, Salahpour A, Miller GW (2017) Membrane transporters as mediators of synaptic dopamine dynamics: implications for disease. Eur J Neurosci 45:20–33. https://doi.org/10.1111/ejn.13357
Longhena F, Faustini G, Missale C, Pizzi M, Bellucci A (2018) Dopamine transporter/alpha-synuclein complexes are altered in the post mortem caudate putamen of Parkinson’s disease: an in situ proximity ligation assay study. Int J Mol Sci. https://doi.org/10.3390/ijms19061611
Longhena F, Faustini G, Varanita T, Zaltieri M, Porrini V, Tessari I, Poliani PL, Missale C, Borroni B, Padovani A, Bubacco L, Pizzi M, Spano P, Bellucci A (2018) Synapsin III is a key component of alpha-synuclein fibrils in Lewy bodies of PD brains. Brain Pathol. https://doi.org/10.1111/bpa.12587
Lundblad M, Decressac M, Mattsson B, Bjorklund A (2012) Impaired neurotransmission caused by overexpression of alpha-synuclein in nigral dopamine neurons. Proc Natl Acad Sci USA 109:3213–3219. https://doi.org/10.1073/pnas.1200575109
McFarland NR, Fan Z, Xu K, Schwarzschild MA, Feany MB, Hyman BT, McLean PJ (2009) Alpha-synuclein S129 phosphorylation mutants do not alter nigrostriatal toxicity in a rat model of Parkinson disease. J Neuropathol Exp Neurol 68:515–524. https://doi.org/10.1097/NEN.0b013e3181a24b53
Medeiros AT, Soll LG, Tessari I, Bubacco L, Morgan JR (2017) alpha-synuclein dimers impair vesicle fission during clathrin-mediated synaptic vesicle recycling. Frontiers in cellular neuroscience 11:388. https://doi.org/10.3389/fncel.2017.00388
Moss J, Bolam JP (2008) A dopaminergic axon lattice in the striatum and its relationship with cortical and thalamic terminals. J Neurosci 28:11221–11230. https://doi.org/10.1523/JNEUROSCI.2780-08.2008
Oliveras-Salva M, Van der Perren A, Casadei N, Stroobants S, Nuber S, D’Hooge R, Van den Haute C, Baekelandt V (2013) rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol Neurodegen 8:44. https://doi.org/10.1186/1750-1326-8-44
Paxinos G, Franklin K (2012) Paxinos and Franklin’s the mouse brain in stereotaxic coordinates, 4th edn
Phan JA, Stokholm K, Zareba-Paslawska J, Jakobsen S, Vang K, Gjedde A, Landau AM, Romero-Ramos M (2017) Early synaptic dysfunction induced by alpha-synuclein in a rat model of Parkinson’s disease. Sci Rep 7:6363. https://doi.org/10.1038/s41598-017-06724-9
Picconi B, Piccoli G, Calabresi P (2012) Synaptic dysfunction in Parkinson’s disease. Adv Exp Med Biol 970:553–572. https://doi.org/10.1007/978-3-7091-0932-8_24
Pieribone VA, Porton B, Rendon B, Feng J, Greengard P, Kao HT (2002) Expression of synapsin III in nerve terminals and neurogenic regions of the adult brain. J Comp Neurol 454:105–114. https://doi.org/10.1002/cne.10417
Plotegher N, Bubacco L (2016) Lysines, Achilles’ heel in alpha-synuclein conversion to a deadly neuronal endotoxin. Ageing Res Rev 26:62–71. https://doi.org/10.1016/j.arr.2015.12.002
Porton B, Wetsel WC, Kao HT (2011) Synapsin III: role in neuronal plasticity and disease. Semin Cell Dev Biol 22:416–424. https://doi.org/10.1016/j.semcdb.2011.07.007
Poudel KR, Bai J (2014) Synaptic vesicle morphology: a case of protein sorting? Curr Opin Cell Biol 26:28–33. https://doi.org/10.1016/j.ceb.2013.09.001
Roberts RF, Wade-Martins R, Alegre-Abarrategui J (2015) Direct visualization of alpha-synuclein oligomers reveals previously undetected pathology in Parkinson’s disease brain. Brain J Neurol 138:1642–1657. https://doi.org/10.1093/brain/awv040
Royce GJ, Laine EJ (1984) Efferent connections of the caudate nucleus, including cortical projections of the striatum and other basal ganglia: an autoradiographic and horseradish peroxidase investigation in the cat. J Comp Neurol 226:28–49. https://doi.org/10.1002/cne.902260104
Samuel F, Flavin WP, Iqbal S, Pacelli C, Sri Renganathan SD, Trudeau LE, Campbell EM, Fraser PE, Tandon A (2016) Effects of serine 129 phosphorylation on alpha-synuclein aggregation, membrane association, and internalization. J Biol Chem 291:4374–4385. https://doi.org/10.1074/jbc.M115.705095
Sancenon V, Lee SA, Patrick C, Griffith J, Paulino A, Outeiro TF, Reggiori F, Masliah E, Muchowski PJ (2012) Suppression of alpha-synuclein toxicity and vesicle trafficking defects by phosphorylation at S129 in yeast depends on genetic context. Hum Mol Genet 21:2432–2449. https://doi.org/10.1093/hmg/dds058
Segura-Aguilar J, Paris I, Munoz P, Ferrari E, Zecca L, Zucca FA (2014) Protective and toxic roles of dopamine in Parkinson’s disease. J Neurochem 129:898–915. https://doi.org/10.1111/jnc.12686
Shan X, Chi L, Bishop M, Luo C, Lien L, Zhang Z, Liu R (2006) Enhanced de novo neurogenesis and dopaminergic neurogenesis in the substantia nigra of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced Parkinson’s disease-like mice. Stem cells 24:1280–1287. https://doi.org/10.1634/stemcells.2005-0487
Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M (1997) Alpha-synuclein in Lewy bodies. Nature 388:839–840. https://doi.org/10.1038/42166
Sudhof TC (2013) Neurotransmitter release: the last millisecond in the life of a synaptic vesicle. Neuron 80:675–690. https://doi.org/10.1016/j.neuron.2013.10.022
Sudhof TC, Rothman JE (2009) Membrane fusion: grappling with SNARE and SM proteins. Science 323:474–477. https://doi.org/10.1126/science.1161748
Surmeier DJ, Obeso JA, Halliday GM (2017) Selective neuronal vulnerability in Parkinson disease. Nat Rev Neurosci 18:101–113. https://doi.org/10.1038/nrn.2016.178
Tofaris GK, Garcia Reitbock P, Humby T, Lambourne SL, O’Connell M, Ghetti B, Gossage H, Emson PC, Wilkinson LS, Goedert M, Spillantini MG (2006) Pathological changes in dopaminergic nerve cells of the substantia nigra and olfactory bulb in mice transgenic for truncated human alpha-synuclein(1-120): implications for Lewy body disorders. J Neurosci 26:3942–3950. https://doi.org/10.1523/JNEUROSCI.4965-05.2006
Vargas KJ, Makani S, Davis T, Westphal CH, Castillo PE, Chandra SS (2014) Synucleins regulate the kinetics of synaptic vesicle endocytosis. J Neurosci 34:9364–9376. https://doi.org/10.1523/JNEUROSCI.4787-13.2014
Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U, Lucic V, Chandra SS (2017) Synucleins have multiple effects on presynaptic architecture. Cell Rep 18:161–173. https://doi.org/10.1016/j.celrep.2016.12.023
Wang L, Das U, Scott DA, Tang Y, McLean PJ, Roy S (2014) alpha-synuclein multimers cluster synaptic vesicles and attenuate recycling. CB 24:2319–2326. https://doi.org/10.1016/j.cub.2014.08.027
Yamada M, Iwatsubo T, Mizuno Y, Mochizuki H (2004) Overexpression of alpha-synuclein in rat substantia nigra results in loss of dopaminergic neurons, phosphorylation of alpha-synuclein and activation of caspase-9: resemblance to pathogenetic changes in Parkinson’s disease. J Neurochem 91:451–461. https://doi.org/10.1111/j.1471-4159.2004.02728.x
Zaltieri M, Grigoletto J, Longhena F, Navarria L, Favero G, Castrezzati S, Colivicchi MA, Della Corte L, Rezzani R, Pizzi M, Benfenati F, Spillantini MG, Missale C, Spano P, Bellucci A (2015) alpha-synuclein and synapsin III cooperatively regulate synaptic function in dopamine neurons. J Cell Sci 128:2231–2243. https://doi.org/10.1242/jcs.157867
Funding
This work was supported by the Michael J. Fox Foundation USA Target Advancement Program, Grant ID #10742.
Author information
Authors and Affiliations
Contributions
Conceptualization GF, PS and AB; Methodology, GF, FL, TV and AB; Investigation, GF, FL, TV, LB and AB; Writing-original draft, GF, PS and AB; Writing-review and editing, GF, LB, MP, CM, FB, AnB, and AB; Funding Acquisition, AB; Resources, LB, FB, AnB and AB; Supervision, AnB, AB.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Faustini, G., Longhena, F., Varanita, T. et al. Synapsin III deficiency hampers α-synuclein aggregation, striatal synaptic damage and nigral cell loss in an AAV-based mouse model of Parkinson’s disease. Acta Neuropathol 136, 621–639 (2018). https://doi.org/10.1007/s00401-018-1892-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-018-1892-1