Abstract
Xanthan gum (XG) is widely used in cosmetic and pharmaceutic products (creams, pastes) and in oil industry (drilling fluids) as a stabilizing and/or thickening agent. In literature, its rheological behavior is mainly presented as that of a shear-thinning or a yield stress fluid. Here, in order to clarify this rheological behavior, we study in detail the flow characteristics during continued flow under given conditions (i.e., controlled stress) for a mass concentration ranging from 0.2 to 5%. We are thus able to identify the apparent flow curve of the material after a short flow duration and the flow curve in steady state (i.e., after a long flow duration). The validity of this flow curve, determined from standard rheometry, is confirmed by magnetic resonance velocimetry. These materials start to exhibit a yield stress behavior beyond some critical xanthan or salt concentration. In that case, a significant increase (by a factor up to 5) of the apparent viscosity is observed during flow in some range of stresses, before reaching a steady state. This original rheopectic effect might be due, after some time of flow associated with deformation and reconfiguration of the XG molecules, to the progressive formation of intermolecular links such as hydrogen bonds and/or intermolecular association due to acetate residues.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Xanthan gum (XG) is a very high molecular weight polysaccharide (with a typical value of \(2\times {10}^{6}\) g/mol) (Jindal and Singh Khattar 2018), which is widely used in cosmetic and pharmaceutic products (creams, pastes) and in oil industry (drilling fluids) as a stabilizing and/or thickening agent (Candido da Silva et al. 2017; Hublik 2012; Palaniraj and Jayaraman 2011). XG is even more used, possibly combined with another hydrocolloid (guar gum, locust bean gum) as an additive in food industry, to gel, stabilize, or increase the viscosity of soups, dressings, beverages, baked products, etc. There seems to be no consensus on the exact mechanisms at the origin of these gelling or stabilizing properties, which is certainly in part due to the fact that rheophysical properties of xanthan gum solutions in water are not so well known. In particular, the question arises as to whether the stability of the mixtures is due to the high fluid viscosity induced by the dispersion of macromolecules, the existence of a network of xanthan macromolecules links, or some interaction between the other suspended elements and the xanthan gum molecules inducing a yield stress (Cao et al. 1990; Doublier et al. 2002; Giboreau et al. 1994; Parker et al. 1995).
In contrast with standard polysaccharides which present classic behavior as shear-thinning and strong dependence of viscoelastic properties with angular frequency, XG generally shows a strong shear-thinning behavior even at low concentration, and only a slight dependence of viscoelastic properties with frequency for high concentration. These exceptional properties of XG have been thoroughly shown in literature leading to describe XG solutions in water as “weak gels” (Clark and Ross-Murphy 1987; Doublier et al. 2002; Giboreau et al. 1994). This weak gel behavior is generally attributed to XG molecules which, in contrast to flexible molecules of standard polysaccharides, are ordered and semi-rigid with a helicoidal structure. Thus, at sufficient concentration, they do not present an entanglement network as standard polysaccharides (Clark and Ross-Murphy 1987) but some jammed structures whose mechanical properties depend much on this jamming.
It has long been recognized that xanthan gum solutions are shear-thinning materials whose apparent viscosity increases with the xanthan concentration (Song et al. 2006; Zhong et al. 2013). This was observed through the increase of the stress level in flow curve for a given shear rate or directly by the increase of the viscosity. Also, the effects of temperature and salt addition were studied (Reinoso et al. 2019; Wyatt and Liberatore 2009; Zhong et al. 2013). A systematic study of the effects of these parameters providing interesting temperature-concentration-shear intensity equivalences, through the evidence of master curves with appropriate scaling of the variables (Choppe et al. 2010). The question of the existence of a yield stress often emerges when the concentration is sufficiently increased. Some authors tend to consider that they do not exhibit a yield stress, and only a shear-thinning behavior more marked for higher concentration (Parker et al. 1995) with a Newtonian plateau at low shear rates (Giboreau et al. 1994; Wyatt and Liberatore 2009) of level also increasing with concentration. Other authors consider the material to exhibit a true yield stress (Whitcomb and Macosko 1978), often from rough studies in a limited range of shear rates(Khalil and Jan 2012; Rodríguez de Castro et al. 2018), and in general beyond some critical concentration(Dakhil et al. 2019; Song et al. 2006), which was associated with a sufficient number of hydrogen bonds (Song et al. 2006). Finally, various techniques for obtaining the yield stress were explored by Ong, which do not always give consistent results, and some thixotropic behavior was suggested (Ong et al. 2019), showing that more studies are needed to clarify the steady-state and transient flow properties of these materials.
Indeed, various problems may be observed in some previous studies, such as measurements in a limited range of shear rates, from which pseudoplastic and yield stress fluid behavior types cannot easily be distinguished; measurements with smooth surfaces, allowing wall slip especially at low shear rates so that yielding does not appear (Cloitre and Bonnecaze 2017; Zhang et al. 2017); fast ramps of stress or shear rates, which do not allow to observe steady states. Here, we intend to clarify the rheological behavior of xanthan solutions with the help of specific knowledge for the proper characterization of yield stress fluids (Bonn et al. 2017; Coussot 2005; Ovarlez 2011) and in a large range of concentration, in order to see the possible behavior transition, in the spirit of Song et al. (2006)’swork, but in a wider range and with more careful observation of transient and steady regimes. This is achieved essentially by using creep tests over sufficient time for large deformation and steady-state flow to be reached. The steady-state flow characteristics were also ascertained thanks to MRI (magnetic resonance imaging) rheometry tests. This makes it possible to observe the existence of a true yield stress beyond some concentration, and two regimes of behavior in the liquid regime. We start by presenting the materials and procedures, then we present and discuss the data.
Materials and methods
Solution preparation
We used a commercial food grade XG powder from Aroma Zone (France) to prepare various mass fractions (range between 0.2 and 5%) of aqueous XG solutions. The powder is dispersed in deionized water at room temperature (20 °C) and mixed thoroughly with a paddle stirrer at 2000 rpm for 30 min. The solution is then left at rest for one night to hydrate and the experiments are performed the following day. Prior to any measurement, the samples are centrifuged (1000 times the gravity) in order to get rid of air bubbles. CaCl2 (Fisher Scientific) has been also used to study the impact of salt concentration on the rheological behavior. In this case, the desired amount of CaCl2 solution 1 M was added during the stirring.
Rheometry
Most of the rheological tests are performed with a stress-controlled rheometer (Malvern Kinexus), equipped with a cone and plate geometry of diameter 50 mm and an angle of 1° which ensures the homogeneity of the stress into the sample. In order to check that some artifacts (evaporation, wall slip, etc.) did not affect the results, we also carried out some tests with a Couette geometry with serrated wall surfaces, inner diameter, and height of 25 mm and 37.5 mm. The rheological measurements, performed under the same conditions, were almost identical for both geometries. This independence of the results with regards to the geometry has also been checked through tests with parallel disks geometries.
Most rheological studies of aqueous XG solutions tend to consider that they are mainly insensitive to their flow history, but some slightly thixotropic (slight evolution of the viscosity with the flow duration has been reported in some case (Ong et al. 2019)). In order to clarify this point, we set up a protocol in which we control carefully the history of the material: a preshear (100 s−1 during 60 s) is applied prior to any measurement, in order to erase the flow history, which brings the sample in a reference state. Then, the material is left at rest for a certain amount of time (typically 10 s), which potentially allows the structure of the material to rebuild from this reference state (N’gouamba et al. 2020). We checked that this procedure ensures good reproducibility of the initial state of the solutions and we did not observe the aging effect most of the time even though some negligible increase of elastic modulus have been observed when we added salt. Various rheometrical procedures (increasing–decreasing stress ramps, creep tests, oscillations) have been used which are described also in the Results and Discussion section.
MRI velocimetry
MRI (magnetic resonance imaging) velocimetry measurements in Couette cell have been carried out in a Bruker BioSpin GmbH. The NMR method for the velocity measurement is a “pulsed gradient spin-echo velocity imaging” sequence, generating 2D spatially resolved velocity maps from which 1D velocity profiles across the gap of the Couette cell are extracted. The Couette cell characteristics are the following: the inner cylinder is made of polyether ether ketone (PEEK) and has a diameter of 14 mm and a length (H) of 60 mm, and the gap size is e = 2 mm. The inner cylinder is mounted to a motor, and the rotation velocity varied in the range of 10 to 600 rpm with a radial resolution of 55 μm. The outer cup is kept static. The Couette cell is immerged into a static field of 300 MHz (7 T). In the plane perpendicular to the axis of the cylinder, the acquisition window is a parallelogram of radial length 7 mm, tangential width 18 mm.
The local flow curve of the material is deduced from the orthoradial velocity ( \({v}_{\theta }\)) profiles measured by NMR velocimetry (see above). The local shear rate at a distance r from the central axis writes \(\dot{\gamma }= \frac{{v}_{\theta }}{r}-\frac{d{v}_{\theta }}{dr}\). The local shear stress at the same distance r is deduced from torque (T) measurements (with the conventional rheometer under exactly the same flow conditions) according to \(\tau = \frac{T}{2\pi H{r}^{2}}\).
Results and discussion
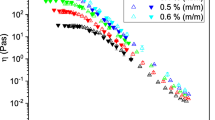
As a first approach to the rheological behavior of XG, we look at data obtained from sweep tests under controlled stress at different concentrations. Here, we imposed stress ramps with logarithmically increasing stress over a total duration of 120 s for each ramp. Thus, in such a test, a steady state at each given stress value is not necessarily reached. The increasing and decreasing stress curves very well superimpose between 0.1 s−1 and the largest shear rate reached, typically a few thousands s−1 (see Fig. 1), which tends to suggest that steady-state flow is reached in this range. For lower shear rates, the stress curve for the decreasing ramp is situated above the increasing ramp curve. This is reminiscent of the behavior observed for yield stress fluids (Coussot 2005) for which, in such a test, the fast increase of the stress corresponds to the progressively increasing deformation (as a result of the ramp) in the solid regime, before reaching the plateau associated with the transition to the liquid regime then flow in this regime. Here, this effect is minor at small concentrations and progressively becomes quite clear and similar to that observed for well-known yield stress fluids. This is consistent with a pseudoplastic behavior at a small concentration, becoming yield stress behavior beyond some concentration.
Such a description is also consistent with the characteristics of the elastic and loss moduli variations for amplitude ramps (see Fig. 2). Here, we performed sweep strain tests at a frequency of 1 Hz, for the same range of concentrations. For low concentrations, the elastic and viscous moduli are almost equal at small deformations, indicating a material in which elastic and viscous effects are both important, while the elastic modulus drops for deformation larger than about 1, which means that the material tends to simple viscous behavior. As the concentration is increased the elastic to viscous modulus ratio progressively increases at low deformation, indicating that the elasticity becomes dominant in this regime, while the elastic modulus drops and the viscous modulus increases beyond some deformation amplitude, which both correspond to typical characteristics of yield stress fluids (N’gouamba et al. 2019). The transition to a clearly yield stress fluid behavior with the increase of concentration can be considered to be around 1% for both tests (dynamic tests and stress sweep).
Adding salt significantly increases the apparent viscosity as already observed in similar XG polyelectrolyte (Wyatt and Liberatore 2009). More precisely, the salt addition induces an increase in the yield stress of the material (see Fig. 3). Remarkably, the flow curves obtained by addition of salt are similar to those obtained without salt by adding a constant stress component (see Fig. 3). This means that the addition of salt has simply a “plastic” effect on the energy dissipation, i.e., essentially independent of the shear rate. This fundamentally differs from an effect, as is often observed for a yield stress fluid, of concentration increase, where the stress is approximately multiplied by a factor (see, e.g., (Boujlel et al. 2012)). This means that the salt has no direct impact on the jammed structure and the unjamming processes, and no systematic (at all flow rates) impact on the viscous dissipation, it essentially induces an additional interaction between the close elements, which breaks and reforms along the relative motion of the elements. This interaction is likely a reduction of electrostatic repulsion.
Actually, we have no clear evidence that the above flow curves correspond to steady-state flows, so that the real existence of yield stress beyond some concentration is not yet proved. In order to clarify this point, we now apply given stress values and follow the evolution of the deformation in time (creep tests). Typical results are shown in Fig. 4. For a concentration smaller than 1%, a steady-state flow is rapidly reached, i.e., the deformation increases linearly in time (see Fig. 4a) for any stress level. For a concentration equal to or larger than 1%, below critical stress (about 3 Pa, for example, Fig. 4b), the deformation increases much more slowly and its rate of increase continuously decreases. Indeed, we have γ≈kt1/3 which implies γ ∞ t−2/3, showing that the shear rate tends towards zero for sufficiently long times. Beyond this critical stress, after some time, the deformation increases linearly with time. Under these conditions, we have a rheological behavior which resembles that of a yield stress fluid, i.e., with two regimes, one in which the fluid tends to stop flowing, even with some residual flow at a rate decreasing in time, and one in which the flow steadily flows.
Creep tests for different concentrations of xanthan gum in water (a 0.2%; b 3%): shear strain vs time for different stress values. Blue symbols (half-filled) correspond to the liquid regime where the steady flow is reached quite instantaneously, red symbols (open) correspond to the liquid regime but with a transient regime, and black symbols (x center) correspond to the solid regime with limited deformation
Actually, we can observe another original trend in these creep tests: in some range of stresses above the yield stress, although the steady-state flow seemed to be reached rapidly after the beginning of the test, the flow apparently slows down and finally, the deformation reaches a new straight line of slope 1 associated with a flow rate smaller than the initial one (see Fig. 4b).
In order to have a clearer view of this effect, we represent the creep tests data in terms of the apparent viscosity as a function of time (see Fig. 5a, b, c). The same observations as above obviously emerge: for low concentration, the viscosity rapidly reaches a plateau at any stress level (see Fig. 5a); we can note however that for stress value below 0.3 Pa, the viscosity apparently increases but since we are in the range of low deformation it is not possible to conclude about a slight shear-thickening effect or a tendency to stoppage associated with the existence of very low yield stress; for concentrations equal or larger than 1% (see Fig. 5b, c), the viscosity reaches a plateau for stress values above the yield stress while it tends to infinity for stresses below the yield stress. Let us remark that in some papers (Whitcomb and Macosko 1978; Wyatt and Liberatore 2009), for low concentrations (typically below 0.5%), a viscosity plateau was observed at low shear rates in consistency with the behavior of standard polymer solutions, which appears in contradiction with our data for which even at low stresses the viscosity in the steady state increases for stress decrease (see Figs. 5a, b). Actually, our creep test data show that this viscosity plateau for XG solutions just corresponds to the transient behavior of the material, and not to the steady-state viscosity. Indeed, we can see in Fig. 5a and b that for low imposed stress values (less than 0.3 Pa for 0.2% and less than 3 Pa for 1%), the initial viscosity is almost constant, which would give a plateau if represented as a function of the shear rate or shear stress. However, if we then follow this viscosity in time, which is the objective of creep tests over sufficient time, we see that the viscosity increases and the plateau would disappear. Again, this is consistent with the assumption that jamming effects are dominant on the material behavior.
The most original trend is that for concentration larger than 1%, we can see that in some range of stresses from just above the yield stress (for example, between 5 and 22 Pa for 3%), the viscosity starts from a low value, apparently follows a plateau, and at some deformation starts increasing significantly, up to ten times its initial value (see Fig. 5c). It is worth emphasizing that this effect is reversible: when one stops the flow and starts again a test with the same sample at the same stress level one obtains the same trend. For larger stress values, the viscosity remains approximately constant during the flow.
It is worth noting that this effect occurs after deformations (say, of the order of 100) much larger than the critical one associated with the solid–liquid transition, which is between 1 and 10 according to creep tests data (see Fig. 5). As a consequence, the viscosity increase effect may be considered as rheopexy, in which the material becomes progressively more viscous with flow duration. In this context, it is interesting to plot the apparent flow curve of the material at the main steps of the process, namely in the very first time (≈ 2 s) of the flow and in the steady-state flow. We then find (see Fig. 6) that the fluid apparently exhibits two main flow regimes associated with two different flow curves: initially, it flows in the first regime (the lower flow curve) but, below some stress value, after some deformation, it steadily flows in the second regime, while it goes on flowing in the first regime for larger stresses.
Stress vs shear rate for an XG solution at 3% under different flow conditions: creep tests or sweep tests. Upward and downward ramps correspond to flow curves already plotted in Fig. 1
Such transient effects are often associated with the developments of heterogeneities, either of the fluid component distribution or of the flow characteristics (shear localization). It does not seem possible to have fluid density heterogeneity as at such large concentrations the elements are part of a jammed structure, at the origin of the yield stress. The possibility of shear localization may be clarified from MRI measurements which provide the steady-state velocity profiles under different rotation velocities of the inner cylinder (see typical data in Fig. 7), from which we can extract the flow properties (see the “Materials and methods” section). For some rotation velocities, it seems that wall slip affected the flow but this does not impair the data analysis since we directly measure the flow characteristics inside the sample by MRI velocimetry, and the stress imposed is transmitted from the inner to the outer cylinder, whatever the flow characteristics, as long as the gap is filled with fluid. It appears that the flow characteristics perfectly fall along the steady-state flow curve obtained from macroscopic tests (see Fig. 8a, b). This confirms that the apparent flow characteristics after a sufficiently large flow duration effectively correspond to homogeneous steady-state flows.
We can finally suggest the following scheme: we are dealing with a material exhibiting yield stress due to the existence of a jammed structure of elastic XG molecules. When a sufficiently large stress is applied to the material, the structure breaks. This breakage allows the relative motion of the blobs, which corresponds to some viscous behavior. For sufficiently low shear rates, after some time of flow associated with deformation and reconfiguration of the XG molecules, some intermolecular links via hydrogen bonds (Harrison et al. 1999) and/or intermolecular association due to acetate residues (Marcotte et al. 2001) can be reformed, which tend to increase the viscosity. For sufficiently high shear rates this effect would not occur or be negligible.
We thus have a rheopectic fluid whose behavior evolution is essentially governed by flow duration. Indeed, beyond its yield stress, in some range of shear rates, it behaves as low viscous fluid, and its viscosity increases in time (during flow) until it reaches a steady state; when it is sheared at higher shear rates, the viscosity remains low; during an increasing ramp of stress or shear rate over a short time, the fluid viscosity remains low (i.e., in the first regime) (see Fig. 6); during a decreasing ramp over a short time, the material remains in its low viscous regime (see Fig. 6).
Conclusion
We have studied experimentally the rheological behavior of aqueous xanthan gum solutions for different mass and salt concentrations. Unlike most studies on polymer solutions which focus on viscoelastic properties by means of oscillatory shear measurements, here, we mainly carried out steady-state shear flow measurements. We were thus able to show clearly that for high concentration (above 1%), aqueous XG solution exhibits true yield stress, unlike most macromolecular solutions. However, our investigation of the liquid regime in the steady-state reveals that, for low values of shear stress, these XG solutions present a striking rheopectic behavior, i.e., an increase of the apparent viscosity with time for a given shear stress. The validity of our rheological measurements by mean of conventional rheometry was checked by MRI velocimetry in order to ensure that this effect was not associated with some artifact such as shear localization. We thus suggest a scenario in which the intermolecular interactions (hydrogen bonds or association due to acetate residues) of XG molecules, at high concentration, can be reformed for a low value of shear stress in the liquid regime (Figs. 9 and 10 in the Appendix).
This study thus allows to clarify the behavior of XG solutions in their liquid regime, which is of significant interest for the various applications in food, cosmetic, and drilling, etc. Thus, we emphasize the importance of studying in detail the “true” steady-state flow characteristics of materials, through continued flows.
Future possible work could attempt to describe more quantitatively the role of the reformation of intermolecular bonds in the rheopexy. This may require coupling rheological tests with some structural measurements such as small-angle x-ray or neutron scattering.
References
Bonn D, Denn MM, Berthier L, Divoux T, Manneville S (2017) Yield stress materials in soft condensed matter. Rev Mod Phys 89(3):1–40. https://doi.org/10.1103/RevModPhys.89.035005
Boujlel J, Maillard M, Lindner A, Ovarlez G, Chateau X, Coussot P (2012) Boundary layer in pastes—displacement of a long object through a yield stress fluid. J Rheol 56(5):1083–1108. https://doi.org/10.1122/1.4720387
Candido da Silva LC, Targino BN, Furtado MM, de Oliveira Pinto MA, Rodarte MP, Hungaro HM (2017) Xanthan: biotechnological production and applications. In: Microbial Production of Food Ingredients and Additives, vol 2020. Elsevier Inc. https://doi.org/10.1016/b978-0-12-811520-6.00013-1
Cao Y, Dickinson E, Wedlock DJ (1990) Creaming and flocculation in emulsions containing polysaccharide. Top Catal 4(3):185–195. https://doi.org/10.1016/S0268-005X(09)80151-3
Choppe E, Puaud F, Nicolai T, Benyahia L (2010) Rheology of xanthan solutions as a function of temperature, concentration and ionic strength. Carbohyd Polym 82(4):1228–1235. https://doi.org/10.1016/j.carbpol.2010.06.056
Clark AH, Ross-Murphy SB (1987) Structural and mechanical properties of biopolymer gels. Adv Polym Sci 83:60–192
Cloitre M, Bonnecaze RT (2017) A review on wall slip in high solid dispersions. Rheol Acta 56:283–305. https://doi.org/10.1007/s00397-017-1002-7
Coussot P (2005) Rheometry of pastes, suspensions, and granular materials: applications in industry and environment. Wiley
Dakhil H, Auhl D, Wierschem A (2019) Infinite-shear viscosity plateau of salt-free aqueous xanthan solutions. J Rheol 63(1):63–69. https://doi.org/10.1122/1.5044732
Doublier J-L, Maingonnat J-F, Cuvelier G (2002) Des sauces aux émulsions alimentaires. In: Comprendre la Rhéologie : De la circulation du sang à la prise du béton. EDP Sciences, pp 116–136
Giboreau A, Cuvelier G, Launay B (1994) Rheological behaviour of three biopolymer/water systems, with emphasis on yield stress and viscoelastic properties. J Texture Stud 25(2):119–138. https://doi.org/10.1111/j.1745-4603.1994.tb01321.x
Harrison G, Franks GV, Tirtaatmadja V, Boger DV (1999) Suspensions and polymers - common links in rheology. Korea-Australia Rheol J 11(3):197–218
Hublik G (2012) Xanthan. Polymer science: A comprehensive reference. Elsevier, pp 221–229. https://doi.org/10.1016/B978-0-444-53349-4.00262-4
Jindal N, Singh Khattar J (2018) Microbial polysaccharides in food idustry. In: biopolymers for food design. Elsevier Inc. https://doi.org/10.1016/B978-0-12-811449-0.00004-9
Khalil M, Jan BM (2012) Herschel-Bulkley Rheological parameters of a novel environmentally friendly lightweight biopolymer drilling fluid from xanthan gum and starch. J Appl Polym Sci 124:595–606. https://doi.org/10.1002/app
Marcotte M, Hoshahili ART, Ramaswamy HS (2001) Rheological properties of selected hydrocolloids as a function of concentration and temperature. Food Res Int 34(8):695–703. https://doi.org/10.1016/S0963-9969(01)00091-6
N’gouamba E, Goyon J, Coussot P (2019) Elastoplastic behavior of yield stress fluids. Phys Rev Fluids 4(12):123301. https://doi.org/10.1103/PhysRevFluids.4.123301
N’gouamba E, Goyon J, Tocquer L, Oerther T, Coussot P (2020) Yielding, thixotropy, and strain stiffening of aqueous carbon black suspensions. J Rheol 64:955. https://doi.org/10.1122/8.0000028
Ong EES, O’Byrne S, Liow JL (2019) Yield stress measurement of a thixotropic colloid. Rheol Acta 58(6–7):383–401. https://doi.org/10.1007/s00397-019-01154-y
Ovarlez G (2011) Caractérisation rhéologique des fluides à seuil. Rhéologie 20:28–43
Palaniraj A, Jayaraman V (2011) Production, recovery and applications of xanthan gum by Xanthomonas campestris. J Food Eng 106(1):1–12. https://doi.org/10.1016/j.jfoodeng.2011.03.035
Parker A, Gunning PA, Ng K, Robins MM (1995) How does xanthan stabilize salad dressing? Food Hydrocolloids 9(4):333–342. https://doi.org/10.1016/S0268-005X(09)80263-4
Reinoso D, Martín-Alfonso MJ, Luckham PF, Martínez-Boza FJ (2019) Rheological characterisation of xanthan gum in brine solutions at high temperature. Carbohydr Polym 203(June 2018):103–109. https://doi.org/10.1016/j.carbpol.2018.09.034
Rodríguez de Castro A, Ahmadi-Sénichault A, Omari A (2018) Using xanthan gum solutions to characterize porous media with the yield stress fluid porosimetry method: robustness of the method and effects of polymer concentration. Transp Porous Media 122(2):357–374. https://doi.org/10.1007/s11242-018-1011-8
Song KW, Kim YS, Chang GS (2006) Rheology of concentrated xanthan gum solutions: Steady shear flow behavior. Fibers Polym 7(2):129–138. https://doi.org/10.1007/BF02908257
Whitcomb PJ, Macosko CW (1978) Rheology of xanthan gum. J Rheol 22(5):493–505. https://doi.org/10.1122/1.549485
Wyatt NB, Liberatore MW (2009) Rheology and viscosity scaling of the polyelectrolyte xanthan gum. J Appl Polym Sci 114(6):4076–4084. https://doi.org/10.1002/app
Zhang X, Lorenceau E, Basset P, Bourouina T, Rouyer F, Goyon J, Coussot P (2017) Wall slip of soft-jammed systems : a generic simple shear process. Phys Rev Lett 119:208004. https://doi.org/10.1103/PhysRevLett.119.208004
Zhong L, Oostrom M, Truex MJ, Vermeul VR, Szecsody JE (2013) Rheological behavior of xanthan gum solution related to shear thinning fluid delivery for subsurface remediation. J Hazard Mater 244–245(November):160–170. https://doi.org/10.1016/j.jhazmat.2012.11.028
Acknowledgements
The authors acknowledge the support of the French National Research Agency within the frame of the grant ANR-17-CE05-0023-04.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
Additional rheological data for 3% and 4% XG solutions
Rights and permissions
About this article
Cite this article
N’gouamba, E., Essadik, M., Goyon, J. et al. Yielding and rheopexy of aqueous xanthan gum solutions . Rheol Acta 60, 653–660 (2021). https://doi.org/10.1007/s00397-021-01293-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00397-021-01293-1