Abstract
Von Economo neurons (VENs) are large spindle-shaped neurons localized to anterior cingulate cortex (ACC) and fronto-insular cortex (FI). VENs appear late in development in humans, are a recent phylogenetic specialization, and are selectively destroyed in frontotemporal dementia, a disease which profoundly disrupts social functioning and self-awareness. Agenesis of the corpus callosum (AgCC) is a congenital disorder that can have significant effects on social and emotional behaviors, including alexithymia, difficulty intuiting the emotional states of others, and deficits in self- and social-awareness that can impair humor, comprehension of non-literal or affective language, and social judgment. To test the hypothesis that VEN number is selectively reduced in AgCC, we used stereology to obtain unbiased estimates of total neuron number and VEN number in postmortem brain specimens of four normal adult controls, two adults with isolated callosal dysgenesis, and one adult whose corpus callosum and ACC were severely atrophied due to a non-fatal cerebral arterial infarction. The partial agenesis case had approximately half as many VENs as did the four normal controls, both in ACC and FI. In the complete agenesis case the VENs were almost entirely absent. The percentage of neurons in FI that are VENs was reduced in callosal agenesis, but was actually slightly above normal in the stroke patient. These results indicate that the VEN population is selectively reduced in AgCC, but that the VENs do not depend on having an intact corpus callosum. We conclude that in agenesis of the corpus callosum the reduction in the number of VENs is not the direct result of the failure of this structure to develop, but may instead be another consequence of the genetic disruption that caused the agenesis. The reduction of the VEN population could help to explain some of the social and emotional deficits that are seen in this disorder.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Studies of split-brain patients have shown that callosal lesions, callosotomy, and agenesis of the corpus callosum (AgCC) can all have significant effects on social and emotional behaviors, including a reduction in affective range [10, 32], an inability to express one’s emotional state (a condition called alexithymia [19, 56]), and difficulty intuiting the emotional states of others [5, 62]. AgCC in particular is associated with deficits in self- and social-awareness that can impair such capacities as humor [6], non-literal or affective language [34], and social judgment [5], suggesting that the abnormal development of the corpus callosum and surrounding midline brain structures may be particularly detrimental to socially relevant aspects of emotional cognition. While symptoms of social impairment in AgCC have traditionally been interpreted as resulting from limitations in inter-hemispheric transfer, here we examine the alternative possibility that other circuitry involved in social functioning may be disrupted in this condition.
VENs are large bipolar neurons (Fig. 1) localized to two brain regions that are known to be involved in social and emotional cognition: the anterior cingulate cortex (ACC) and the fronto-insular cortex (FI). VENs are radially oriented and have sparsely branching dendritic trees with symmetric apical and basal dendrites that could rapidly relay the output of a cortical column [59]. In human fMRI experiments, ACC and FI are both activated by paradigms evoking social emotions such as empathy [47], guilt [46], unfairness [48], humor [60], embarrassing situations or violations of social norms [4], and romantic love [3, 23]. The VENs could serve as a fast relay of these social emotions to other brain structures [2, 30]. In frontotemporal dementia (FTD), a disorder that profoundly disrupts social functioning and self-awareness, there is a 74% reduction in the VEN population relative to controls, and many of the remaining VENs are severely dysmorphic [44, 45]. Patients with FTD experience focal degeneration of both ACC and FI, accompanied by severe deficits in self-awareness, empathy, “theory of mind,” and moral reasoning [26, 28, 36, 49, 51]. The similarity in the social deficits in FTD and AgCC suggests that they may share common defects in neural circuitry. VENs may be particularly vulnerable to neuropathologies such as AgCC because they emerge late both in human development and in primate phylogeny. Examination of a broad spectrum of primates has shown that VENs are a distinctive feature of great apes and humans, and are more abundant in humans than in great apes [1, 29], suggesting that they represent a recent phylogenetic specialization that must have arisen after the hominoids split from other Old-World primates approximately 12–15 million years ago. Interestingly, VENs are also present in large-brained cetaceans [18] and elephants [17], suggesting adaptive convergence for VEN evolution. The VENs may be a circuit specialization that supports the capacity to adapt rapidly to a social environment that is complex and in constant flux [2]. In addition to the late appearance of VENs in primate evolution, VENs emerge late in human development [2] compared with other neuronal types [35, 39]. They first appear in very small numbers at the 35th week of gestation. At full term only about 10% of the adult number of VENs are present. The late arrival of these neurons, both evolutionarily and developmentally, could make them especially vulnerable to errors of neurogenesis. If VENs are selectively affected in AgCC, it could help to explain some symptoms of irregular social and emotional behavior.
High magnification photomicrographs of VENs. Normal 58-year-old man (C1) (a). Normal 50-year-old woman (C3) (b). Normal 50-year-old man (C4) (c). Scale bars = 25 μm. While pyramidal neurons (dashed arrow in c) are characterized by a triangular-shaped soma with a single apical dendrite and numerous basal dendrites, VENs (solid arrows) are characterized by a large spindle-shaped soma with a single large apical and a single large basal dendrite
To test the hypothesis that VEN number is selectively reduced in AgCC, we used stereology to obtain unbiased estimates of total neuron number and VEN number in postmortem brain specimens of two adults with isolated callosal agenesis. We predict that if VENs are selectively vulnerable in AgCC, then the proportion of VENs relative to total neuron number will be reduced in the disease state compared to normal controls. We also compared the AgCC specimens with an individual who suffered a non-fatal cerebral infarction late in life that left the corpus callosum and ACC severely atrophied. This comparison is designed to help understand differences in VEN vulnerability to callosal pathologies that occur congenitally versus those that are acquired later in life.
Materials and methods
Case information
Histological sections of AgCC and normal brains were studied at the Yakovlev-Haleem Brain Collection at the National Museum of Health and Medicine, Washington, DC. In this collection of more than 1,300 serially sectioned brains (most of which were prepared in the 1940s–1960s), there were several documented cases of callosal agenesis. Most of the AgCC cases were also associated with other anomalies such as trisomy, microcephaly, and spina bifida, but there were two cases of isolated AgCC in which both ACC and FI were well preserved with a sufficient number of sections for stereology in at least one hemisphere.
The first case is of a 48-year-old man who died of a coronary thrombosis. Absence of the entire corpus callosum was discovered at autopsy. As in most cases of AgCC, the anterior commissure was present, while the hippocampal, habenular, and posterior commissures were absent [52]. The brain exhibited enlarged ventricles, radiating posterior cingulate gyri, and longitudinal callosal bundles (bundles of Probst), all of which are features common in AgCC [25, 27]. There is no documentation about this individual’s occupation or mental health, though the case file describes him as “well developed” and “presumably mentally competent”. Unfortunately, only the left hemisphere was suitable for this study. The right hemisphere had been cut sagittally and therefore could not be used for counting VENs as their elongated shape and size relative to the section thickness make them identifiable only when they are oriented within the plane of section. In FI and ACC this is principally in the coronal plane.
The second case is of a 71-year-old woman who died from renal and respiratory infection. Autopsy revealed partial AgCC in which the genu and rostral third of the body of the corpus callosum were present, while the remaining body and splenium were absent. As in the previous case the anterior commissure was present, but all of the other small forebrain commissures were absent. Enlarged ventricles and radiating cingulate gyri were observed, as were the bundles of Probst. The museum record does contain some biographical information. She was born in 1882 and completed 2 years of high school before beginning the work, first as a telephone operator and then as a clerk in a county courthouse. In childhood, she is described as “extremely sensitive, shy, and retiring,” and in adulthood as “reserved and prudish.” At the age of 40 she had a “brief attack of hysteria”, and was later admitted to a state hospital at age 45 after developing violent crying episodes at work. Although there were no other physical or neurological abnormalities detected, she was assessed as having a “mental age [of] 12; IQ 77” and remained institutionalized.
The cerebral infarction specimen was obtained from a 55-year-old woman who, 15 years earlier, suffered a non-fatal localized cerebral infarction of a communicating branch of the anterior cerebral artery. The stroke resulted in complete atrophy of the cingulate gyri, and nearly completes atrophy of the corpus callosum, with only a few interhemispheric fibers visible on histological sections (see Fig. 6a). The specimen was obtained at autopsy from the Mount Sinai School of Medicine Division of Neuropathology and consisted of a coronal slab approximately 2 cm thick containing a portion of fronto-insular cortex on the left side.
Four neurologically normal control cases were also measured: one man aged 58 years (C1) who died of myocardial infarction, one man aged 86 years with no indications of dementia (C2) who died of myocardial infarction, one woman aged 50 years (C3) who died of myocarditis, and one man aged 50 years (C4) who died of myocardial infarction.
Histology and stereology
Each brain had been immersion-fixed in either formalin or freshly depolymerized paraformaldehyde. With the exception of the cerebral infarct specimen, all brains were embedded in celloidin and sectioned at 35 μm in the coronal plane. Every tenth section was Nissl stained using cresyl violet, except for the complete AgCC case which was stained every 50th section. The cerebral infarction specimen was sectioned at 100 μm on a freezing microtome. Because this specimen contained only a portion of FI on one side, the specimen was measured only for the ratio of total neurons to VENs.
VENs are located in layer V of ACC and FI (see Fig. 2 for lamination pattern). They are radially oriented (long axis perpendicular to the pia) and are morphologically distinct from pyramidal neurons based on their bipolar cell body with single large apical dendrite and a single large basal dendrite. These two large dendrites bifurcate only sparsely and never close to the soma. In many regions of the cortex, layer VI is dense with small, spindle-shaped neurons, but these cells are not considered VENs. Our regions of interest for ACC and FI include only layers III–V. With the exception of the complete AgCC case, ROIs were defined by the extent of VEN-containing regions in FI and ACC. In the complete AgCC case, where so few VENs were evident, the ROIs were defined to encompass an area around the existing VENs large enough to obtain adequate stereological sampling of both VENs and other neurons; this zone typically extended 3–4 mm on either side of the observed VENs.
Location and morphology of von Economo neurons (VENs). Photomicrograph indicating the lamination pattern of fronto-insular cortex in a normal human. VENs are located in layer V. Scale bar = 1 mm. Modified from Von Economo and Koskinas [58]
Stereological estimates of total neuron and VEN number were made using a Nikon or Reichert microscope with a digital camera and motorized stage interfaced to a desktop computer running StereoInvestigator software (MBF Bioscience, Wiliston, VT). Absolute cell number was estimated using an optical fractionator probe [61] at 60× magnification with a counting frame of 140 × 100 μm and an optical disector height of 25 μm (with 5 μm guard zones). Sampling frequencies were selected by conducting a preliminary population estimate before each stereological run and then choosing a virtual grid size appropriate for counting 300–600 neurons [43]. In most cases, FI was sampled every tenth section, while ACC—which is much larger—was sampled every 40th section. However, in the complete AgCC case, which had been stained at intervals of 50 sections, all available sections were used for stereology.
The regions of interest used for total neuron counts were the same regions used in the VEN counts, rather than regions defined by the morphological boundaries of FI or ACC. In estimating total neuron number, neurons were distinguished from glial cells by their well-defined cytoplasm and distinct nucleus with a darkly stained nucleolus. For each of the stereological estimates the Gundersen (m = 1) coefficient of error, which reflects the technical reliability of the sampling, was computed [16].
Statistical testing for differences between each of the pathological cases and the sample of normal cases was performed with the independent-samples t test, specifically the special case of comparing a single individual with a sample [50]. In this case, the degrees of freedom are one less than the control sample (df = 3 in our case).
Results
Results of the stereological estimates (Table 1) indicated a substantial difference in the number of VENs in AgCC cases compared to normal controls. The partial AgCC case exhibited approximately 30–50% fewer VENs than the control cases in both FI and ACC (Fig. 3a). In the case of complete agenesis, the VEN population was estimated to be only a few thousand cells. Among independent-samples t tests, only the comparison between left FI in controls versus complete AgCC reached statistical significance (t = 3.515, df = 3, P = 0.039), while the P values for all other tests of absolute VEN number ranged between 0.101 and 0.173. It is also interesting to note that a right-dominant asymmetry in VEN number, which has previously been reported for normal adults and children [1, 2], was common to the partial agenesis case as well as the control cases.
Stereologic estimates of VEN numbers in FI and ACC. Absolute number of VENs as estimated by the optical fractionater (a). The two cases of callosal agenesis exhibit fewer VENs than the two normal controls. The partial AgCC case contains approximately half as many VENs as the control cases, while the VENs are almost entirely absent in the case of complete agenesis. In the control group, error bars represent ±1 standard error of the mean. The right-dominant asymmetry in VEN number present in the controls and partial AgCC is consistent with previous reports of VEN asymmetry in normal adults and children [1, 2]. The right hemisphere of the complete AgCC case was not suitable for stereology. Percentage of neurons in FI and ACC that are VENs, obtained from stereological estimation (b). In the partial AgCC case, the percentage of neurons that are VENs is slightly lower than in controls, while in the case of complete AgCC the ratio is dramatically lower. In the case of the ACC infarction specimen, the VEN percentage is higher than in controls. Of the seven possible statistical tests comparing diseased states with controls, four tests achieve statistical significance. In the ACC infarction subject, only the left FI was suitable for stereology. The right hemisphere of the complete AgCC case was not suitable for stereology
There is a large amount of variation in the estimated number of total neurons within the VEN-containing regions (Table 1), but this is mainly a product of variation in the volumetric extent of the VENs. By obtaining the ratio of VENs to total neurons we can normalize for this size difference and provide an appropriate test of selective VEN reduction. In the four control cases, the ratio of VENs to all neurons was estimated to be approximately 1:100 in FI, and in ACC between 1:100 and 1:200 (Table 2; Fig. 2b). In the partial agenesis case, this ratio is lower (~1:150 in FI and ~1:250 in ACC), whereas in the case of complete agenesis the ratio was estimated to be ~1:1,400 in FI and ~1:950 in ACC.
These ratio differences can be assessed statistically using the special case of the independent-samples t test (a single individual compared with a control sample), the results of which are presented graphically in Fig. 2b. In the partial agenesis case, left FI has a significantly lower VEN to total neuron ratio (i.e., fewer VENs) than control specimens (left FI: t = 7.748, df = 3, P = 0.0045). In the left hemisphere of the complete AgCC case, both FI and ACC exhibited significantly higher ratios than control cases (left FI: t = 20.127, df = 3, P < 0.001; left ACC: t = –2.574, df = 3, P = 0.082).
Unlike in the AgCC cases, in the stroke case, the ratio of VENs to total neurons was not reduced. It was in fact slightly higher than in control cases for left FI (the only region available for study). This result was statistically significant (t = −10.299, df = 3, P = 0.002). This strongly suggests that the VENs in FI were not selectively harmed by the destruction of the corpus callosum in this case.
Among the control group, none of the variables correlated with age, nor did the woman exhibit any significant differences in stereological estimates or neuron-to-VEN ratios when compared with the three men.
We also compared the topographic extent of the VEN-containing regions in the normal and diseased states (Fig. 4a–c). In normal controls, VENs in ACC are distributed throughout the subgenual and supragenual gyrus and are concentrated in the crowns of the gyri. In the partial agenesis case, the VENs in ACC lie within a malformed cingulate gyrus that is inverted on the left side, while the right side appears more similar to the distribution found in controls. The single hemisphere of the complete AgCC case has a well-formed cingulate gyrus that appears morphologically normal.
Tracings of coronal sections from specimens examined in this study illustrating the location of VENs in: a normal control (a), the partial AgCC case (b), and the complete AgCC case (c). In normal controls, VENs in ACC (grey) are distributed throughout the subgenual and supragenual gyrus and are concentrated in the crowns of the gyri. In FI (black) the VENs occupy a limited space antero-posteriorly, but they extend axially in a ribbon of cortex lying dorso-medial to the temporal pole. In the partial agenesis case, the VENs in ACC lie within a malformed cingulate gyrus that is inverted on the left side (white arrow). Also in the partial AgCC brain, some of the VENs are located in the normal FI gyrus (black arrow) while other VENs appear to be abnormally located in what would be morphologically defined as the medial orbitofrontal gyri (dashed arrows). In the available right hemisphere of the complete AgCC case the VENs appear to occupy their normal position in ACC and FI despite the absence of the corpus callosum. Scale bars = 5 cm
In the control cases, VENs within FI occupy a limited space antero-posteriorly, but they extend axially in a long ribbon lying dorso-medial to the temporal pole (Fig. 5a). In one hemisphere of the complete agenesis case the VENs occupy a similar position, but in the partial AgCC brain they appear to be abnormally located in what would be morphologically defined as the medial orbitofrontal gyri (Fig. 5b). The distribution of VENs in the stroke case appears to be normal despite the morphological abnormalities caused by the infarction and subsequent period of prolonged degeneration (Fig. 6).
Coronal tracings of the ventro-medial frontal cortex in a normal 50-year-old woman (a) and a 71-year-old woman with partial AgCC (b). For these tracings, all VENs were plotted on the section and indicated by a filled square. Normally, VENs are distributed throughout the fronto-insular cortex lying dorsomedial to the temporal pole. In the partial agenesis case many of the VENs are abnormally located in what would be morphologically defined as the medial orbitofrontal gyri
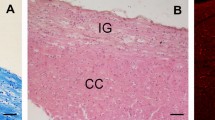
The 55-year-old non-fatal cerebral infarction specimen. Histological section prepared using the Gallyas fiber stain (a). Rectangle indicates the region of interest pictured in (b). Note that the corpus callosum (top right corner of the inset box) is severely reduced to only a few fibers. Scale bar = 1 cm. Tracing of the VEN-containing region in left FI (b). As in Fig. 5, all VENs were plotted on the section and are indicated by black squares. Despite the complete absence of a cingulate gyrus, this specimen exhibits a large number of VENs distributed normally along the mediolateral extent of the FI gyrus
Discussion
With approximately 180 million fibers interconnecting homologous areas of association cortex [57], the corpus callosum is by far the largest interhemispheric commissure. Callosal agenesis occurs in roughly 1 in 4,000 individuals, and its genetic and developmental etiology has become increasingly well understood [32]. The large majority of AgCC cases occur in conjunction with other abnormalities (AgCC is a component of at least 28 genetically identifiable syndromes) but it can also infrequently occur in isolation. Among these individuals some are essentially asymptomatic in daily life, and many cases of AgCC remain undiagnosed.
Early research on AgCC emphasized a lack of disconnection syndromes, such as hemialexia, that were typical of callosotomy patients [15, 42]. However, subsequent experiments with high-functioning acallosal individuals have found specific, often subtle, deficits in several areas [7, 12, 20, 22, 41]. Individuals with AgCC often have deficits in complex novel problem solving [5, 8, 13, 15, 41] as well as linguistic deficits that include difficulty in processing some phonetic and semantic aspects of language [9, 21, 40, 53–55] and meaningless or “out-of-place” conversation [21, 31].
Callosotomy patients maintain normal social interactions or recover them within months of their operation [14, 24]. Recent studies have suggested that AgCC is associated with deficits in social cognition that often have a more severe impact on daily living than deficits in sensory integration or motor coordination [31]. For example, acallosal individuals tend to exhibit poor psychosocial insight as judged by their diminished understanding of scenes depicting social interactions [5, 33]. Individuals with AgCC also have difficulty comprehending non-literal or affective language [34] and humor [6], both of which require social and emotional insight.
The results of our stereologic analysis support the hypothesis that VEN number is selectively reduced in callosal agenesis. The case of partial AgCC exhibited up to a 50% reduction in the number of VENs in FI and ACC compared to controls, while the VENs were virtually wiped out in complete AgCC. The maintenance of a sizable VEN population in partial AgCC compared with complete AgCC may indicate that the corpus callosum provides sustaining connections for the VENs [37, 38], and that even a partial corpus callosum could be sufficient to preserve the VEN population. Although only one of the six comparisons of absolute VEN number between controls and AgCC cases was statistically significant, the results are qualitatively striking and suggest that further analysis is warranted.
A more important measure of VEN reduction is the number of VENs as a proportion of total neurons. Our results indicate that the number of VENs relative to total neurons decreases significantly in callosal agenesis. In normal controls the ratio of VENs to total neurons was estimated to be approximately 1:100 in FI and approximately 1:130 in ACC. In the case of partial AgCC this ratio is approximately 1:150 in FI and 1:250 in ACC. In the complete AgCC case the ratio is estimated to be approximately 1:1,400 in FI and approximately 1:950 in ACC. Of the seven possible statistical comparisons between normal controls and AgCC cases, four were statistically significant. We interpret these results to indicate that VEN populations are at greater risk from the malformations associated with AgCC than are other ACC or FI neurons.
Although we were able to measure total neuron-to-VEN ratio only in left FI of the stroke case, our results indicate that this individual died with slightly more VENs relative to other neurons compared with control subjects. This result indicates that destruction of the corpus callosum does not in itself result in left FI VEN loss. Rather, AgCC and VEN loss may be independent consequences of a common developmental defect. Furthermore, our study does not address the mechanism by which VEN number is reduced. This could be the result of migration deficits, failures during neurogenesis, or actual cell death related to the presence of AgCC. The same mechanisms could be responsible for the shift in the location of the VENs in FI in the partial AgCC case relative to normal cases. In immunocytochemical studies done in normal brains in our laboratory (Nicole Tetreault and John Allman), the VENs selectively express the product of the gene Disc1 (disrupted in schizophrenia). Disc1 regulates neuronal migration and the dendritic morphology of postnatally generated neurons in mice [11]. The targets of VEN projections remain unknown, and although we have reason to believe that VENs are long-distance projection neurons, it is not known if they project inter-hemispherically through the corpus callosum. Therefore, our analyses cannot distinguish whether the reduction in VEN fraction seen in AgCC is a direct result of the interruption of callosal connections, or if the reduction is a secondary consequence of developmental malformations associated with the disorder. In either case, our results offer important evidence of a localized neuronal origin for the social and emotional deficits that are associated with AgCC. This result is consistent with that observed in frontotemporal dementia [44]. It remains to be seen if VEN populations are reduced in other neuropathologies in which social/emotional deficits are a component.
The principal limitation to our analysis is the small sample size of AgCC cases. AgCC is a rare disorder and there are very few histological specimens available for the study. As far as we are aware, the specimens we measured at the Yakovlev collection are the only complete histological series available for AgCC in the United States. Moreover, since AgCC is often associated with other neurological abnormalities (e.g., seizures, hydrocephaly, mental retardation, and sensorimotor deficits), histological specimens of isolated AgCC are especially difficult to find. However, it is these cases that are the most valuable, since psychological abnormalities in these individuals can be more confidently attributed to callosal agenesis specifically rather than to other confounding factors. We have restricted our analysis to two cases of isolated AgCC. Hopefully in the future more of these unique cases will become available and our experiment can be replicated with a larger sample.
Conclusion
Callosal agenesis is one of many neurological disorders that are accompanied by deficits in social and emotional cognition. In particular, AgCC is associated with impairments in empathy, humor, and social intuition. We have demonstrated here that the VEN population is severely reduced in cases of AgCC, and that VENs are selectively vulnerable in this pathology compared with other neuron types. The convergence of our data with other studies on VEN numbers in health and disease provides indirect evidence that VENs play an important role in social cognition. If VEN populations are selectively reduced in AgCC their numbers may also be reduced in other neurological disorders, and this may help to explain the social and emotional deficits associated with neuropathologic changes in diseases such as autism, schizophrenia, and FTD.
References
Allman JM, Hakeem A, Watson KK (2002) Two phylogenetic specializations in the human brain. Neuroscientist 8:335–346
Allman JM, Watson KK, Tetreault NA, Hakeem AY (2005) Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci 9:367–373. doi:10.1016/j.tics.2005.06.008
Bartels A, Zeki S (2000) The neural basis of romantic love. Neuroreport 11:3829–3834. doi:10.1097/00001756-200011270-00046
Berthoz S, Armony JL, Blair RJ, Dolan RJ (2002) An fMRI study of intentional and unintentional (embarrassing) violations of social norms. Brain 125:1696–1708. doi:10.1093/brain/awf190
Brown WS, Paul LK (2000) Cognitive and psychosocial deficits in agenesis of the corpus callosum with normal intelligence. Cognit Neuropsychiatry 5:135–157. doi:10.1080/135468000395781
Brown WS, Paul LK, Symington M, Dietrich R (2005) Comprehension of humor in primary agenesis of the corpus callosum. Neuropsychologia 43:906–916. doi:10.1016/j.neuropsychologia.2004.09.008
Chiarello C (1980) A house divided? Cognitive functioning with callosal agenesis. Brain Lang 11:128–158. doi:10.1016/0093-934X(80)90116-9
David AS, Wacharasindhu A, Lishman WA (1993) Severe psychiatric disturbance and abnormalities of the corpus callosum: review and case series. J Neurol Neurosurg Psychiatry 56:85–93
Dennis M (1981) Language in congenitally acallosal brain. Brain Lang 12:33–53. doi:10.1016/0093-934X(81)90004-3
Devinsky O, Laff R (2003) Callosal lesions and behavior: history and modern concepts. Epilepsy Behav 4:607–617. doi:10.1016/j.yebeh.2003.08.029
Duan X, Chang JH, Ge S et al (2007) Disrupted-in-schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell 130:1146–1158. doi:10.1016/j.cell.2007.07.010
Ettlinger G (1977) Agenesis of the corpus callosum. In: Vinken PJ, Bruyn GW (eds) Handbook of clinical neurology, vol 30. Elsevier, Amsterdam, pp 285–297
Fischer M, Ryan SB, Dobyns WB (1992) Mechanisms of interhemispheric transfer and patterns of cognitive function in acallosal patients of normal intelligence. Arch Neurol 49:271–277
Gilliam F, Wyllie E, Kotagal P, Geckler C, Rusyniak G (1996) Parental assessment of functional outcome after corpus callosotomy. Epilepsia 37:753–757. doi:10.1111/j.1528-1157.1996.tb00647.x
Gott PS, Saul RE (1978) Agenesis of the corpus callosum: limits of functional compensation. Neurology 28:1272–1279
Gundersen HJ, Jensen EB, Kieû K, Nielsen J (1999) The efficiency of systematic sampling in stereology—reconsidered. J Microsc 193:199–211. doi:10.1046/j.1365-2818.1999.00457.x
Hakeem AY, Sherwood CC, Bonar CJ, Hof PR, Allman JM (2008) Von Economo neurons in the elephant brain. Anat Rec (in press)
Hof PR, Van der Gucht E (2007) Structure of the cerebral cortex of the humpback whale, Megaptera novaeangliae (Cetacea, Mysticeti, Balaenopteridae). Anat Rec 290:1–31. doi:10.1002/ar.20407
Hoppe KB, Bogen JE (1977) Alexithymia in 12 commissurotomized patients. Psychother Psychosom 28:148–155
Jeeves MA (1994) Callosal agenesis—a natural split brain: overview. In: Lassonde M, Jeeves MA (eds) Callosal agenesis—a natural split brain?. Plenum Press, New York, pp 285–299
Jeeves MA, Temple CM (1987) A further study of language function in callosal agenesis. Brain Lang 32:325–335. doi:10.1016/0093-934X(87)90131-3
Jeret JS, Serur D, Wisniewski KE, Lubin RA (1987) Clinicopathological findings associated with agenesis of the corpus callosum. Brain Dev 9:255–264
Karama S, Lecours AR, Leroux J, Bourgouin P, Beaudoin G, Joubert S et al (2002) Areas of brain activation in males and females during viewing of erotic film excerpts. Hum Brain Mapp 16:1–13. doi:10.1002/hbm.10014
Lassonde M, Sauerwein C (1997) Neuropsychological outcome of corpus callosotomy in children and adolescents. J Neurosurg Sci 41:67–73. doi:10.1097/00006123-199707000-00014
Loeser JD, Alvord EC (1968) Agenesis of the corpus callosum. Brain 91:553–570. doi:10.1093/brain/91.3.553
Lough S, Kipps CM, Treise C, Watson P, Blair JR, Hodges JR (2006) Social reasoning, emotion and empathy in frontotemporal dementia. Neuropsychologia 44:950–958. doi:10.1016/j.neuropsychologia.2005.08.009
Meyer BU, Roricht S, Niehaus L (1998) Morphology of acallosal brains as assessed by MRI in six patients leading a normal daily life. J Neurol 245:106–110. doi:10.1007/s004150050187
Miller BL, Seeley WW, Mychack P, Rosen HJ, Mena I, Boone K (2001) Neuroanatomy of the self: evidence from patients with frontotemporal dementia. Neurology 57:817–821
Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR (1999) A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA 96:5268–5273. doi:10.1073/pnas.96.9.5268
Nimchinsky EA, Vogt BA, Morrison JH, Hof PR (1995) Spindle neurons of the human anterior cingulate cortex. J Comp Neurol 355:27–37. doi:10.1002/cne.903550106
O’Brien G (1994) The behavioral and developmental consequences of corpus callosum agenesis and Aicardi Syndrome. In: Lassonde M, Jeeves MA (eds) Callosal agenesis: a natural split brain?. Plenum, New York, pp 235–246
Paul LK, Brown WS, Adolphs R, Tyszka JM, Richards LJ, Mukherjee P et al (2007) Agenesis of the corpus callosum: genetic, developmental and functional aspects of connectivity. Nat Rev Neurosci 8:287–299. doi:10.1038/nrn2107
Paul LK, Schieffer B, Brown WS (2004) Social processing deficits in agenesis of the corpus callosum: narratives from the thematic appreciation test. Arch Clin Neuropsychol 19:215–225. doi:10.1016/S0887-6177(03)00024-6
Paul LK, Van Lancker-Sidtis D, Schieffer B, Dietrich R, Brown WS (2003) Communicative deficits in agenesis of the corpus callosum: nonliteral language and affective prosody. Brain Lang 85:313–324. doi:10.1016/S0093-934X(03)00062-2
Rakic P (1984) Emergence of neuronal and glial cell lineages in primate brain. In: Black IB (ed) Cellular and molecular biology of neural development. Plenum Press, New York
Rankin KP, Gorno-Tempini ML, Allison SC, Stanley CM, Glenn S, Weiner MW et al (2006) Structural anatomy of empathy in neurodegenerative disease. Brain 129:2945–2956. doi:10.1093/brain/awl254
Rose JE, Woolsey CN (1948) Structure and relations of limbic cortex and anterior thalamic nuclei in rabbit and cat. J Comp Neurol 89:279–347. doi:10.1002/cne.900890307
Rose JE, Woolsey CN (1958) Cortical connections and functional organization of thalamic auditory system of cat. In: Harlow HF, Woolsey CN (eds) Biological and biochemical bases of behavior. University of Wisconsin Press, Madison, pp 127–150
Samuelsen GB, Larsen KB, Bogdanovic N, Laursen H, Graem N, Larsen JF et al (2003) The changing number of cells in the human fetal forebrain and its subdivisions: a stereological analysis. Cereb Cortex 13:115–122. doi:10.1093/cercor/13.2.115
Sanders RJ (1989) Sentence comprehension following agenesis of the corpus callosum. Brain Lang 37:59–72. doi:10.1016/0093-934X(89)90101-6
Sauerwein HC, Nolin P, Lassonde M (1994) Cognitive functioning in callosal agenesis. In: Lassonde M, Jeeves MA (eds) Callosal agenesis: a natural split brain?. Plenum, New York, pp 221–233
Saul RE, Sperry RW (1968) Absence of commissurotomy symptoms with agenesis of the corpus callosum. Neurology 18:307
Schmitz C, Hof PR (2005) Design-based stereology in neuroscience. Neuroscience 130:813–831. doi:10.1016/j.neuroscience.2004.08.050
Seeley WW, Carlin DA, Allman JM, Macedo MN, Bush C, Miller BL et al (2006) Early frontotemporal dementia targets neurons unique to apes and humans. Ann Neurol 60:660–667. doi:10.1002/ana.21055
Seeley WW, Allman JM, Carlin DA et al (2007) Divergent social functioning in behavioral variant frontotemporal dementia and Alzheimer disease: reciprocal networks and neuronal evolution. Alzheimer Dis Assoc Disord 21:S50–S57
Shin LM, Dougherty DD, Orr SP, Pitman RK, Lasko M, Macklin ML et al (2000) Activation of anterior paralimbic structures during guilt-related script-driven imagery. Biol Psychiatry 48:43–50. doi:10.1016/S0006-3223(00)00251-1
Singer T, Seymour B, O’Doherty J, Kaube H, Dolan RJ, Frith CD (2004) Empathy for pain involves the affective but not sensory components of pain. Science 303:1157–1162. doi:10.1126/science.1093535
Singer T, Seymour B, O’Doherty JP, Stephan KE, Dolan RJ, Frith CD (2006) Empathic neural responses are modulated by the perceived fairness of others. Nature 439:466–469. doi:10.1038/nature04271
Snowden JS, Gibbons ZC, Blackshaw A, Doubleday E, Thompson J, Craufurd D et al (2003) Social cognition in frontotemporal dementia and Huntington’s disease. Neuropsychologia 41:688–701. doi:10.1016/S0028-3932(02)00221-X
Sokal RR, Rohlf FJ (1995) Biometry: the priciples and practice of statistics in biological research. W.H. Freeman, New York
Sturm VE, Rosen HJ, Allison S, Miller BL, Levenson RW (2006) Self-conscious emotion deficits in frontotemporal lobar degeneration. Brain 129:2508–2516. doi:10.1093/brain/awl145
Taylor M, David AS (1998) Agenesis of the corpus callosum: a United Kingdom series of 56 cases. J Neurol Neurosurg Psychiatry 64:131–134
Temple CM, Ilsley J (1993) Phonemic discrimination in callosal agenesis. Cortex 29:341–348
Temple CM, Jeeves MA, Vilarroya O (1989) Ten pen men: rhyming skills in two children with callosal agenesis. Brain Lang 37:548–564. doi:10.1016/0093-934X(89)90111-9
Temple CM, Jeeves MA, Vilarroya OO (1990) Reading in callosal agenesis. Brain Lang 39:235–253. doi:10.1016/0093-934X(90)90013-7
TenHouten WD, Hoppe KD, Bogen JE, Walter DO (1986) Alexithymia: an experimental study of cerebral commissurotomy patients and normal control subjects. Am J Psychiatry 143:312–316
Tomasch J (1954) Size, distribution, and number of fibers in the human corpus callosum. Anat Rec 119:119–135. doi:10.1002/ar.1091190109
Von Economo C, Koskinas G (1925) Die Cytoarchitectonik der Hirnrinde des erwachsenen Menschen. Springer
Watson KK, Jones TK, Allman JM (2006) Dendritic architecture of the von Economo neurons. Neuroscience 141:1107–1112. doi:10.1016/j.neuroscience.2006.04.084
Watson KK, Matthews BJ, Allman JM (2006) Brain activation during sight gags and language-dependent humor. Cereb Cortex (in press)
West MJ, Slomianka L, Gundersen HJG (1991) Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec 231:482–497. doi:10.1002/ar.1092310411
Zaidel DW (1995) A view of the world from a split-brain perspective. In: Critchley EMR (ed) The neurological boundaries of reality. Jason Aronson, Northvale, pp 161–174
Acknowledgments
We thank Archibald Fobbs, Curator of the Yakovlev Brain Collection, National Museum of Health and Medicine for his generous assistance in providing access to the acallosal and control specimens, and to Dr. D. Wolfe of the Mount Sinai School of Medicine for generously providing access to the cerebral infarction specimen. We also wish to thank Ralph Adolphs, Warren Brown and J. Michael Tyszka for their valuable discussions of this project. This research was generously funded by grants from the James S. McDonnell Foundation, the Gordon and Betty Moore Foundation, the David and Lucile Packard Foundation, and the Gustavus and Louise Pfeiffer Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kaufman, J.A., Paul, L.K., Manaye, K.F. et al. Selective reduction of Von Economo neuron number in agenesis of the corpus callosum. Acta Neuropathol 116, 479–489 (2008). https://doi.org/10.1007/s00401-008-0434-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0434-7