Abstract
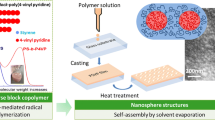
In this contribution, we reported the synthesis of polyhedral oligomeric silsesquioxane (POSS)-capped poly(N-vinyl pyrrolidone) (PVPy) via reversible addition-fragmentation chain transfer/macromolecular design via interchange of xanthate (RAFT/MADIX) polymerization. First, a POSS macromer bearing xanthate moiety was synthesized and was then used as the chain transfer agent to mediate the radical polymerization of N-vinylpyrrolidone (NVP). By controlling the mass ratios of the POSS-CTA to NVP, a series of the POSS-capped PVPy amphiphiles were successfully synthesized with various molecular weights. It was found that in bulks, the POSS-capped PVPy was microphase-separated and the POSS end groups were self-organized into the spherical microdomains with the size of 10~100 nm in diameter. In the solvent selective for PVPy (e.g., water), the POSS-capped PVPy was capable of self-assembling into the spherical micelles with an average diameter of 20~50 nm as evidenced by dynamic laser scattering (DLS) and transmission electron microscopy (TEM). Owing to the amphiphilicity, POSS-capped PVPy also displayed the self-assembly behavior in poly(vinylidene fluoride) (PVDF), in which the POSS cages were aggregated into 10~30 nm microdomains. In the nanocomposites of PVDF with POSS-capped PVPy, the spherical POSS microdomains were readily etched by using hydrofluoric acid, leaving the nanopores in the materials.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Organic-inorganic hybrids have attracted considerable interest of investigators owing to their excellent comprehensive thermomechanical properties resulting from the synergism of organic and inorganic components [1,2,3,4,5]. It is realized that the thermomechanical properties of organic-inorganic hybrids are quite dependent on the morphologies of the materials, which can be modulated in terms of the inter-component interactions through the control over the architecture of the organic-inorganic hybrids. Polyhedral oligomeric silsesquioxanes (POSS) are a class of interesting inorganic compounds [1, 2], which generally have cage-like stereo structures. Typical POSS molecule has cube-octameric structures with the nanometer size (i.e., ~ 0.53 nm in diameter) and the formula of R8Si8O12. The well-defined nanostructure inspires of utilizing POSS as the building blocks to access the organic-inorganic nanocomposites with improved and new properties. In principle, POSS can be introduced into organic polymer systems through physical and chemical approaches [6,7,8,9]. Generally, a simple physical mixing of polymer with POSS would give rise to the occurrence of macroscopic phase separation. The poor miscibility results from the big difference in solubility parameters between POSS and polymers. By comparison, POSS can be effectively incorporated into organic polymers via chemical approaches such as copolymerization or reactive blending. Owing to the formation of chemical linkage between POSS and polymers, the tendency of macroscopic phase separation can be significantly suppressed. In the past years, there has been ample literature to report the preparation of the POSS-containing hybrids with a variety of architectures [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31]. In most of these previous reports, POSS molecules were used as copolymerization monomers or reactive agents to be incorporated into polymers, in which POSS cages were randomly distributed along the main chains of polymers. Considering the structural and elemental feature of POSS macromers, investigators have recently explored the synthesis of the organic-inorganic hybrids in which POSS cages were precisely in the designated positions, such as POSS-capped polymer telechelics (or semi-telechelics) [12, 14,15,16,17, 32,33,34,35,36], bead-like polymers with POSS in the main chains [19,20,21, 37, 38]. The purpose of these studies is to examine the self-assembly behavior of this class of well-defined organic-inorganic hybrids. For instance, Mather et al [15] first reported the synthesis of POSS-capped poly(ethylene oxide) telechelics via the direct reaction of isocyanatopropyldimethylsilylheptacyclohexyl POSS with poly(ethylene glycol) (PEG); it was found that the crystallization and rheological behavior of PEO was significantly changed while the POSS cages were capped at two ends of PEO chains [16, 32]. Taking advantage of copper (I)-catalyzed Huisgen cycloaddition reaction (i.e., click chemistry) between 3-azido propylhepta(3,3,3-trifluropropyl) POSS and alkyne-terminated PEO/α-cyclodextrin polypseudorotaxanes, Zeng et al [39] obtained organic-inorganic polyrotaxanes; it was found that the POSS cages can significantly alter the crystal structures of the polyrotaxanes. More recently, Wang et al. [40] reported the synthesis of POSS-capped poly(N-isopropyl acrylamide) telechelics via a reversible addition-fragmentation chain transfer RAFT polymerization approach. In their work, a diPOSS-functionalized trithiocarbonate was first synthesized and then used as the chain transfer agent to mediate the radical polymerization of N-isopropyl acrylamide. Such a “POSS-spreading” strategy ensures that the POSS cages were precisely capped at two ends of poly(N-isopropyl acrylamide) chains. Owing to the highly hydrophobicity of the POSS termini, the POSS-capped poly(N-isopropyl acrylamide) telechelics can form the physical hydrogels in water, which displayed fast deswelling and reswelling properties.
Poly(N-vinylpyrrolidone) (PVPy) is a typical water-soluble polymer and it has found wide-spread applications in the chemical, pharmaceutical, and material fields owing to the versatile properties of this polymer. It is of interest to conjugate POSS with PVPy to endow the polymer with amphiphilicity. The organic-inorganic hybrids involving PVPy and POSS can be self-organized into a variety of nanostructures in bulks and aqueous solutions. It is anticipated that the conjugation of PVPy with POSS can further extend the application of the polymeric materials via the control over the formation of the hybrids with various morphologies. Notably, Liu et al [41] ever reported the synthesis of organic-inorganic PVPy copolymers with double-decker silsesquioxane in the main chains. It was found that the organic-inorganic PVPy copolymers were microphase-separated in bulks. In aqueous media, the organic-inorganic PVPy copolymers can self-assemble into the spherical nanoobjects with the size of 20~50 nm in diameter. However, POSS-containing PVPy hybrids were scarcely reported vis-à-vis other POSS-containing polymers. Therefore, it is of interest to explore the synthesis of the POSS-containing PVPy hybrids with specific architectures.
In this contribution, we reported the synthesis of POSS-capped PVPy, a novel organic-inorganic amphiphile via reversible addition-fragmentation chain transfer/macromolecular design with interchange of xanthate (RAFT/MADIX) polymerization. Thereafter, the self-assembly behavior of the POSS-capped PVPy amphiphiles in bulks, aqueous solutions and poly(vinylidene fluoride) (PVDF) was investigated. In particular, the self-assembly behavior of POSS-capped PVPy in PVDF was utilized to prepare the nanoporous PVDF materials. To the best of our knowledge, there has been no previous report in this regard. The self-assembly behavior of the POSS-capped PVPy in bulks and aqueous solution were investigated by means of electron microscopy, UV-vis spectroscopy and dynamic laser scattering (DLS).
Experimental
Materials
N-vinylpyrrolidone (NVP) was purchased from Aldrich reagent, China, and it was passed through a basic alumina column to remove the inhibitor before use. 2,2-Azobisisobutylnitrile (AIBN) was obtained from Shanghai Reagent, China, and was recrystallized from ethanol twice. Phenyltrimethoxysilane (98%) was obtained from Zhejiang Chem-Tech Co., China. Trichlorosilane, 2-bromopropiomyl bromide, and triethylamine were purchased from TCI Co. Shanghai, China. Allyl alcohol (99%) was obtained from Shanghai Reagent, China, and it was distilled over calcium hydride. Karstedt catalyst was synthesized in the lab by following the method of literature with slight modification [42]. Ethyl xanthate potassium was synthesized as reported by Stenzel et al [43]. Allyl 2-bromopropanoate was prepared via the reaction of allyl alcohol with 2-bromopropiomyl bromide. Sodium hydroxide, anhydrous magnesium sulfate, and hydrofluoric acid were obtained from Shanghai Reagent, China. Organic solvents such as tetrahydrofuran (THF), methanol, diethyl ether, toluene, dichloromethane, acetone, and N,N-dimethylformamide (DMF) were obtained from Shanghai Reagent, China. Before use, toluene and THF were refluxed over sodium and then distilled.
Synthesis of hydroheptaphenyl POSS (POSS-H)
Hydroheptaphenyl POSS was synthesized by following the method reported previously [44]. First, phenyltrimethoxysilane (45.540 g, 0.230 mol), deionized water (5.260 g, 0.290 mol), sodium hydroxide (0.960 g, 0.099 mol), and 250 mL of anhydrous THF were added into a 500-mL round-bottom flask. Under refluxing condition, the reaction was carried out for 5 h and then cooled to room temperature. At room temperature, the reaction was performed for additional 15 h. The solvent together other volatile components were removed via rotary evaporation, heptaphenyltricycloheptasiloxane trisodium silanolate [Na3O12Si7(C6H5)7] (43.330 g) was obtained with the yield of 79.1%. Second, the above Na3O12Si7(C6H5)7 and 250 mL of anhydrous THF were added to a flask with vigorous stirring. Cooling to 0 °C, trichlorosilane (8.000 g, 0.590 mol) was added to the mixture; the system was maintained at 0 °C for 3 h and at room temperature for 21 h with vigorous stirring. The insoluble components were filtered out and the solvent was removed via rotary evaporator. The solids were washed with methanol (3 × 100 mL). After drying in vacuo at 40 °C for 24 h, the product (i.e., POSS-H) (32.420 g) was obtained with the yield of 63.2%. 1H NMR (ppm, CDCl3): 4.50 (s, 1H, -SiH), 7.18~7.83 (m, 35H, protons of phenyl groups).
Synthesis of bromopropionate POSS (POSS-COOCHCH3Br)
POSS-H (10.000 g, 10.400 mmol), allyl 2-bromopropanoate (9.000 g, 46.600 mmol), and anhydrous toluene (100 mL) were added to a flask. This system was purged with highly pure nitrogen for 30 min and then Karstedt catalyst (300 μL) was injected with a syringe. Heating the mixture up to 90 °C, the reaction was performed for 36 h with vigorous stirring. After concentrated via a rotary evaporator, the reacted mixture was dropped into methanol (300 mL) to obtain the precipitates. After drying in vacuo at 40 °C for 24 h, the product (9.120 g) was obtained with the yield of 75.2%. 1H NMR (ppm, CDCl3): 0.81~0.98 [m, 2.0H, -OCH2CH2CH2OCOCH(CH3)Br], 1.75~1.80 [t, 3H, -CH2CH2OCOCH(CH3)Br], 1.81~1.96 [s, 2H, -CH2CH2CH2OCOCH(CH3)Br], 4.20~4.28 [s, 2H, -CH2CH2CH2OCOCH(CH3)Br], 4.34~4.40 [s, 1H, -CH2CH2CH2OCOCH(CH3)Br], and 7.18~7.83 (m, 35H, protons of aromatic rings).
Synthesis of POSS-CTA
To a flask containing a magnetic stirrer, POSS-COOCHCH3Br (6.000 g, 5.200 mmol), ethyl xanthate potassium (3.000 g, 17.600 mmol) and the mixture of dichloromethane with acetone (50/50, 120 mL) were added with vigorous stirring. The reaction was performed at room temperature for 48 h. The insoluble solids were filtered out; the reacted mixture was concentrated via rotary evaporation and was dropwise added to methanol (300 mL) to obtain the precipitates. The precipitates were washed with methanol three times and dried in vacuo at 40 °C overnight. The product (i.e., POSS-CTA) (5.440 g) was obtained with the yield of 77.8%. 1H NMR (ppm, CDCl3): 1.50~1.70 [s, 3H, -SCSOCH2CH3], 4.55~4.65 [t, 2H, -SCSOCH2CH3], 1.50~1.70 [s, 3.0H, -SCSOCH2CH3], and 4.55~4.65 [t, 2.0H, -SCSOCH2CH3].
Synthesis of POSS-capped PVPy
Typically, POSS-CTA (1.000 g, 8.400 mmol), NVP (3.500 g, 27.150 mmol), AIBN (27.400 mg, 1.680 mmol), and 1,4-dioxane (6 mL) were added to a flask containing a magnetic stirrer. The mixture was bubbled with highly pure nitrogen for 30 min and then the flask was sealed. The polymerization was carried out at 70 °C for 20 h to attain a desired conversion of NVP. The polymerized mixture was dropwise added to diethyl ether (100 mL) to afford the precipitates. After drying in vacuo at 40 °C for 24 h, the polymer (3.700 g) was obtained with the conversion of NVP to be c.a. 82.2%. 1H NMR (ppm, CDCl3): 3.45~3.60 [2H, -C(N)HCH2-], 3.30 [2H, -NCH2CH2-], 2.41 [2H, -CH2CH2CO-], 2.02 [2H, -CH2CH2CO-] and 4.92 [2H, -C(N)HCH2-]. GPC (DMF, PS standards): Mn = 4200 Da with Mw/Mn = 1.19.
Preparation of PVDF and POSS-PVPy blends
Desired amount of PVDF and POSS-PVPy4K were dissolved in N.N-dimethylformamide (DMF) at the concentration of 10 wt% and the solutions were cast onto petri dishes. The majority of solvent was evaporated at 60 °C for 12 h and the residual solvent was removed in vacuo at 60 °C for 1 week until the constant weight. The films of samples were subjected to DSC measurements and used for the preparation of nanoporous PVDF films (See infra).
Preparation of nanoporous PVDF
The above films of the blends of PVDF with POSS-PVPy4K were immersed into the aqueous solutions of hydrofluoric acid at the concentration of 40 wt% for 72 h. The etched films were taken out and then washed with a great amount of deionized water. After drying in vacuum oven, the nanoporous films were subjected to morphological observation by means of scanning electron microscope.
Measurements and techniques
Proton nuclear magnetic resonance (1H NMR) spectroscopy was carried out on a Varian Mercury Plus 400 MHz NMR spectrometer and deuterium chloroform was used as the solvent to dissolve the samples. The molecular weights were measured on a Waters 1515 GPC system equipped with Waters 2414 Refractive Index detector. N,N-dimethylformamide containing LiBr at the concentration of 0.01 M was used as the eluent and the molecular weights were expressed relative to polystyrene standards. Differential scanning calorimetry (DSC) was performed on a TA Q2000 DSC apparatus. The samples (about 5 mg in weight) were first heated up to 200 °C and held at this temperature for 3 min, followed by quenching to − 60 °C. After that, the samples were re-heated to 200 °C at the heating rate of 20 °C × min−1 and then cooled to − 60 °C at a cooling rate of 10 °C × min−1. Thermogravimetric analysis (TGA) was conducted on a TA Q5000 thermal gravimetric analyzer under air atmosphere from room temperature to 800 °C at a heating rate of 20 °C × min−1. The samples (c.a. 5 mg in weight) were heated from room temperature to 800 °C in air atmosphere. UV-vis spectroscopy was carried out on an Agilent Cary 60 UV-vis spectrophotometer. The aqueous suspensions of POSS-capped PVPy polymers with the concentration in the range of 0.001~0.2 mg × mL were prepared; the THF solution of pyrene at the concentration of 60 mg × L−1 was then added to the above suspensions of POSS-capped PVPy and the THF was removed by rotary evaporation. The concentration of pyrene in the above specimens was controlled to be 3.00 mg × L−1. Before UV-vis measurements, all the suspensions were dispersed under ultrasonic irradiation for 2 h. The above suspensions were also subjected to dynamic laser scattering (DLS) measurements on a Malvern nanoZS90 analyzer with a He-Ne laser operated at a wavelength of λ = 633 nm. In the measurements, the data were collected at a fixed scattering angle of 90o. For each sample, three parallel measurements were carried out to report the average values of hydrodynamic radii. The morphological observations were conducted on a JEOL JEM 2100F transmission electron microscope (TEM) at the voltage of 200 kV. To investigate the morphologies of the polymers in bulks, the suspensions of POSS-capped PVPy dissolved in DMF at the concentration of 2 mg × ml−1 were dropped onto carbon-coated copper grids. Before the measurements, the solvent was fully removed. To investigate the morphology of self-assembly, the suspensions of POSS-capped PVPy polymers in water at the concentration of 0.2 g × L−1 were dropped onto carbon-coated copper grids and the water was removed via freeze-drying approach. The morphologies of the blends of PVDF with POSS-PVPy4K and the nanoporous films of PVDF were observed by means of scanning electron microscopy (SEM). The samples were examined on a Sirion 200 field-emission scanning electron microscope at an activation voltage of 5 kV. Before the morphological observation, the surfaces of samples were coated with thin layers of gold of about 100 Å.
Results and discussion
Synthesis of POSS-capped PVPy
The route for synthesis of POSS-CTA was shown in Scheme 1. First, heptaphenyltricycloheptasiloxane trisodium silanolate [Na3O12Si7(C6H5)7] was prepared via hydrolysis and rearrangement of phenyltrimethoxysiloxane in the presence of sodium hydroxide and then the silylation reaction of Na3O12Si7(C6H5)7 with trichlorosilane (HSiCl3) was carried out to afford a hydroheptaphenyl POSS (denoted POSS-H) [44]. Second, the hydrosilylation reaction of POSS-H with allyl 2-bromopropanoate was performed to obtain a 2-bromopropanoate-bearing POSS macromer (denoted POSS-COOCHCH3Br). Lastly, the as-obtained POSS-COOCHCH3Br was allowed to react with potassium ethyl xanthate to afford a xanthate-functionalized POSS, which would be used as the chain transfer agent (denoted POSS-CTA) for the RAFT/MADIX polymerization of NVP. Figure 1 shows the 1H NMR spectra of POSS-H, POSS-COOCHCH3Br, and POSS-CTA. For POSS-H, the signal of resonance at 4.50 ppm is assignable to the proton of Si-H bond; those in the range of 7.0~7.8 ppm to the protons of phenyl groups connected to silicon atoms of silsesquioxane cage. After the hydrosilylation reaction, the resonance at 4.50 ppm completely disappeared, indicating that the hydrosilylation was carried to completion. In the meantime, there newly appeared several signals of resonance at 0.95, 1.78, 1.92, 4.22, and 4.36 ppm and they are assignable to the protons of methyl, methylene, and methine groups of propyl 2-bromopropionate, respectively as indicated. For POSS-CTA, there were two new signals of resonance at 1.55 and 4.58 ppm, assignable to the protons of methyl and methylene groups of ethyl xanthate moiety. The results of 1H NMR spectroscopy indicate that POSS-CTA was successfully obtained. In this work, we investigated the kinetics of radical polymerization of NPV which was mediated by the use of POSS-CTA by means of gel permeation chromatography (GPC). The results showed that that the reaction followed a pseudo-first-order kinetics of polymerization and in a living and controlled manner (See Fig. S1 through S3 in Supporting Information).
In this work, we synthesized a series of POSS-capped PVPy samples with various lengths of PVPy chains by controlling the mass ratio of NVP to POSS-CTA. Representatively shown in Fig. 2 is the 1H NMR spectrum of POSS-PVPy5K. For comparison, the spectrum of POSS-CTA was also included. Compared to POSS-CTA, there appeared several new resonances at 4.92, 3.49, 3.30, 2.41, and 2.02 ppm; they are attributable to the protons of methylene groups of PVPy chains as indicated. The 1H NMR spectroscopy indicates that the product combined the structural feature from POSS and PVPy chain, i.e., that POSS-capped PVPy was successfully obtained. All the POSS-capped PVPy samples were subjected to gel permeation chromatography (GPC) to measure their molecular weights and the GPC curves are shown in Fig. 3. The data of molecular weights are summarized in Table 1. Notably, all the samples displayed the unimodal distribution of molecular weights and the values of PDI were measured to be in the range of 1.13~1.28.
To demonstrate the existence of the terminal POSS cages, all the POSS-capped PVPy samples were subjected to thermal gravimetric analysis (TGA). The measurements were carried out in air atmosphere and the TGA curves are shown in Fig. 4. For pure PVPy, the initial degradation occurred at c.a. 200 °C. It is noted that the pure polymer can be completely decomposed at 800 °C. For the POSS-capped PVPy samples, the temperatures of initial degradation (Td’s) were significantly enhanced, increasing with increasing the percentage of POSS (i.e., decreasing the lengths of PVPy chains). The enhanced Td’s are attributable to the presence of POSS cages in the samples. The POSS cages with the higher thermal stability could behave as the barrier to suppress the release of the gaseous products of decomposition. More importantly, all the POSS-capped PVPy samples cannot be completely decomposed at the temperature as high as 800 °C, which was in marked contrast to pure PVPy. The residue of decomposition resulted from the inorganic silsesquioxane cages. Assuming that only single ends are connected to single POSS cages and all the organic components are completely decomposed whereas the silsesquioxane cages were converted into silica, it is possible to estimate the average lengths of PVPy chains according to the yields of decomposition at 800 °C; the results of the molecular weights are also summarized in Table 1. It is plausible to propose that the results from TGA are much closer to the real molecular weights. The TGA results further indicate that the POSS-capped PVPy samples were successfully obtained.
Self-assembly in bulks
The morphology of POSS-caped PVPy was investigated by transmission electron microscopy (TEM) and the TEM images are shown in Fig. 5. All the POSS-capped PVPy samples displayed the heterogonous morphologies at the nanometer scale, depending on the percentage of POSS. It is seen that the spherical dark microdomains with the size of 10~100 nm were dispersed into the continuous matrix. The heterogeneous morphologies are indicative of the occurrence of microphase separation in the POSS-capped PVPy samples. In terms of the difference in electron density between POSS and PVPy and the percentage of POSS in the samples, the dark microdomains are attributable to the aggregates of POSS whereas the continuous matrices to PVPy. Considering that the nanosize of the single silsesquioxane was about 1.0 nm, it is judged that the POSS microdomains were the aggregates of tens (or hundreds) of POSS cages via POSS-POSS interactions. The formation of the microphase-separated morphologies is ascribed to the immiscibility of PVPy with POSS, which resulted in the self-assembly behavior of the POSS-capped PVPy in bulks. The formation of POSS microdomains at the nanometer scale could significantly affect the packing of PVPy chains in bulks, which was readily investigated by means of Fourier transform infrared (FTIR) spectroscopy (See infra).
Self-assembly in aqueous solutions
It is known that PVPy is a typical water-soluble polymer whereas POSS is highly hydrophobic. Therefore, the assembly of PVPy with POSS via the formation of chemical linkage would endow the POSS-capped PVPy samples with the self-assembly behavior in water. Depending on the lengths of PVPy chains, the POSS-capped PVPy samples would be self-assembled into the micelles with various morphologies. In aqueous media, the POSS aggregates would form the cores whereas the PVPy chains constitute the coronas of the micelles. In this work, we first measured the critical micelle concentrations (CMC) and then observed the morphologies of the self-assembled nanoobjects. With pyrene as the probe molecule, the CMCs were measured by means of UV-vis spectrophotometry [45,46,47]. Figure 6 shows the plots of absorbance of pyrene at λ = 340 nm as functions of logarithmic concentration of the POSS-capped PVPy polymers. It was seen that the absorbance remained unchanged at relatively lower concentrations but it abruptly increased while the concentration was increased to the specific values. Thereafter, the absorbance also linearly increased with increasing the logarithmic values of concentrations. The abrupt increase in the intensity at λ = 340 nm suggest that there was a considerable aggregation of pyrene molecules at the specific concentrations. It is proposed that the micelles were formed at the concentrations, which were thus taken as the CMCs. The crossover points of the two straight lines were taken as the onset concentrations of micellization, i.e., CMCs. The CMCs were measured to be 0.049, 0.042, 0.036, and 0.030 mg × mL−1 for POSS-PVPy11K, POSS-PVPy7K, POSS-PVPy5K, and POSS-PVPy4K, respectively. Notably, the CMCs increased with increasing the lengths of PVPy chains, indicating that hydrophobicity of the organic-inorganic amphiphiles decreased with increasing the lengths of the water-soluble chains (viz. PVPy).
At a concentration above the CMCs (viz. 0.20 g × L−1), the aqueous suspensions of the POSS-capped PVPy samples were subjected to dynamic light scattering (DLS) to measure the sizes of the self-assembled nanoobjects. Shown in Fig. 7 are the plots of hydrodynamic radius (Rh) distribution as functions of Rh at room temperature. Notably, all the samples displayed the unimodal distribution of nanoparticle sizes. The Rh values were measured to be 68.9, 62.3, 55.1, and 27.4 nm for POSS-PVPy4K, POSS-PVPy5K, POSS-PVPy7K, and POSS-PVPy11K, respectively. It is noted that the Rh values decreased with increasing the lengths of PVPy chains. The morphologies of the micelles were further investigated by means of transmission electron microscopy (TEM); the specimens were prepared via freeze-drying the aqueous suspensions and the TEM images are presented in Fig. 8. The spherical nanoparticles with the size of 20~50 nm in diameter were obtained for all the POSS-capped PVPy samples. Notably, the sizes of the nanoparticles increased with increasing the percentage of POSS (i.e., with decreasing the lengths of PVPy chains). The decrease in lengths of PVPy chains (i.e., the increase in percentage of POSS) resulted in the decrease in the surface free energy of POSS microdomains. To counterpart the tendency to enhance the surface free energy, the POSS cages were thus re-organized into the nanoparticles with the increased sizes. The TEM results were in good agreement with those of DLS.
Self-assembly in poly(vinylidene fluoride)
In a previous work [48], we have demonstrated that PVPy was miscible with poly(vinylidene fluoride) (PVDF) in amorphous state. In contrast, POSS was immiscible with PVDF. The difference in the miscibility of PVDF with PVPy and POSS suggests that POSS-capped PVPy would display the self-assembly behavior in its blends with PVDF homopolymer, in which POSS cages would be self-organized into the microdomains whereas PVPy chains remained intimately mixed with PVDF. Owing to the rigid and stereo cage-like structures, the POSS microdomains (or POSS aggregates) could be good position-occupants in PVDF. If the POSS microdomains can be preferentially etched, the nanopores will thus be left in the materials. The self-assembly behavior could be utilized to access the nanoporous PVDF. In this work, we first examined the self-assembly behavior of POSS-capped PVPy in PVDF and then explored the preparation of the nanoporous PVDF via etching the blends with hydrofluoric acid.
In this work, one of POSS-capped PVPy samples (i.e., POSS-PVPy4K) was used to prepare the blends with PVDF. At room temperature, the blends were cloudy or translucent owing to the crystallization of PVDF. Upon heating up to the temperature above the melting point of PVDF (e.g., 180 °C), the films with the thickness of about 25 μm became transparent, suggesting that no macroscopic phase separation occurred in the blends. The blends were subjected to differential scanning calorimetry (DSC) and the DSC curves are shown in Fig. 9. For plain PVDF, single endothermic and exothermic melting peaks were exhibited at 170 and 136 °C in the heating and cooling curves, respectively. The former is attributable to the melting transition whereas the latter to the crystallization transition of PVDF. The fact that no cold crystallization peak was exhibited in the heating scan indicates that the crystallization was undergone completion in the quenching process. Upon adding POSS-capped PVPy, the melting and crystallization peaks gradually shifted to lower temperatures. Notably, the Tm’s and Tc’s decreased with increasing the content of POSS-capped PVPy. This observation is interpreted on the basis of the miscibility of PVPy with PVDF. The depression in melting points of crystalline components has been taken as the typical evidences of miscibility in polymer blends [49, 50]. In the present case, PVPy had the glass transition temperature (ca. Tg = 180 °C) much higher than that of PVDF (about Tg = −40 °C) [47]. As a miscible component, PVPy would give rise to the enhancement in Tg’s of the blends in comparison to plain PVDF. As a consequence, the crystallization of PVDF in the miscible blends became more difficult than plain PVDF, i.e., the crystallization temperatures (Tc’s) shifted to lower temperatures with increasing the content of POSS-capped PVPy. The X-ray diffraction (XRD) results showed that but the crystal structures remained unchanged while the crystallinity of PVDF decreased with increasing the content of POSS-PVPy in the blends (See Fig. S4). The decreased crystallinity of PVDF is attributable to the miscibility of PVDF with PVPy chains of POSS-PVPy. It is proposed that the inclusion of the higher Tg and miscible component (viz. PVPy) prevented PVDF from crystallization and thus the crystallinity was decreased. The similar results have been reported in other miscible blends of PVDF [48,49,50].
The miscibility of PVPy and the immiscibility of POSS with PVDF resulted that the POSS-PVPy4K were self-assembled into the POSS microdomains in PVDF, which was readily confirmed with the morphological observation by means of scanning electron microscopy (SEM). Representatively shown in Fig. 10 is the SEM micrograph of the blend containing 30 wt% of POSS-PVPy4K. Notably, a heterogeneous morphology was exhibited; the spherical microdomains with the size of 10~30 nm were dispersed in the continuous matrix. The spherical microdomains are attributable to the aggregates of tens of POSS cages whereas the matrix to PVDF which was miscible with PVPy. To etch the POSS microdomains, the blends of PVDF with POSS-PVPy4K were immersed into aqueous solution of hydrofluoric acid (HF). Representatively shown in Fig. 11 is the SEM image of the blends of PVDF with 30 wt% of POSS-capped PVPy which was etched for 72 h. After removal of the POSS microdomains, notably, the nanopores with the size comparable to that of the POSS microdomains were exhibited. The fraction of nanopores in the blends was further analyzed by the use of ImageJ software [51] and the values are summarized in Fig. 12. It is seen that the percentage of nanopores increased with increasing the content of POSS-PVPy4K. For instance, the nanoporous PVDF with 12 vol% of nanopores was obtained by the use of the blend containing 40 wt% of POSS-PVPy4K. The above results indicate that the nanoporous PVDF materials were successfully obtained. Nanoporous polymer films have found wide-spread applications in the aspect of ion conductance, catalyst substrates, templates, filtration of viruses, superhydrophobic surfaces, and cell culture devices [52,53,54,55]. In the past years, there have been some reports on the preparation of nanoporous PVDF membranes via various approaches [56,57,58]. In this work, we presented a new approach to access nanoporous PVDF films by utilizing the self-assembly behavior of POSS-capped PVPy in PVDF.
Conclusions
In this work, we reported the synthesis of POSS-capped poly(N-vinyl pyridine) (PVPy) amphiphiles. First, a macromer bearing xanthate moiety was synthesized and used as the chain transfer agent to mediate the radical polymerization of N-vinylpyrrolidone (NVP). By controlling the mass ratios of this xanthate-functionalized POSS (denoted POSS-CTA) to NVP, we successfully synthesized a series of the POSS-capped PVPy amphiphiles with various molecular weights via reversible addition-fragmentation chain transfer/macromolecular design via interchange of xanthate (RAFT/MADIX) polymerization. The self-assembly behavior of the POSS-capped PVPy amphiphiles was investigated in this work. In bulks the POSS-capped PVPy was microphase-separated and the POSS end groups were self-organized into the spherical microdomains with the size of 10~100 nm in diameter. In the solvent selective for PVPy (e.g., water), the POSS-capped PVPy can be self-assembled into the spherical micelles with an average diameter of 20~50 nm as evidenced by dynamic laser scattering (DLS) and transmission electron microscopy (TEM). More importantly, the POSS-capped PVPy amphiphiles were capable of displaying the self-assembly behavior in poly(vinylidene fluoride) (PVDF). It was found that in the blends of PVDF with the POSS-capped PVPy the spherical POSS microdomains were formed with the size of 10~30 nm in diameter. After removal of the POSS microdomains via etching with hydrofluoric acid, the nanopores were generated with the size comparable to that of the POSS microdomains, which provides a new approach to prepare nanoporous PVDF.
References
Giannelis EP, Krishnamoorti R, Manias E (1999) Polymer-silicate nanocomposites: model systems for confined polymers and polymer brushes. Adv Polym Sci 138:107–147
Abe Y, Gunji T (2004) Oligo- and polysiloxanes. Prog Polym Sci 29:149–182
Pielichowski K, Njuguna J, Janowski B, Pielichowski J (2006) Polyhedral oligomeric silsesquioxanes (POSS)-containing nanohybrid polymers. Springer Berlin Heidelberg
Baney RH, Itoh M, Sakakibara A, Suzuki T (1995) Silsesquioxanes. Chem Rev 94:1409–1430
Provatas A, Matisons JG (1997) Silsesquioxanes: synthesis and applications. Trends Polym Sci 5:327–332
Schwab JJ, Lichtenhan JD (1998) Polyhedral oligomeric silsesquioxane (POSS)-based polymers. Appl Organomet Chem 12:707–713
Li G, Wang L, Ni H, Pittman CU (2001) Polyhedral oligomeric silsesquioxane (POSS) polymers and copolymers: a review. J Inorg Organomet Polym 11:123–154
Phillips SH, Haddad TS, Tomczak SJ (2004) Developments in nanoscience: polyhedral oligomeric silsesquioxane (POSS)-polymers. Curr Opin Solid State Mater Sci 8:21–29
Wang F, Lu X, He C (2011) Some recent developments of polyhedral oligomeric silsesquioxane (POSS)-based polymeric materials. J Mater Chem 21:2775–2782
Lickiss PD, Rataboul F (2008) Chapter I: fully condensed polyhedral oligosilsesquioxanes (POSS): from synthesis to application. In Adv Organomet Chem. Elsevier Science & Technology 57:1–116
Kuo SW, Chang FC (2011) POSS related polymer nanocomposites. Prog Polym Sci 36:1649–1696
Ni Y, Zheng S (2007) Nanostructured thermosets from epoxy resin and an organic-inorganic amphiphile. Macromolecules 40:7009–7018
Zeng K, Zheng S (2007) Nanostructures and surface dewettability of epoxy thermosets containing hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(ethylene oxide). J Phys Chem B 111:13919–13928
Zeng K, Wang L, Zheng S (2009) Rapid deswelling and reswelling response of poly(N-isopropylacrylamide) hydrogels via formation of interpenetrating polymer networks with polyhedral oligomeric silsesquioxane-capped poly(ethylene oxide) amphiphilic telechelics. J Phys Chem B 113:11831–11840
Kim BS, Mather PT (2002) Amphiphilic telechelics incorporating polyhedral oligosilsesquioxane: 1. synthesis and characterization. Macromolecules 35:8378–8384
Kim BS, Mather PT (2006) Morphology, microstructure, and rheology of amphiphilic telechelics incorporating polyhedral oligosilsesquioxane. Macromolecules 39:9253–9260
Zhang W, Müller AHE (2010) A “click chemistry” approach to linear and star-shaped telechelic POSS-containing hybrid polymers. Macromolecules 43:3148–3152
Zhang W, Fang B, Walther A, Müller AHE (2009) Synthesis via raft polymerization of tadpole-shaped organic/inorganic hybrid poly(acrylic acid) containing polyhedral oligomeric silsesquioxane (POSS) and their self-assembly in water. Macromolecules 42:2563–2569
Wang L, Zhang C, Zheng S (2011) Organic-inorganic poly(hydroxyether of bisphenol A) copolymers with double-decker silsesquioxane in the main chains. J Mater Chem 21:19344–19352
Wei K, Wang L, Li L, Zheng S (2014) Synthesis and characterization of bead-like poly(N-isopropylacrylamide) copolymers with double decker silsesquioxane in the main chains. Polym Chem 6:256–269
Wei K, Wang L, Zheng S (2013) Organic-inorganic polyurethanes with 3,13-dihydroxypropyloctaphenyl double-decker silsesquioxane chain extender. Polym Chem 4:1491–1501
Choi J, Harcup J, Yee AF, Zhu Q, Laine RM (2001) Organic/inorganic hybrid composites from cubic silsesquioxanes. J Am Chem Soc 123:11420–11430
Huang J, He C, Xiao Y, Mya KY, Dai J, Siow YP (2003) Polyimide/POSS nanocomposites: interfacial interaction, thermal properties and mechanical properties. Polymer 44:4491–4499
Neumann D, Fisher M, Tran L, Matisons JG (2002) Synthesis and characterization of an isocyanate functionalized polyhedral oligosilsesquioxane and the subsequent formation of an organic-inorganic hybrid polyurethane. J Am Chem Soc 124:13998–13999
Costa ROR, Vasconcelos WL, Tamaki R, Laine RM (2001) Organic/inorganic nanocomposite star polymers via atom transfer radical polymerization of methyl methacrylate using octafunctional silsesquioxane cores. Macromolecules 34:5398–5407
Choi J, Tamaki R, Kim SG, Laine RM (2003) Organic/inorganic imide nanocomposites from aminophenylsilsesquioxanes. Chem Mater 15:3365–3375
Huang KW, Kuo SW (2010) High-performance polybenzoxazine nanocomposites containing multifunctional poss cores presenting vinyl-terminated benzoxazine groups. Macromol Chem Phys 211:2301–2311
Liu Y, Yang X, Zhang W, Zheng S (2006) Star-shaped poly(ε-caprolactone) with polyhedral oligomeric silsesquioxane core. Polymer 47:6814–6825
Pyun J, Matyjaszewski K, Wu J, Kim GM, Chun SB, Mather PT (2003) ABA triblock copolymers containing polyhedral oligomeric silsesquioxane pendant groups: synthesis and unique properties. Polymer 44:2739–2750
Li S, Liu Y, Ji S, Zhou Z, Li Q (2014) Synthesis and self-assembly behavior of thermoresponsive poly(oligo(ethylene glycol) methyl ether methacrylate)-POSS with tunable lower critical solution temperature. Colloid Polym Sci 292:2993–3001
Tsuchiya K, Ishida Y, Kameyama A (2017) Synthesis of diblock copolymers consisting of POSS-containing random methacrylate copolymers and polystyrene and their cross-linked microphase-separated structure via fluoride ion-mediated cage scrambling. Polym Chem 8:2516–2527
Kim BS, Mather PT (2006) Amphiphilic telechelics with polyhedral oligosilsesquioxane (POSS) end-groups: dilute solution viscometry. Polymer 47:6202–6207
Wang L, Gong W, Zheng S (2009) Poly(hydroxyether of bisphenol A)-alt-polydimethylsiloxane: a novel thermally crosslinkable alternating block copolymer. Polym Int 58:124–132
Zeng K, Wang L, Zheng S, Qian X (2009) Self-assembly behavior of hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(ɛ-caprolactone) in epoxy resin: nanostructures and surface properties. Polymer 50:685–695
Zheng Y, Wang L, Zheng S (2012) Synthesis and characterization of heptaphenyl polyhedral oligomeric silsesquioxane-capped poly(N-isopropylacrylamide)s. Eur Polym J 48:945–955
Li L, Zhang C, Zheng S (2017) Synthesis of POSS-terminated polycyclooctadiene telechelics via ring-opening metathesis polymerization. J Polym Sci Part A Polym Chem 55:223–233
Wei K, Wang L, Zheng S (2013) Organic-inorganic copolymers with double-decker silsesquioxane in the main chains by polymerization via click chemistry. J Polym Sci Part A Polym Chem 51:4221–4232
Xu S, Zhao B, Wei K, Zheng S (2018) Organic-inorganic polyurethanes with double decker silsesquioxanes in the main chains: morphologies, surface hydrophobicity, and shape memory properties. J Polym Sci Part B Polym Phys 56:893–906
Zeng K, Zheng S (2010) Synthesis and characterization of organic/inorganic polyrotaxanes from polyhedral oligomeric silsesquioxane and poly(ethylene oxide)/α-cyclodextrin polypseudorotaxanes via click chemistry. Macromol Chem Phys 210:783–791
Wang L, Zeng K, Zheng S (2011) Hepta(3,3,3-trifluoropropyl) polyhedral oligomeric silsesquioxane-capped poly(N-isopropylacrylamide) telechelics: synthesis and behavior of physical hydrogels. ACS Appl Mater Interfaces 3:898–909
Liu N, Zheng S (2016) Organic-inorganic poly(N-vinylpyrrolidone) copolymers with double-decker silsesquioxane in the main chains: synthesis, glass transition, and self-assembly behavior. J Polym Sci Part A Polym Chem 54:2949–2961
Steffanut P, Osborn JA, Decian A, Fisher J (2015) Efficient homogeneous hydrosilylation of olefins by use of complexes of Pt0 with selected electron-deficient olefins as ligands. Chem Eur J 4:2008–2017
Stenzel MH, Cummins L, Roberts GE, Davis TP, Vana P, Barner-Kowollik C (2003) Xanthate mediated living polymerization of vinyl acetate: a systematic variation in MADIX/RAFT agent structure. Macromol Chem Phys 204:1160–1168
Cao Y, Xu S, Li L, Zheng S (2017) Physically cross-linked networks of POSS-capped poly(acrylate amide)s: synthesis, morphologies, and shape memory behavior. J Polym Sci Part B Polym Phys 55:587–600
Kalyanasundaram K, Thomas JK (1977) Environmental effects on vibronic band intensities in pyrene monomer fluorescence and their application in studies of micellar systems. J Am Chem Soc 99:2039–2044
Wilhelm M, Zhao C, Wang Y, Xu R, Winnik MA, Mura JL, Riess G, Croucher MD (1991) Poly(styrene-ethylene oxide) block copolymer micelle formation in water: a fluorescence probe study. Macromolecules 24:1033–1040
Astafieva I, Zhong XF, Eisenberg A (1993) Critical micellization phenomena in block polyelectrolyte solutions. Macromolecules 26:7339–7352
Li L, Li J, Zheng S (2018) Poly(vinylidene fluoride)-block-poly(N-vinyl pyrrolidone) diblock copolymers: synthesis via sequential RAFT/MADIX polymerization and self-assembly behavior. Polymer 142:61–71
Nishi T, Wang TT (1975) Melting point depression and kinetic effects of cooling on crystallization in poly(vinylidene fluoride)-poly(methyl methacrylate) mixtures. Macromolecules 8:909–915
Imken RL, Paul DR, Barlow JW (1976) Transition behavior of poly(vinylidene fluoride)/poly(ethyl methacrylate) blends. Polym Eng Sci 16:593–601
Rasband WS (2007) Image J, National Institutes of Health: Bethesda, MD, 2007. Please see https://rsb.info.nih.gov/ij/index.html, Accessed, July 2017
Wan LS, Li JW, Ke BB, Xu ZK (2012) Ordered microporous membranes templated by breath figures for size-selective separation. J Am Chem Soc 134:95–98
Boker A, Lin Y, Chiapperini K, Horowitz R, Thompson M, Carreon V, Xu T, Abetz C, Skaff H, Dinsmore AD, Emrick T, Russell TP (2004) Hierarchical nanoparticle assemblies formed by decorating breath figures. Nat Mater 3:302–306
Harris DJ, Lewis JA (2008) Marangoni effects on evaporative lithographic patterning of colloidal films. Langmuir 24:3681–3685
Lee JH, Ro HW, Huang R, Lemaillet P, Germer TA, Soles CL, Stafford CM (2012) Anisotropic, hierarchical surface patterns via surface wrinkling of nanopatterned polymer films. Nano Lett 12:5995–5999
Li B, Wang B, Liu Z, Qing G (2016) Synthesis of nanoporous PVDF membranes by controllable crystallization for selective proton permeation. J Membrane Sci 517:111–120
Rahimpour A, Madaeni SS, Zereshki S, Mansourpanah Y (2009) Preparation and characterization of modified nano-porous PVDF membrane with high antifouling property using UV photo-grafting. Appl Surface Sci 255:7455–7461
Lee MK, Lee J (2014) A nano-frost array technique to prepare nanoporous PVDF membranes. Nanoscale 6:8642–8648
Acknowledgments
The financial supports from Natural Science Foundation of China (No. 21774078, 51133003 and 21274091) and Anhui Province Key Laboratory of Environment-friendly Polymer Materials were gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 481 kb)
Rights and permissions
About this article
Cite this article
Zhang, Y., Zhao, B., Li, L. et al. Polyhedral oligomeric silsesquioxane-capped poly(N-vinyl pyrrolidone) amphiphiles: synthesis, self-assembly, and use as porogen of nanoporous poly(vinylidene fluoride). Colloid Polym Sci 297, 141–153 (2019). https://doi.org/10.1007/s00396-018-4440-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-018-4440-6