Abstract
Poly(ethylene-co-vinyl alcohol) (EVOH) monoliths were fabricated from two kinds of EVOH with different ethylene contents by thermally induced phase separation (TIPS) technique using a mixed solvent of isopropanol (IPA) and water. The pore and skeleton sizes could be controlled by changing the fabrication parameters such as the concentration of the polymer, the ratio of IPA and water, and the cooling temperature. The EVOH monoliths possessed relatively high specific surface area and uniform mesopore structure. Furthermore, the monolith was applied for a support matrix of enzyme immobilization. The monolith was successfully activated by carbonyldiimidazole and subsequently reacted with urease. The good reusability of the urease-immobilized monolith was demonstrated. The present EVOH monoliths have good hydrophilicity and reactive hydroxyl groups; therefore, it possesses large potential for various bio-related applications such as enzyme immobilization and protein purification.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Mesoporous materials with the structural capabilities at the scale of a few nanometers and large surface area can meet the demands of growing applications such as protein immobilization, adsorption, ion-exchange, catalysis, sensors, etc. Fabrication of mesoporous materials based on inorganic or organic-inorganic hybrid frameworks has been widely adopted using organic molecules as templates [1–4]. However, little research has been done on purely organic mesoporous materials by similar methods; such trials often lead to collapse of the mesopore structure during template removal process [5, 6]. Thus, fabrication of mesoporous polymer materials with a template-free method is a challenge work.
Development of monoliths with porous structure has been an ever-growing field over the last decade. Due to their high permeability, fast mass transfer performance, high stability, and easy chemical modification, monoliths have drawn considerable attention in applications such as separation media [7, 8], enzyme immobilization [9–12], chromatography [8], ion-exchange [13, 14], and catalysis [15–17]. Recently, we have developed a new method to fabricate polymeric monoliths from the corresponding polymer solutions by thermally induced phase separation (TIPS) or non-solvent induced phase separation. These methods are very convenient to prepare monoliths with interconnected porous structure without any templates [18–24].
EVOH is a crystalline copolymer with hydrophilic vinyl alcohol and hydrophobic ethylene segments [25]. EVOH has drawn much attention as a unique biomedical material because EVOH has many advantages such as hydrophilicity, biocompatibility, thermal stability, and chemical resistance. Unlike PVA, EVOH is not soluble in water, although it shows good hydrophilicity. EVOH is industrially prepared by special radical copolymerization of ethylene and vinyl acetate, followed by alkaline hydrolysis. Thus, its monolithic materials cannot be easily obtained from its monomers. Recently, we reported the fabrication of metal nanoparticles-EVOH composite monoliths by TIPS method [26, 27]. To widen the application of EVOH monolith, the precise tuning of its pore and skeleton sizes should be further discussed.
Urease is an enzyme that catalyzes the hydrolysis of urea to ammonia and carbon dioxide [28]. Immobilized urease is specially applied for removal of urea for blood detoxification in artificial kidneys [29]. It could also be used for construction of biosensors to detect urea contained in urine or serum [30, 31]. Various kinds of matrix have been reported for urease immobilization, such as gels, membranes, nanoparticles, and beads [32–34].
In this study, we emphasize the fabrication of a mesoporous EVOH monolith by TIPS and its application for urease immobilization. Two kinds of EVOH with different ethylene contents were applied for the fabrication of EVOH monolith. The morphology of the EVOH monolith was controlled by changing the fabrication parameters. Additionally, urease was successfully immobilized onto the chemically activated EVOH monolith. EVOH monolith with a hydrophilic segment has large potential over hydrophobic polymeric monoliths for bio-related applications such as tissue engineering, protein purification, drug delivery, and enzyme immobilization.
Experimental
Materials
Two kinds of EVOH with ethylene content of 27 and 44 mol% (abbreviated as EVOH 27 and EVOH 44, respectively), urease, bovine serum albumin (BSA), and QuantiPro™ BCA Assay Kit were supplied from Sigma Co. Carbonyldiimidazole (CDI), IPA, urea, acetone, and acetonitrile (ACN) were purchased from Wako Co. All reagents were used as received without further purification.
Fabrication of EVOH monolith
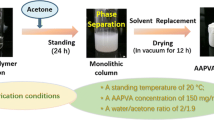
The general procedure for fabrication of an EVOH monolith is illustrated in Fig. 1. For the fabrication of an EVOH 27 monolith from an EVOH 27 solution (140 mg/mL), the experiment process is as follows. An EVOH 27 solution was prepared by dissolving 140 mg of EVOH 27 in a mixture of 0.65 mL of IPA and 0.35 mL of water at 75 °C, and the solution was cooled at 20 °C. During the cooling stage, the phase separation took place to form the monolith, which was immersed into a large amount of acetone to remove the embedded solvent and subsequently dried under vacuum.
Characterization
Scanning electron microscope (Hitachi Co., SU3500) was used to observe the monolith cross-sections. Before the measurement, the monolith was fractured to small pieces, mounted on a SEM stub, and sputtered with pure gold in vacuo. SEM images were recorded at an accelerating voltage of 15 kV. Nitrogen adsorption/desorption isotherms for the EVOH monolith were measured with a NOVA-4200e surface area and pore size analyzer (Quantachrome Instruments) at 77 K. The multipoint Brunauer Emmett Teller (BET) method and nonlocal density functional theory (NLDFT) method were used to determine the specific surface area and the pore size distribution plot, respectively. Fourier transform infrared (FT-IR) measurements were performed with Thermo Scientific (Yokohama, Japan) Nicolet iS5 by the attenuated total reflectance method. Elemental analysis was carried out on a CHN corder MT-5 (Yanaco New Science Inc.). UV was measured by an Infinite 200 (Tecan) microplate reader.
Immobilization of urease on EVOH monolith
Immobilization of urease on the EVOH monolith was carried out in the following procedure (Fig. 2). EVOH monolith (0.1 g) was immersed in 5 mL of ACN solution containing CDI (0.2 g). The reaction was carried out at 40 °C for 4 h. Afterwards, the monolith was washed thoroughly with acetone and subsequently dried under vacuum. The resulting CDI-modified EVOH monolith (CDI-EVOH monolith) was stored in 4 °C for further uses. Urease immobilization was conducted at 4 °C for 12 h. Fifty milligrams of the CDI-EVOH monolith was immersed in 10 mL of 5.0 mg/mL urease solution (dissolved in 0.1 M phosphate buffer (pH 7.0)). Then, the monolith was washed with the buffer for at least three times. The amount of urease immobilized on the monolith was determined by measuring the concentration change of the initial and final urease solutions using the bicinchoninic acid (BCA) method [35].
Activity assay of immobilized urease
Urease activity was measured by using the Berthelot reaction according to the literature [36]. Urease is a nickel-dependent enzyme that catalyzes hydrolysis of urea to form ammonia and carbon dioxide. The liberated ammonium ion reacts with salicylate and hypochlorite to give a green dye. For the evaluation of the enzyme activity, two kinds of solution were prepared: solution 1 containing 0.50 M sodium salicylate and 0.0010 M sodium nitroprusside; solution 2 containing 0.030 M sodium hypochlorite. Activity of the immobilized urease was measured as follows: 50 mg of the urease-immobilized monolith was added to 1.0 mL 0.20 mg/mL urea solution (dissolved in pH 7.6 phosphate buffer). After the reaction at 37 °C for 10 min, 0.40 mL of the reaction solution was mixed with 0.10 mL of 0.5 M NaOH, 0.50 mL of solution 1, and 0.50 mL of solution 2. The mixture was then incubated at 37 °C for 10 min. The absorbance at 625 nm was measured for determination of the concentration of the formed green dye.
The reusability of the urease-immobilized EVOH monolith (urease-EVOH monolith) was evaluated by successive activity assay. After each activity assay, the monolith (50 mg) was removed from the reaction mixture and washed thoroughly with 2 mL of a phosphate buffer (pH 7.6) for 10 min. Relative residual activity of the immobilized urease was calculated by comparing each repetition activity with the first cycle activity.
Results and discussion
Fabrication and characterization of EVOH monolith
Generally, a mediate affinity of polymers toward solvent molecules is a key factor to fabricate monoliths by TIPS method. EVOH is insoluble in neither IPA nor water. Interestingly, EVOH 27 became soluble in a mixture of IPA and water (65:35 vol%) at 75 °C. The EVOH solution with the concentration of 140 mg/mL in this mixed solvent was kept at 20 °C. During the cooling course, the phase separation took place to form the monolith (Fig. 1). This unique behavior is probably based on cosolvent effect [37, 18]; a poly(methyl methacrylate) monolith was prepared on the basis of the cosolvency by using a mixture of ethanol and water as a phase separation solvent [18].
The inside morphology of the EVOH monolith was observed by SEM, which shows the three-dimensional open pore structure of the monolith (Fig. 1). The average pore and skeleton sizes of the monolith were 2.9 and 1.9 μm, respectively. The nitrogen adsorption/desorption isotherms for the monolith showed a type IV isotherm with a relatively wide type H1 hysteresis loop in the P/P0 range from 0.5 to 1.0 (Fig. 3). By using the nonlocal density functional theory (NLDFT) method, the pore size distribution (PSD) plot for the EVOH monolith was obtained, which reveals uniform pores with diameter of ca. 5.0 nm. The specific surface area was measured as 93.1 m2 g−1 by multipoint BET method. These data indicate the formation of relatively uniform mesopores and macropores in the EVOH monolith.
By changing the polymer concentration and the composition of the mixed solvent, the range of the monolith formation was examined. Table S1 summarizes the monolith formation for EVOH 27. When the water ratio in the mixed solvent was 35 %, the monolith was formed in the polymer concentration from 80 to 180 mg/mL. In case of the polymer concentration of 100 mg/mL, the formation of the monolith with bicontinuous structure was observed in the water ratio was 30, 35, or 55 %, whereas the polymer was soluble even at room temperature in the range of the water ratio from 40 to 50 %. In the higher polymer concentration (150 mg/mL), the monolith was formed in the range of the water ratio from 35 to 55 %. These data clearly show the strong dependence of the monolith formation on the polymer concentration and solvent composition. The effect of the polymer concentration may be explained as follows. The lower concentration may result in insufficient numbers of the EVOH chains to form the continuous skeleton structure, and the phase separation is difficult to occur because of higher viscosity in case of the higher concentration.
EVOH 44 was also available for the monolith fabrication under the similar conditions (Table S2). When the concentration of EVOH 44 was in the range from 140 to 200 mg/mL, the monolith with the bicontinuous structure was obtained, which range was smaller than that for EVOH 27. In case of the concentration less than 120 mg/mL, the monolith formation was not observed. This may be due to the difference of the hydrophilicity of EVOH.
Morphology control of EVOH monolith
Effects of various fabrication parameters on the morphology of the EVOH monolith have been systematically investigated. The effect of the cooling temperature on the morphology of the EVOH monolith is shown in Fig. 4. When the cooling temperature changed from 20 to 0 °C, the average pore size of the monolith decreased from 2.9 to 0.5 μm and the skeleton size decreased from 1.9 to 0.4 μm (Fig. 5a) for EVOH 27. This may be because at higher cooling temperature, the phase separation process takes place more slowly, leading to the formation of large pores [19]. As for EVOH 44, the pore and skeleton sizes of the monolith were larger than those for EVOH 27, probably due to the different affinity and/or solubility of the polymer toward the phase separation solvent. In all cases examined, the relatively uniform structure was observed. These dates suggest that the pore and skeleton sizes could be controlled by changing the cooling temperature.
The polymer concentration also affected on the morphology of the EVOH monolith (Fig. S2). As the concentration increased from 140 to 180 mg/mL, the average pore sizes decreased from 2.9 to 1.1 μm and the skeleton sizes decreased from 1.9 to 0.8 μm (Fig. 6a) for EVOH 27. This could also be explained by the relationship of viscosity and phase separation. At higher polymer concentration, the phase separation process occurs more quickly in the viscous polymer solution, resulting in the formation of smaller skeleton pores. For EVOH 44, the similar behavior of the monolith formation was observed, and the change of the pore and skeleton sizes against the polymer concentration was smaller than that for EVOH 27.
Immobilization of urease on EVOH monolith
In this study, EVOH monolith was applied for matrix of immobilized enzymes. Hydrophilic matrices are often suitable for immobilized enzymes, especially, their use in aqueous media; thus, EVOH 27 exhibiting higher hydrophilicity was selected. In the following experiments, the material used was the monolith prepared by the following conditions: EVOH 27 with the concentration of 150 mg/mL, the water ratio of 35 %, the cooling temperature of 20 °C.
CDI is a well-known reagent to activate a hydroxyl group for introduction of functional molecules [38, 39]. As shown in Fig. 1, the hydroxyl group of EVOH on the monolith surface was modified by CDI for introduction of urease on the monolith. Figure 7 shows the FT-IR spectra of EVOH and CDI-EVOH monolith. A broad peak at 3325 cm−1 due to the hydroxyl group decreased after the activation by CDI. The imidazolyl-carbamate group of the product was confirmed by a peak at 1750 cm−1 due to asymmetric stretch of the carbonyl group, a peak at 1460 cm−1 to CH2 scissor of deformation of CDI, peaks at 1394 and 1246 cm−1 to C-N stretches of CDI. These data show the successful modification of EVOH monolith by CDI.
The modification by CDI would be mainly made on the monolith surface. As a measure of the modification monitoring, the elemental analysis of the CDI-EVOH monolith was carried out (Fig. 8). The nitrogen content in the monolith increased with increasing the CDI concentration, suggesting the control of the surface composition as a function of the CDI concentration. SEM observation shows that the inside morphology of the monolith hardly changed after the modification (Fig. S1).
The mechanical strength of the CDI-EVOH monolith prepared by using 60 and 80 mg/mL CDI was found to be inferior to that by the lower concentration of CDI or of the unmodified monolith by qualitative evaluation. Thus, the monolith modified by using 40 mg/mL CDI, which possessed relatively good toughness and high content of the activated group, was used for immobilization of urease. The amount of urease onto the monolith was measured as 4 mg urease per 1 g of the monolith.
The immobilized urease showed good catalytic activity for hydrolysis of urea, which may be because urea can reach the catalyst active site of urease in a short time due to the good hydrophilicity and interconnected pore structures of the EVOH monolith. Furthermore, the immobilized urease could be repeatedly used (Fig. 9). Ninety three percent of the relative activity remained after eight successive cycles. The large stability for recycle usage might be most attributable to the stable covalent bond between urease and the EVOH monolith, preventing urease escape from the matrix during urea decomposition process. This good reusability of the present urease-EVOH monolith is significant for potent bio-related industrial applications of the present monolith.
Conclusions
A mesoporous EVOH monolith was fabricated from the EVOH solution in a mixed solvent of IPA and water by TIPS. The morphology of the monolith could be controlled by altering the fabrication conditions such as the mixed ratio of IPA and water, the cooling temperature, and the concentration of EVOH. The resulting EVOH monolith possessed a large specific surface area and uniform mesoporous structure. Urease was immobilized on the EVOH monolith activated by CDI. The good reusability of the urease-EVOH monolith was demonstrated.
Unlike PVA, EVOH is insoluble in water, although it shows high hydrophilicity. Thus, the crosslinking is not required for usage of the EVOH monolith in aqueous media. Additionally, nonionic and hydrophilic EVOH is a suitable material for suppression of nonspecific adsorption of biomolecules such as proteins and DNA. Based on these characteristics, the present EVOH monolith may be useful for a variety of bio-related applications. Further studies on applications of the EVOH monolith for enzyme immobilization and protein purification are underway in our laboratory.
References
Wan Y, Zhao D (2007) On the controllable soft-templating approach to mesoporous silicates. Chem Rev 107:2821–2860
Li W, Zhao D (2013) An overview of the synthesis of ordered mesoporous materials. Chem Commun 49:943–946
Kresge CT, Leonowicz ME, Roth WJ, Vartuli JC, Beck JS (1992) Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 359:710–712
Langley PJ, Hulliger J (1999) Nanoporous and mesoporous organic structures: new openings for materials research. Chem Soc Rev 28:279–291
Chandra D, Jena BK, Raj CR, Bhaumik A (2007) Functionalized mesoporous cross-linked polymer as efficient host for loading gold nanoparticles and its electrocatalytic behavior for reduction of H2O2. Chem Mater 19:6290–6296
Modak A, Mondal J, Sasidharan M, Bhaumik A (2011) Triazine functionalized ordered mesoporous polymer: a novel solid support for Pd-mediated C-C cross-coupling reactions in water. Green Chem 13:1317–1331
Urban J, Svec F, Fréchet JMJ (2010) Efficient separation of small molecules using a large surface area hypercrosslinked monolithic polymer capillary column. Anal Chem 82:1621–1623
Urban J, Svec F, Fréchet JMJ (2010) Hypercrosslinking: new approach to porous polymer monolithic capillary columns with large surface area for the highly efficient separation of small molecules. J Chromatogr A 1217:8212–8221
Kato M, Inuzuka K, Sakai-Kato K, Toyo’oka T (2005) Monolithic bioreactor immobilizing trypsin for high-throughput analysis. Anal Chem 77:1813–1818
Pierre SJ, Thies JC, Dureault A, Cameron NR, Van Hest JCM, Carette N, Michon T, Weberskirch R (2006) Covalent enzyme immobilization onto photopolymerized highly porous monoliths. Adv Mater 18:1822–1826
Svec F (2006) Less common applications of monoliths: I. Microscale protein mapping with proteolytic enzymes immobilized on monolithic supports. Electrophoresis 27:947–961
Benčina M, Benčina K, Štrancar A, Podgornik A (2005) Immobilization of deoxyribonuclease via epoxy groups of methacrylate monoliths: use of deoxyribonuclease bioreactor in reverse transcription-polymerase chain reaction. J Chromatogr A 1065:83–91
Nandi M, Okada K, Uyama H (2011) Functional mesoporous polymer monolith for application in ion-exchange and catalysis. Funct Mater Lett 4:407–410
Lubbad SH, Bandari R, Buchmeiser MR (2011) Ring-opening metathesis polymerization-derived monolithic strong anion exchangers for the separation of 5′-phosphorylated oligodeoxythymidylic acids fragments. J Chromatogr A 1218:8897–8902
Kočí P, Schejbal M, Trdlička J, Gregor T, Kubíček M, Marek M (2007) Transient behaviour of catalytic monolith with NOx storage capacity. Catal Today 119(1–4):64–72
Tomašić V (2007) Application of the monoliths in DeNOx catalysis. Catal Today 119:106–113
Kundu D, Patra AK, Sakamoto J, Uyama H (2014) A palladium-loaded mesoporous polymer monolith as reusable heterogeneous catalyst for cross-coupling reactions. React Funct Polym 79:8–13
Shinya Y, Wenjuan H, Urara H, Hiroshi U (2014) Facile fabrication of poly(methl methacrylate) monolith via thermally induced phase separation by utilizing unique cosolvency. Polymer (United Kingdom) 55:3212–3216
Xin Y, Fujimoto T, Uyama H (2012) Facile fabrication of polycarbonate monolith by non-solvent induced phase separation method. Polymer (United Kingdom) 53:2847–2853
Park SB, Sakamoto J, Sung MH, Uyama H (2013) Macroscopic cavities within a microporous 3-D network: a poly(γ-glutamic acid) monolith prepared by combination of particulate templates and a phase separation technique. Polymer (United Kingdom) 54:6114–6118
Sun X, Fujimoto T, Uyama H (2013) Fabrication of a poly(vinyl alcohol) monolith via thermally impacted non-solvent-induced phase separation. Polym J (Tokyo, Jpn) 45:1101–1106
Okada K, Nandi M, Maruyama J, Oka T, Tsujimoto T, Kondoh K, Uyama H (2011) Fabrication of mesoporous polymer monolith: a template-free approach. Chem Commun 47:7422–7424
Xin Y, Uyama H (2012) Fabrication of polycarbonate and poly(3-hydroxybutyrate-co-3-hydroxyhexanoate) blend monolith via non-solvent-induced phase separation method. Chem Lett 41:1509–1511
Han W, Xin Y, Hasegawa U, Uyama H (2014) Enzyme immobilization on polymethacrylate-based monolith fabricated via thermally induced phase separation. Polym Degra Stab 109:362–366
Lv R, Zhou J, Du Q, Wang H, Zhong W (2006) Preparation and characterization of EVOH/PVP membranes via thermally induced phase separation. J Membr Sci 281:700–706
Wang G, Yoshikawa H, Tamiya E, Uyama H (2015) Mesoporous poly(ethylene-co-vinyl alcohol) monolith captured with silver nanoparticles as a SERS substrate: facile fabrication and ultra-high sensitivity. RSC Adv 5:25777–25780
Wang G, Kundu D, Uyama H (2015) One-pot fabrication of palladium nanoparticles captured in mesoporous polymeric monoliths and their catalytic application in C-C coupling reactions. J Colloid Interface Sci 451:184–188
Mobley HLT, Island MD, Hausinger RP (1995) Molecular biology of microbial ureases. Microbiol Rev 59:451–480
Chang TM (1966) Semipermeable aqueous microcapsules (“artificial cells”): with emphasis on experiments in an extracorporeal shunt system. Artif Intern Organs 12:13–19
Lee WY, Kim SR, Kim TH, Lee KS, Shin MC, Park JK (2000) Sol-gel-derived thick-film conductometric biosensor for urea determination in serum. Anal Chim Acta 404:195–203
Shalini J, Sankaran KJ, Lee CY, Tai NH, Lin IN (2014) An amperometric urea bisosensor based on covalent immobilization of urease on N2 incorporated diamond nanowire electrode. Biosens Bioelectron 56:64–70
Chen JP, Chiu SH (1999) Preparation and characterization of urease immobilized onto porous chitosan beads for urea hydrolysis. Bioprocess Biosyst Eng 21:323–330
Kayastha AM, Srivastava PK (2001) Pigeonpea (Cajanus cajan L.) urease immobilized on glutaraldehyde-activated chitosan beads and its analytical applications. Appl Biochem Biotechnol Enzym Eng Biotechnol 96:41–53
Krajewska B, Leszko M, Zaborska W (1990) Urease immobilized on chitosan membrane: preparation and properties. J Chem Technol Biotechnol 48:337–350
Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC (1985) Measurement of protein using bicinchoninic acid. Anal Biochem 150:76–85
Chaney AL, Marbach EP (1962) Modified reagents for determination of urea and ammonia. Clin Chem 8:130–132
González-Benito J, Koenig JL (2006) Nature of PMMA dissolution process by mixtures of acetonitrile/alcohol (poor solvent/nonsolvent) monitored by FTIR-imaging. Polymer (United Kingdom) 47:3065–3072
Bethell GS, Ayers JS, Hancock WS, Hearn MT (1979) A novel method of activation of cross-linked agaroses with 1,1′-carbonyldiimidazole which gives a matrix for affinity chromatography devoid of additional charged groups. J Biol Chem 254:2572–2574
Li YC, Liao YT, Chang HH, Young TH (2013) Covalent bonding of GYIGSR to EVAL membrane surface to improve migration and adhesion of cultured neural stem/precursor cells. Colloids Surf B 102:53–62
Acknowledgments
This study is financially supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 25288090) and a Project for Creating Start-ups from Advanced Research and Technology, MEXT.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, G., Uyama, H. Reactive poly(ethylene-co-vinyl alcohol) monoliths with tunable pore morphology for enzyme immobilization. Colloid Polym Sci 293, 2429–2435 (2015). https://doi.org/10.1007/s00396-015-3637-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3637-1