Abstract
We describe diffuse glioma-like infiltrates in excised tubers in five out of forty Tuberous sclerosis complex (TSC) patients undergoing excision of a tuber at our institution within the last 10 years. All patients presented with refractory seizures. Resection specimens from four patients had the pathognomonic histologic features of neuroglial hamartomas (tubers) and in one case there was cortical microdysgenesis lacking cells typical of TSC. All lesions were associated with an infiltrate of atypical, mostly elongate, glioma-like small cells, which were immunoreactive for GFAP in three, and pS6 (a marker for activity of the mTOR pathway), in two cases. MAP-2 and CD34, were negative and MIB-1 (Ki67) immunostains ranged from <1–21%. Array-based comparative genomic hybridization revealed that these proliferative phenomena were associated with 21 different copy number aberrations in comparison with a tuber without atypical infiltrates. Postoperatively (follow-up period ranging from 8 to 34 months) none of the patients have any evidence of a glioma. We report that tubers resected for treatment of seizures are sometimes associated with glioma-like lesions, which are indistinguishable from infiltrating gliomas by morphology and immunohistochemistry. Genomic analysis with SNP arrays revealed copy number changes which may be associated with the pathogenesis of such infiltrates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tuberous sclerosis complex (TSC) is an autosomal dominant multisystem genetic disorder often characterized by severe epilepsy. With the evolution of neurosurgical interventions for treatment of epilepsy refractory to medical therapy, TSC-associated resection specimens are commonly seen in surgical neuropathological practice, particularly in centers where epilepsy surgery is performed [58]. In this context, neuroglial hamartomas (historically referred to as “tubers”) are a frequently encountered histologic substrate of a clinical seizure disorder. Pathological features of tubers include architectural disarray of the cortex with loss of lamination, occurrence in the cortex and underlying white matter of abnormally enlarged neuron-like cells with glassy eosinophilic cytoplasm and large euchromatic neuron-like single or multiple nuclei (so-called tuberous sclerosis cells, often also referred to as giant cells) and enlarged reactive astrocytes [47, 57]. In the majority of cases encountered in our practice, these characteristic features confirm the clinical and radiological diagnosis of tuber. However, we have found several cases where these hamartomatous features were paired with a disturbing hypercellularity and/or an infiltrate of small atypical glioma-like cells. These findings prompted us to perform additional diagnostic workup using immunohistochemistry and array-based comparative genomic hybridization, in order to further characterize these lesions and evaluate the possibility of a diffusely infiltrating glioma.
Herein, we present the cases of five patients with such glioma-like infiltrates.
Material and methods
Institutional Review Board approval was obtained for the study. A retrospective review of H&E stained slides of excision specimens identified through the electronic surgical neuropathology files from 1997 to 2007 was performed. Pertinent clinical information including imaging findings and status at last follow-up was obtained from the neurosurgical offices.
All pertinent histopathologic slides, including immunostains as well as H&E stains and stains for myelin (Luxol fast blue/H&E) were jointly reviewed by the three neuropathologist authors (IF, CC, DCM). Additionally performed immunostains for hamartin, tuberin, pS6, CD34, and MAP-2 were reviewed by two neuropathologist authors (IF, CC). Proliferation indices for Ki67 (MIB1) immunostains were counted with Image Pro Express, Media Cybernetics image analysis software. One high-power field (10× × 40×) in the area of maximal labeling with Ki-67 (excluding areas around depth-electrode tracks) was counted for each case.
Immunostains were all performed with a Ventana automated immunostainer (Ventana Medical Systems, Tucson, AZ). Primary antibodies included those for vimentin (Ventana), GFAP (Dako, Carpinteria, CA), Synaptophysin (Dako), neurofilament protein (antibody RMDO20, Zymed, Carlsbad, CA), Neu-N (Chemicon, Temecula, CA), leukocyte common antigen (LCA, CD45, Ventana), CD68 (antibody KP1, Dako), Ki67 (MIB1, Ventana), CD34 (Ventana), MAP-2 (Sigma), and phosphorylated ribosomal protein S6 (Cell Signaling, Danvers, MA). Primary antibodies for hamartin and tuberin were kindly provided by Mizuguchi [42]. Both antibodies were raised by immunizing rabbits against peptides synthesized according to the sequence deduced from human tuberous sclerosis gene 1 and 2 cDNA, respectively, as described in detail elsewhere [42].
Array-based comparative genomic hybridization (Affymetrix mapping 500K array set)
Sufficient amounts of paraffin-embedded tissue for DNA extraction were available from four cases. Areas with glioma-like infiltrates were dissected from 50 μm paraffin-embedded sections with the aid of a microscope. One case of a tuber without a glioma-like infiltrate was used as a control.
DNA extraction
Samples were deparaffinized with xylene. DNA extraction was done with a QIAamp® micro kit (QIAGEN) according to the manufacturer’s instructions. The extractions were modified to include an initial incubation at 95°C for 15 min followed by 5 min at room temperature as described previously [61], before being digested with proteinase K for 3 days at 56°C in a water-bath with addition of fresh enzyme at 24 h intervals.
DNA quality assessment and preparation
The extracted DNA was quantified using a Nanodrop machine (Thermo Scientific). Multiplex PCR was done to assess the quality of DNA. The QIAGEN® multiplex PCR kit was used with a set of primers to amplify various regions of the GAPDH gene. Products were visualized with ethidium bromide on a 1% gel.
Preparation and application of DNA to the mapping arrays
Samples were analyzed on the Affymetrix GeneChip® human mapping 250K Nsp assay kit and the mapping 250K Sty assay kit [59] and hybridized to the 250K arrays. The only deviation from the manufacturer’s protocol consisted of the addition of five cycles to the PCR cycling conditions. The 500K assay was done according to the manufacturer’s protocol, beginning with 250 ng DNA. Exactly 90 μg of PCR product were fragmented and labeled, using additional PCRs as necessary for formalin-fixed samples.
Copy number analysis
Raw intensity files (.cel) of Affymetrix 500K mapping arrays were analyzed using Affymetrix Genotyping Console v2.1 under default parameters. A reference set of 100 HAPMAP samples (GEO record GSE5173) were used to obtain ratiometric measures from the single color array profiles. Copy number regions of amplifications and deletions were reported when event loci overlapped in multiple test samples but not in the control.
Results
Patients
Over a period of 10 years, 40 patients diagnosed with TSC underwent a resection of one or more tubers at our institution. Among these were five patients whose lesions were found to have hypercellularity with appearances resembling infiltrating fibrillary astrocytomas and were thus diagnosed as lesions suspicious for glioma, or (in one instance) as a diffusely infiltrating glioma. One patients’ clinical history and outcome was published previously [58]. The clinical histories are also summarized in Table 1.
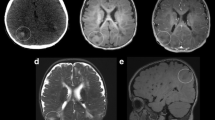
Patient age at the time of surgery ranged from 5 to 17 years. Four patients were male, one female. All patients presented with refractory partial seizures and met the clinical criteria for a diagnosis of TSC; all patients had a history of seizures since infancy and global developmental delay. Four patients had cardiac rhabdomyomas, and one patient had ash-leaf spots and adenoma sebaceum. MRI studies revealed cortical tubers (consisting of cortical and subcortical foci of increased T2 signal intensity) in all patients, and additionally, contrast enhancing subependymal nodules in three patients and subependymal giant cell astrocytomas in three patients.
Patients 1–4 underwent a three-stage craniotomy. This consisted of [1] an initial surgery for implantation of subdural grid and strip electrodes (Adtech, Inc., Racine, WI), followed by extraoperative video-EEG monitoring to specifically localize the ictal onset zone and map sensory and motor cortex [2], seizure focus resection and electrode reimplantation, a second extraoperative monitoring period, and [3] a final operation for further resection and electrode removal. Patient 5 underwent a 3-stage left frontal craniotomy with invasive monitoring and resection of the epileptogenic tuber. Unfortunately, additional monitoring after tuber resection captured persistent ictal activity from uncovered multiple left-sided secondary seizure foci. The patient underwent an anterior corpus callosotomy at the third stage as a palliative procedure.
Patient 4 had undergone previous resections; 6 years prior, he had undergone resections of tubers in the left parietal, temporal, and occipital lobes at an outside institution. Four years before surgery at NYU, multiple subpial transections in the left temporal lobe were performed, which resulted in a modest improvement in seizure frequency.
Two of the five patients (patients 2 and 3) were known to have mutations of the TSC2 gene. The other three patients did not have genetic testing.
At last clinical follow-up ranging from 8 to 34 months, none of the patients had any new areas of enhancement or progressive T2 signal change suspicious for a glioma. Two patients were free of seizures and three patients had a reduction in seizures compared to the pre-operative period.
Histological findings
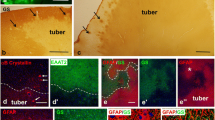
In all but one patient, typical neuroglial hamartomas (tubers) could be identified, consisting of cortical architectural disarray with enlarged, dysmorphic neurons (TSC cells, Fig. 1b) which were variably labeled with neuronal markers (Neu-N, synaptophysin, neurofilament protein, MAP-2) and the glial marker GFAP. Additional immunostains for hamartin, tuberin, and pS6 also labeled the cytoplasm of enlarged, dysmorphic cells in all cases (Fig. 1g, h). A CD34 immunostain demonstrated labeling of a single enlarged neuronal-type cell in a granular cytoplasmic fashion in one case, as previously described for ganglion cells in ganglioglioma [6, 10]. In the other four cases, CD34 stained vascular endothelia only (Fig. 1f).
Case 3: MRI revealed multifocal bihemispheric cortical/subcortical regions of hyperintensity on T2 (a). Histologically, there were large “balloon cells” (arrows) typical of a “tuber” (b, H&E, 40×), but there was also striking cellularity (c, H&E 40×). There were cells resembling gliomatosis-like cells with elongate irregular nuclei (arrows), which were labeled by a GFAP immunostain (d, GFAP 40×). LCA revealed a scattered inflammatory infiltrate (e, 40×). CD34 labels endothelial cells only (f, 40×). TSC cells are highlighted in the background of infiltrating cells by the pS6 immunostain (g, 20×). Tuberin also marks TSC cells (h, 40×)
In addition, in each patient specimen there was an infiltrate of atypical, irregularly shaped gliomatosis-cerebri-like cells with mostly elongate, but in some cases focally oval to round nuclei. These cells resembled “naked nuclei” with no discernible cytoplasm on H&E stains which were seen to infiltrate among cortical neurons in well-ordered as well as dysplastic areas, and were also present in the underlying white matter (Figs. 1c, 2a). Immunohistochemical characteristics of these infiltrates are summarized in Table 2.
Case 5: a foci of atypical elongate cells with scant surrounding cytoplasm infiltrating among neuronal cells (arrows, H&E 40×), not labeled with a GFAP immunostain (b, 40×). A MIB-1 immunostain labels upto 10% of nuclei (c, Ki-67, 40×).d MAP-2 highlights dysmorphic neuronal cells (40×). e p53 labels occasional nuclei (40×). f Many infiltrating cells are labeled with a pS6 immunostain, as are the giant cells of the tuber (inset, 40×)
There was immunopositivity of a portion of these cells with GFAP in three cases, but no labelng with Neu-N, synaptophysin, neurofilament protein, MAP-2, CD34, hamartin, tuberin, pS6, CD68 (Kp-1) and CD45 (LCA) immunostains (Figs. 1d–h, 2d, f), while the MIB-1 labeling index was low (<1%) in one case, the other four cases had MIB-1 labeling indices ranging from 8 to 21% (Fig. 2c). P53 immunostains revealed focal immunopositivity in two cases (Fig. 2e). However, in one of these, there was also labeling of TSC cells with p53 (Fig. 2e).
Immunostains for CD68 (Kp-1) and CD45 (LCA) served to highlight inflammatory changes associated with the placement of electrodes for recording purposes and also revealed a mild microglial activation in all resection specimens. However, only a very small percentage of atypical appearing infiltrating cells were labeled with these markers.
Comparative genomic hybridization
Each of the four cases tested and the control case displayed copy number variations on >100 locations of the genome (Fig. 3). Copy number losses were overall more frequent than copy number gains. The control case had fewer losses than all the study group cases and fewer copy number gains than two of the cases with glioma-like infiltrates. A case-by-case summary of chromosomes with copy number variations is given in Table 3.
In comparison to the control, the four cases with glioma-like proliferations had copy number losses involving 15 genes and a copy number gain involving one gene. Additional overlapping copy number losses affecting three of four study group cases were detected in chromosomal regions involving five genes. A list of these genes, encoded proteins and their functions is provided in Table 4. None of these aberrations occurred in genomic regions with known copy number variations.
Copy number losses were identified in genes implicated in cell cycle regulation (CLSPN, PES1, TBRG4), cell adhesion and motility (MYO1G, FAT), regulation of transcription (EIF2C1, EIF2C4, ZMIZ2, PURB, H2AFV, ZBTB38), protein transport (SEC14L3, SEC14L4), metabolism (GAL3ST1, TCN2, PPIA), and CNS vascular development (CCM2). The function of four additional genes with copy number variations identified in samples with glioma-like proliferations compared with the control has not been clarified. These copy number aberrations each spanned lesser than 1 Mb. No variations were detected in the chromosomal regions of the TSC1 and TSC2 genes.
Discussion
With this small case series, we report the presence of histologic features indistinguishable from diffusely infiltrating gliomas in patients with documented TSC and a clinical presentation of refractory seizures.
Histology and immunohistochemistry
“Cellularity” and “glial proliferations” in tubers have been described in the literature [18, 47, 57]; however, the constitutive cells in these cases consisted of enlarged stellate astrocytes and not irregular “naked nuclei” or microglial-like cells as in the cases presented here. Furthermore, the absence of expression of neuronal markers in the infiltrating cells in our case argues against these cells being simply heterotopic (non-migrated) cortical neurons. Likewise, a majority of the cells could not be characterized as microglial in nature by immunohistochemistry. Therefore, the histological findings cannot be wholly ascribed to a reactive inflammatory process. All of our patients had more than one resection. We found these hypercellular zones in the first surgical specimens, and/or at considerable distance from reactions next to prior surgical margins.
In three of five cases, we observed expression of GFAP in a portion of the infiltrating cells, raising the possibility of these infiltrates being low-grade diffuse astrocytoma. Of interest, an isomorphic subtype of astrocytoma with a benign long-term prognosis has been described in patients with long-standing epilepsy [8, 55]. Affected individuals in the reported clinical series were adults without a history of TSC. Similarly to our cases, these lesions are characterized by a homogeneous infiltrate of GFAP-positive, MAP-2 negative astrocytic-type cells without mitotic activity and a low MIB-1 labeling index. Patients in this series had progression-free survival times of upto 13 years, demonstrating a better prognosis of this subtype than for other more “typical” astrocytic tumors composed of MAP-2 positive cells.
In this context, the neuronal marker MAP-2 has been described as a useful tool to distinguish low-grade astrocytic from mixed glioneuronal lesions, i.e., gangliogliomas, which are also associated with epilepsy and can present a differential diagnostic challenge, while diffuse astrocytomas are usually MAP-2 positive, the glial component of gangliogliomas is not [8, 9]. Another helpful marker in this distinction is CD34, which highlights dysmorphic ganglion cells of ganglioglioma [10]. “Isomorphic gliomas” associated with long-term seizures differ from more typical low-grade astrocytomas in their absence of MAP-2 immunoreactivity, as seen in our cases.
There was no expression of CD34 in the resection specimens presented herein except for vascular endothelia and a single dysmorphic ganglion cell in one case, indicating that the infiltrates we observe are not merely the glial component of gangliogliomas [10]. Expression of the CD34 epitope also occurs in approximately half of cortical dysplasias and neuroglial hamartomas. Hence the expression in a large dymsorphic cell indicates its malformative nature [6].
The tumor suppressor genes TSC1 or TSC2 (encoding for hamartin and tuberin, respectively) carry mutations in patients with TSC [2, 52, 56].
Physiologically, the hamartin and tuberin proteins form an intracellular complex which blocks activation of mTOR (mammalian target of Rapamycin) [31]. When function of the TSC protein complex is lost, activation of mTOR leads to phosphorylation of activation of ribosomal protein S6. In the cases presented here, there was an expression of hamartin and tuberin, as well as pS6 in the giant cells of tubers. These findings are in agreement with existing published data on the immunohistochemical profile of brain lesions in TSC [5, 15, 26, 46]. In two cases we found expression of pS6 in the glioma-like infiltrating cells. The significance of this observation is unclear, since this protein is also expressed in gliomas [49].
Four out of five cases had focally high proliferation indices, which is concerning, but does not prove that this is caused by a neoplastic process. After all, the mTOR pathway which is pathologically activated in TSC, has a critical role in regulation of cell growth. Furthermore, the TSC complex positively regulates the cyclin-dependent kinase inhibitor p27, indicating a potential deregulation of cell proliferation with loss of function of the TSC protein complex [50].
A p53 immunostain labeled infiltrating cells in two cases, but the fact that nuclei that were part of what was recognized as “tuber” also were labeled warrants caution in its interpretation. Furthermore, physiological crosstalk between p53 and mTOR pathways is documented, raising the possibility that focal immunopositivity for p53 represents a TSC-associated, non-neoplastic phenomenon [22].
Gliomas (other than SEGAs) occur rarely in tuberous sclerosis patients. Al-Saleem et al. [1] reported a glioblastoma in a 22 month-old child with TSC who had multiple cortical tubers which were separated from the neoplastic process. In contrast, a case report by Eren et al. [21] describes a diffuse fibrillary astrocytoma growing in or apparently arising from a tuber. CT scans in a 4-year-old girl with tuberous sclerosis demonstrated growth of a parieto-occipital tuber over the course of a few months. Histologically, the lesion consisted of mostly spindle cells with atypical nuclei forming a cellular lesion in the background of dysmorphic enlarged neuronal and astrocytic cells interpreted as a pre-existing tuber. In this particular case, the lesion had frankly neoplastic features, was very cellular and included mitotic figures. Since the reports of diffusely infiltrating gliomas in TSC patients are very rare, their occurrence in this setting may be coincidental.
The nature and cause of the excess cellularity in our TSC patients remain unclear. Cells were immunoreactive with GFAP in three cases, and pS6 in two, indicating the activation of mTOR pathway in the infiltrating cells as in the components of the tuber, but not with CD34, MAP-2, or other neuronal markers, which almost exclusively highlighted dysmorphic neurons in the tubers.
It is possible that these are components of “isomorphic gliomas” as described in patients with long-standing epilepsy by Blumcke and others [7, 8]. The short post-operative follow-up in our series hinders the classification of these processes based on the long-term clinical behavior.
However, the lesions we observed could also represent a non-neoplastic tuberous sclerosis-associated phenomenon. It has been demonstrated that tubers are in fact dynamic in nature, expressing markers of cellular proliferation and subject to recruitment of new cells into the lesion [17, 35]. Such additional constituent cells, however, cannot explain the histologic phenomenon we observe, since such “newly recruited” cells have been described to be giant cells or dysmorphic neurons by morphology and stain with neuronal markers.
There is not only a morphological, but also a pathogenetic overlap between TSC-associated lesions and diffusely infiltrating gliomas. For example, the protein products of the TSC1 and TSC2 genes, hamartin and tuberin, are considered to function as tumor suppressors. The TSC proteins form a complex which regulates cell growth through the mTOR signaling pathway [43], which is also implicated in the development of diffusely infiltrating gliomas [16]. Accordingly, the mTOR-inhibiting drug CCI-779 was effective in clinical trials for the treatment of glioblastoma and also for the treatment of tuberous sclerosis in vivo [25, 37].
Array-based comparative genomic hybridization
The high number of copy number variations detected in all samples including the control is consistent with existing data on the frequency of submicroscopic structural variants in the genome. While some of these structural variants are of no consequence, others are etiological factors of disease [23].
Considering the specific gene loci identified in our samples as potential candidate genes responsible for observed proliferative phenomena, it is perhaps significant that not only could some of them be linked to a neoplasm by virtue of their function (for example the cell cycle regulators CLSPN, PES1, and TBRG4), but also based on existing data. For example, a deletion of the purine-rich element binding protein B has been found in 60 patients with myelodysplastic syndrome and acute myelogenous leukemia [38].
One case of a multifocal anaplastic astrocytoma associated with transcobalamin 2 deficiency has been reported, even though such deficiency was caused by a mutation in the functionally connected PMS2 gene in the particular case [27].
Of the genes with to date unknown function, C16ORF45 is of particular interest, because a loss was identified with identical (or at least closely approximated) breakpoints in all four samples, involving a single gene. This homogeneity across our samples indicates that this is not likely a coincidental finding. While the encoded protein has not been identified, a GEO database [4] search revealed that a copy number loss involving this gene has been found in glioblastomas compared to normal control [13, 14].
Data on submicroscopic (less than 1 Mb) copy number variations in TSC patients for comparison with our data are not available in the literature. Karyotype-based CGH data on patients with TSC are sparse. Only data of chromosomal imbalances in subependymal giant cell astrocytomas are published. Rickert and Paulus [48] found no DNA copy number changes and no mutations in TSC-associated genes in eight patients. However, a correlation of our findings with karyotype-based CGH is not possible because the 21 overlapping aberrations detected involve DNA fragments of submicroscopic length.
One study of genomic DNA of seizure patients (not including TSC patients) using array-based CGH revealed frequent gains on chromosomes 1p, 5p, 8q, 10q, and losses on chromosome 7q [32]. None of the involved genes in that study are identical with the candidate genes we identified.
More abundant data exist on genomic aberrations in gliomas. A recent analysis of 112 gliomas using BAC array CGH demonstrated that known genomic imbalances (1p, 19q loss, EGFR amplification) in gliomas tend to cluster, distinguishing three genetic subsets [30], thus validating the use of array-based CGH as a method of genomic profiling. The array used has a resolution of 1 Mb, thus explaining why the study found genomic aberrations previously identified with karyotype-based approaches [32]. Another microarray-based CGH study on 50 gliomas including glioblastomas, mixed oligoastrocytomas, and oligodendrogliomas identified 351 genes linked to gliomagenesis [14]. In a similar approach, Ruano [51] and others found novel amplified genes on chromosomes 4p15 and 13q32–34 in glioblastoma multiforme. The 21 genes with copy number variations in our samples are not among these. Considering that a possible glioma in our samples would be low-grade, it is perhaps not surprising that our findings differ from the above described studies except for the copy number loss in the C16ORF45 gene.
In low-grade gliomas, Wong [60] and associates identified copy number losses in genes involved in cell cycle regulation different from those found in our samples.
The copy number variations we identified do not allow us to distinguish between a neoplasm and a non-neoplastic process in our cases, since no data on TSC cases is available for comparison.
Summary and conclusion
The cases presented here have histologic features suggestive, but not diagnostic of a neoplasm. There is some similarity to previously described “isomorphic gliomas” in patients with long-standing epilepsy. Genomic profiling revealed copy number aberrations that could be the basis of a proliferative phenomenon, although it is also possible that these are associated with TSC. Further studies are needed to clarify the nature and pathogenesis of glioma-like infiltrates in tubers.
References
Al-Saleem T, Wessner LL, Scheithauer BW, Patterson K, Roach ES, Dreyer SJ et al (1998) Malignant tumors of the kidney, brain, and soft tissues in children and young adults with the tuberous sclerosis complex. Cancer 83(10):2208–2216. doi :10.1002/(SICI)1097-0142(19981115)83:10<2208::AID-CNCR21>3.0.CO;2-K
Ali JB, Sepp T, Ward S, Green AJ, Yates JR (1998) Mutations in the TSC1 gene account for a minority of patients with tuberous sclerosis. J Med Genet 35(12):969–972
Avramut M, Achim CL (2003) Immunophilins in nervous system degeneration and regeneration. Curr Top Med Chem 3(12):1376–1382. doi:10.2174/1568026033451871
Barrett T, Troup DB, Wilhite SE, Ledoux P, Rudnev D, Evangelista C et al (2007) NCBI GEO: mining tens of millions of expression profiles—database and tools update. Nucleic Acids Res 35(database issue):D760–D765. doi:10.1093/nar/gkI887
Baybis M, Yu J, Lee A, Golden JA, Weiner H, McKhann G 2nd et al (2004) mTOR cascade activation distinguishes tubers from focal cortical dysplasia. Ann Neurol 56(4):478–487. doi:10.1002/ana.20211
Blumcke I, Giencke K, Wardelmann E, Beyenburg S, Kral T, Sarioglu N et al (1999) The CD34 epitope is expressed in neoplastic and malformative lesions associated with chronic, focal epilepsies. Acta Neuropathol 97(5):481–490. doi:10.1007/s004010051017
Blumcke I, Lobach M, Wolf HK, Wiestler OD (1999) Evidence for developmental precursor lesions in epilepsy-associated glioneuronal tumors. Microsc Res Tech 46(1):53–58. doi :10.1002/(SICI)1097-0029(19990701)46:1<53::AID-JEMT5>3.0.CO;2-0
Blumcke I, Luyken C, Urbach H, Schramm J, Wiestler OD (2004) An isomorphic subtype of long-term epilepsy-associated astrocytomas associated with benign prognosis. Acta Neuropathol 107(5):381–388. doi:10.1007/s00401-004-0833-3
Blumcke I, Muller S, Buslei R, Riederer BM, Wiestler OD (2004) Microtubule-associated protein-2 immunoreactivity: a useful tool in the differential diagnosis of low-grade neuroepithelial tumors. Acta Neuropathol 108(2):89–96. doi:10.1007/s00401-004-0873-8
Blumcke I, Wiestler OD (2002) Gangliogliomas: an intriguing tumor entity associated with focal epilepsies. J Neuropathol Exp Neurol 61(7):575–584
Boyne MT 2nd, Pesavento JJ, Mizzen CA, Kelleher NL (2006) Precise characterization of human histones in the H2A gene family by top down mass spectrometry. J Proteome Res 5(2):248–253. doi:10.1021/pr050269n
Braun GS, Kretzler M, Heider T, Floege J, Holzman LB, Kriz W et al (2007) Differentially spliced isoforms of FAT1 are asymmetrically distributed within migrating cells. J Biol Chem 282(31):22823–22833. doi:10.1074/jbc.M701758200
Bredel M, Bredel C, Juric D, Duran GE, Yu RX, Harsh GR et al (2006) Tumor necrosis factor-alpha-induced protein 3 as a putative regulator of nuclear factor-kappaB-mediated resistance to O6-alkylating agents in human glioblastomas. J Clin Oncol 24(2):274–287. doi:10.1200/JCO.2005.02.9405
Bredel M, Bredel C, Juric D, Harsh GR, Vogel H, Recht LD et al (2005) Functional network analysis reveals extended gliomagenesis pathway maps and three novel MYC-interacting genes in human gliomas. Cancer Res 65(19):8679–8689. doi:10.1158/0008-5472.CAN-05-1204
Catania MG, Johnson MW, Liau LM, Kremen TJ, de Vellis JS, Vinters HV (2001) Hamartin expression and interaction with tuberin in tumor cell lines and primary cultures. J Neurosci Res 63(3):276–283. doi :10.1002/1097-4547(20010201)63:3<276::AID-JNR1021>3.0.CO;2-A
Choe G, Horvath S, Cloughesy TF, Crosby K, Seligson D, Palotie A et al (2003) Analysis of the phosphatidylinositol 3′-kinase signaling pathway in glioblastoma patients in vivo. Cancer Res 63(11):2742–2746
Crino PB (2004) Molecular pathogenesis of tuber formation in tuberous sclerosis complex. J Child Neurol 19(9):716–725
DiMario FJ Jr (2004) Brain abnormalities in tuberous sclerosis complex. J Child Neurol 19(9):650–657
Doi N, Zenno S, Ueda R, Ohki-Hamazaki H, Ui-Tei K, Saigo K (2003) Short-interfering-RNA-mediated gene silencing in mammalian cells requires Dicer and eIF2C translation initiation factors. Curr Biol 13(1):41–46. doi:10.1016/S0960-9822(02)01394-5
Edwards MC, Liegeois N, Horecka J, DePinho RA, Sprague GF Jr, Tyers M et al (1997) Human CPR (cell cycle progression restoration) genes impart a far-phenotype on yeast cells. Genetics 147(3):1063–1076
Eren S, Polat P, Erman Z (2002) An unusual tuberous sclerosis case presenting with fibrillary astrocytoma. Pediatr Neurosurg 37(3):118–121. doi:10.1159/000064397
Feng Z, Zhang H, Levine AJ, Jin S (2005) The coordinate regulation of the p53 and mTOR pathways in cells. Proc Natl Acad Sci USA 102(23):8204–8209. doi:10.1073/pnas.0502857102
Feuk L, Carson AR, Scherer SW (2006) Structural variation in the human genome. Nat Rev Genet 7(2):85–97. doi:10.1038/nrg1767
Filion GJ, Zhenilo S, Salozhin S, Yamada D, Prokhortchouk E, Defossez PA (2006) A family of human zinc finger proteins that bind methylated DNA and repress transcription. Mol Cell Biol 26(1):169–181. doi:10.1128/MCB.26.1.169-181.2006
Galanis E, Buckner JC, Maurer MJ, Kreisberg JI, Ballman K, Boni J et al (2005) Phase II trial of temsirolimus (CCI-779) in recurrent glioblastoma multiforme: a North Central Cancer Treatment Group Study. J Clin Oncol 23(23):5294–5304. doi:10.1200/JCO.2005.23.622
Grajkowska W, Kotulska K, Matyja E, Larysz-Brysz M, Mandera M, Roszkowski M et al (2008) Expression of tuberin and hamartin in tuberous sclerosis complex-associated and sporadic cortical dysplasia of Taylor’s balloon cell type. Folia Neuropathol 46(1):43–48
Gururangan S, Frankel W, Broaddus R, Clendenning M, Senter L, McDonald M et al (2008) Multifocal anaplastic astrocytoma in a patient with hereditary colorectal cancer, transcobalamin II deficiency, agenesis of the corpus callosum, mental retardation, and inherited PMS2 mutation. Neurooncol 10(1):93–97. doi:10.1215/15228517-2007-037
Honke K, Tsuda M, Hirahara Y, Ishii A, Makita A, Wada Y (1997) Molecular cloning and expression of cDNA encoding human 3′-phosphoadenylylsulfate:galactosylceramide 3′-sulfotransferase. J Biol Chem 272(8):4864–4868. doi:10.1074/jbc.272.8.4864
Huang CY, Beliakoff J, Li X, Lee J, Sharma M, Lim B et al (2005) hZimp7, a novel PIAS-like protein, enhances androgen receptor-mediated transcription and interacts with SWI/SNF-like BAF complexes. Mol Endocrinol 19(12):2915–2929. doi:10.1210/me.2005-0097
Idbaih A, Marie Y, Lucchesi C, Pierron G, Manie E, Raynal V et al (2008) BAC array CGH distinguishes mutually exclusive alterations that define clinicogenetic subtypes of gliomas. Int J Cancer 122(8):1778–1786. doi:10.1002/ijc.23270
Jozwiak J, Jozwiak S, Grzela T, Lazarczyk M (2005) Positive and negative regulation of TSC2 activity and its effects on downstream effectors of the mTOR pathway. Neuromolecular Med 7(4):287–296. doi:10.1385/NMM:7:4:287
Kim HS, Yim SV, Jung KH, Zheng LT, Kim YH, Lee KH et al (2007) Altered DNA copy number in patients with different seizure disorder type: by array-CGH. Brain Dev 29(10):639–643. doi:10.1016/j.braindev.2007.04.006
Kinoshita Y, Jarell AD, Flaman JM, Foltz G, Schuster J, Sopher BL et al (2001) Pescadillo, a novel cell cycle regulatory protein abnormally expressed in malignant cells. J Biol Chem 276(9):6656–6665. doi:10.1074/jbc.M008536200
Koesters R, Adams V, Betts D, Moos R, Schmid M, Siermann A et al (1999) Human eukaryotic initiation factor EIF2C1 gene: cDNA sequence, genomic organization, localization to chromosomal bands 1p34–p35, and expression. Genomics 61(2):210–218. doi:10.1006/geno.1999.5951
Lee A, Maldonado M, Baybis M, Walsh CA, Scheithauer B, Yeung R et al (2003) Markers of cellular proliferation are expressed in cortical tubers. Ann Neurol 53(5):668–673. doi:10.1002/ana.10579
Lee J, Kumagai A, Dunphy WG (2003) Claspin, a Chk1-regulatory protein, monitors DNA replication on chromatin independently of RPA, ATR, and Rad17. Mol Cell 11(2):329–340. doi:10.1016/S1097-2765(03)00045-5
Lee L, Sudentas P, Donohue B, Asrican K, Worku A, Walker V et al (2005) Efficacy of a rapamycin analog (CCI-779) and IFN-gamma in tuberous sclerosis mouse models. Genes Chromosomes Cancer 42(3):213–227. doi:10.1002/gcc.20118
Lezon-Geyda K, Najfeld V, Johnson EM (2001) Deletions of PURA, at 5q31, and PURB, at 7p13, in myelodysplastic syndrome and progression to acute myelogenous leukemia. Leukemia 15(6):954–962. doi:10.1038/sj.leu.2402108
Lin SY, Li K, Stewart GS, Elledge SJ (2004) Human Claspin works with BRCA1 to both positively and negatively regulate cell proliferation. Proc Natl Acad Sci USA 101(17):6484–6489. doi:10.1073/pnas.0401847101
Liquori CL, Berg MJ, Squitieri F, Leedom TP, Ptacek L, Johnson EW et al (2007) Deletions in CCM2 are a common cause of cerebral cavernous malformations. Am J Hum Genet 80(1):69–75. doi:10.1086/510439
Maiorana A, Tu X, Cheng G, Baserga R (2004) Role of pescadillo in the transformation and immortalization of mammalian cells. Oncogene 23(42):7116–7124. doi:10.1038/sj.onc.1207916
Mizuguchi M, Takashima S (2001) Neuropathology of tuberous sclerosis. Brain Dev 23(7):508–515. doi:10.1016/S0387-7604(01)00304-7
Narayanan V (2003) Tuberous sclerosis complex: genetics to pathogenesis. Pediatr Neurol 29(5):404–409. doi:10.1016/j.pediatrneurol.2003.09.002
Petit N, Blecon A, Denier C, Tournier-Lasserve E (2006) Patterns of expression of the three cerebral cavernous malformation (CCM) genes during embryonic and postnatal brain development. Gene Expr Patterns 6(5):495–503. doi:10.1016/j.modgep. 2005.11.001
Pezacka EH, Jacobsen DW, Luce K, Green R (1992) Glial cells as a model for the role of cobalamin in the nervous system: impaired synthesis of cobalamin coenzymes in cultured human astrocytes following short-term cobalamin-deprivation. Biochem Biophys Res Commun 184(2):832–839. doi:10.1016/0006-291X(92)90665-8
Plank TL, Logginidou H, Klein-Szanto A, Henske EP (1999) The expression of hamartin, the product of the TSC1 gene, in normal human tissues and in TSC1- and TSC2-linked angiomyolipomas. Mod Pathol 12(5):539–545
Richardson EP Jr (1991) Pathology of tuberous sclerosis. Neuropathologic aspects. Ann N Y Acad Sci 615:128–139. doi:10.1111/j.1749-6632.1991.tb37755.x
Rickert CH, Paulus W (2002) No chromosomal imbalances detected by comparative genomic hybridisation in subependymal giant cell astrocytomas. Acta Neuropathol 104(2):206–208. doi:10.1007/s00401-002-0544-6
Riemenschneider MJ, Betensky RA, Pasedag SM, Louis DN (2006) AKT activation in human glioblastomas enhances proliferation via TSC2 and S6 kinase signaling. Cancer Res 66(11):5618–5623. doi:10.1158/0008-5472.CAN-06-0364
Rosner M, Freilinger A, Hengstschlager M (2006) The tuberous sclerosis genes and regulation of the cyclin-dependent kinase inhibitor p27. Mutat Res 613(1):10–16. doi:10.1016/j.mrrev.2006.03.001
Ruano Y, Mollejo M, Ribalta T, Fiano C, Camacho FI, Gomez E et al (2006) Identification of novel candidate target genes in amplicons of Glioblastoma multiforme tumors detected by expression and CGH microarray profiling. Mol Cancer 5:39. doi:10.1186/1476-4598-5-39
Sancak O, Nellist M, Goedbloed M, Elfferich P, Wouters C, Maat-Kievit A et al (2005) Mutational analysis of the TSC1 and TSC2 genes in a diagnostic setting: genotype–phenotype correlations and comparison of diagnostic DNA techniques in tuberous sclerosis complex. Eur J Hum Genet 13(6):731–741. doi:10.1038/sj.ejhg.5201402
Sasaki T, Shiohama A, Minoshima S, Shimizu N (2003) Identification of eight members of the Argonaute family in the human genome small star, filled. Genomics 82(3):323–330. doi:10.1016/S0888-7543(03)00129-0
Scherer SW, Cheung J, MacDonald JR, Osborne LR, Nakabayashi K, Herbrick JA et al (2003) Human chromosome 7: DNA sequence and biology. Science 300(5620):767–772. doi:10.1126/science.1083423
Schramm J, Luyken C, Urbach H, Fimmers R, Blumcke I (2004) Evidence for a clinically distinct new subtype of grade II astrocytomas in patients with long-term epilepsy. Neurosurgery 55(2):340–347. doi:10.1227/01.NEU.0000129546.38675.1B discussion 7–8
van Slegtenhorst M, de Hoogt R, Hermans C, Nellist M, Janssen B, Verhoef S et al (1997) Identification of the tuberous sclerosis gene TSC1 on chromosome 9q34. Science 277(5327):805–808. doi:10.1126/science.277.5327.805
Vinters HV, Park SH, Johnson MW, Mischel PS, Catania M, Kerfoot C (1999) Cortical dysplasia, genetic abnormalities and neurocutaneous syndromes. Dev Neurosci 21(3–5):248–259. doi:10.1159/000017404
Weiner HL, Carlson C, Ridgway EB, Zaroff CM, Miles D, LaJoie J et al (2006) Epilepsy surgery in young children with tuberous sclerosis: results of a novel approach. Pediatrics 117(5):1494–1502. doi:10.1542/peds.2005-1206
Williams JG, Kubelik AR, Livak KJ, Rafalski JA, Tingey SV (1990) DNA polymorphisms amplified by arbitrary primers are useful as genetic markers. Nucleic Acids Res 18(22):6531–6535. doi:10.1093/nar/18.22.6531
Wong KK, Tsang YT, Chang YM, Su J, Di Francesco AM, Meco D et al (2006) Genome-wide allelic imbalance analysis of pediatric gliomas by single nucleotide polymorphic allele array. Cancer Res 66(23):11172–11178. doi:10.1158/0008-5472.CAN-06-2438
Wu L, Patten N, Yamashiro CT, Chui B (2002) Extraction and amplification of DNA from formalin-fixed, paraffin-embedded tissues. Appl Immunohistochem Mol Morphol 10(3):269–274. doi:10.1097/00022744-200209000-00015
Ye X, Ji C, Yin G, Tang R, Zeng L, Gu S et al (2004) Characterization of a human Sec14-like protein cDNA SEC14L3 highly homologous to human SPF/TAP. Mol Biol Rep 31(1):59–63. doi:10.1023/B:MOLE.0000013504.88003.32
Acknowledgments
Martha-Eugenia Ruiz-Tachiquín has a postdoctoral fellowship from Instituto Mexicano del Seguro Social (IMSS).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fischer, I., Cunliffe, C., Bollo, R.J. et al. Glioma-like proliferation within tissues excised as tubers in patients with tuberous sclerosis complex. Acta Neuropathol 116, 67–77 (2008). https://doi.org/10.1007/s00401-008-0391-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-008-0391-1