Abstract
Employing polymethacrylic acid (PMAA) as the template and N-vinyl pyrrolidone (N-VP) as monomer, the ATRP-template miniemulsion polymerization was carried out in the aqueous medium by using MBP/CuBr/bpy as initiator. The results were characterized by dynamic light scattering (DLS), transmission electron microscope (TEM), and gel permeation chromatography (GPC). It was observed that the stable particles exhibited amphoteric pH sensitivity, namely that in the range of pH 3.0 to 5.0, the particles precipitated, whereas beyond the range the particles were stable and swollen as pH varied. Moreover, the pH range was variable according to the molecular weight of PVP. The results of GPC indicated that the molecular weight of template polymer PMAA was duplicated by the daughter polymer PVP. Being noncross-linked, unlike the common microgels, the hydrodynamic diameter dramatically increased in a very narrow pH range, e.g., pH 5.5 –6 and 2.0–2.5. Finally, the nanoparticles of PMAA/PVP were applied for the controlled release of rifampicin (RFP) and doxorubicin (DOX).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Particles of sensitive polymer allowing a drastical variation of physical state, stimulated by the change of external environment such as temperature [1–5] and pH [6–9], have attracted great interests in the fields of biomedicine and bioapplications. These particles are considered as the ideal carriers for the drug delivery in human body. Therefore, most of sensitive polymers are biocompatible and water-soluble. For example, poly(N-isopropylacrylamide) (PNiPAm) [1] is a thermosensitive polymer, exhibiting the change of water solubility at ca 33 °C, whilst poly(methacrylic acid) (PMAA) [6] is a pH-sensitive polymer which shows the variation of water solubility at different pH. Since the loci of bioapplications are aqueous, the survival of particles consisting of these polymers prior to the drug delivery is always an important topic in the design and preparation of particles, especially for the pH-sensitive particles. A lot of methods have been developed to prepare the particles sizing from nanometers to micrometers such as the emulsion polymerization, dispersion/precipitation polymerization, and self-assembly of block polymers as well [10]. However, comprehensively reviewing these methods, methodologically, two strategies have been applied, namely utilizing the hydrophobic polymer segments or aggregates to control the dissolution of water-soluble polymer and crosslinking the water-soluble polymer chains. The latter is well known as the hydrogel. On the view of metabolism, the best drug delivery system (DDS) is those metabolizable easily and quickly soon after the drug is released. Accordingly, the disadvantages of these two strategies are obvious. For example, except for rare hydrophobic polymer like polylactic acid, most hydrophobic polymers are not degradable. As for the hydrogel, it is also difficult to be degraded due to cross-linking structure. Therefore, it is necessary to develop a new type of DDS that is readily to be metabolized.
On the other hand, since the changes of temperature and pH in the body are very weak, the sharp response of particles to a weak stimulus is needed. It is not difficult for the temperature-sensitive polymer, PNiPAM, because its volume sharply expands at the low critical solution temperature [1–3]. However, for pH-sensitive polymers like polymethacrylic acid, the volume change is largely dependent to the dissociation of carboxylic acid. The volume gradually expands as pH increases until pH = 4.5 near to pKa of methacrylic acid [4, 8, 9]. Moreover, the low pH such as 4.5 is unrealistic in the body on the view of application. Therefore, many approaches have been developed to improve its pH sensitivity [4–12]. A common approach applied to adjust the pH at which the polymer exhibits sharp volume change is the addition of polymers carrying amide or amino groups. For example, the microspheres of poly (MAA-co-acrylamide (AAm)) cross-linked by N, N′-methylene bis (acrylamide) (MBA) exhibit a sharp volume transition at pH 4.5 [6]. In particular, the amphoteric microgel of poly (MAA-co-N, N′-dimethylamino ethylene methacrylate (DMAEMA)) shows a special pH sensitivity. It shrinks in the pH range from pH 6.5 to 8, but greatly expands at pH either less than 6.5 or higher than 8. Additionally, the range of pH can be adjusted with different ratios of MAA and DEAEMA. However, these microgels are all prepared in ethanol and, unfortunately, cross-linked. In aqueous solution, these microgels cannot be prepared. Therefore, it is necessary to develop a method to prepare the pH-sensitive microgel without cross-linking and simultaneously adjust the pH to the bio-environmental pH, i.e., pH 6–7, at which the response takes place.
It is well known that the insoluble complexes can form with two soluble polymers such as polyacrylic acid–polyethylene oxide (PAAc-PEO) and PAAc-polyvinyl pyrrolidone (PVP) in the aqueous solution [11, 12]. Inspired by it and combined with the knowledge of template polymerization [13] and the mechanism of particle formation [14–16], in this paper, a new method is proposed to prepare the pH-sensitive particles consisted of two water-soluble polymers without cross-linking in the aqueous solution. As shown in Scheme 1, a template polymer H-bond interacts with a monomer. After the template polymer saturated adsorbs the monomer, a globule forms. Selecting an appropriate initiation system that is able to dominantly partition within the globule, the polymerization of monomer will take place solely in the globule and create a nanoparticle. Since the saturated concentration of monomer in the globule is determined by the unit ratio of template polymer and monomer, the length of daughter polymer is adjustable. Accordingly, if a pH-sensitive polymer such as PMAA is selected as the template polymer, whilst PVP is chosen as the daughter polymer, the pH sensitivity of particles will be variable according to length of PVP. This approach has been proven to be feasible [17]. In our previous work, the formation of globule with PVP and MAA was confirmed. Various initiators were applied for the preparation of stable latex including the oil-soluble initiator, azobisisobutyronitrile (AIBN), water-soluble initiator, potassium persulfate (KPS), and redox initiator. Finally, it was observed that the stable well-defined nanoparticles of PVP/PMAA (PVP as template) were prepared only by using the initiation system for atom transfer radical polymerization (ATRP) [17]. Moreover, the molecular weight of father polymer, PVP, was duplicated by the daughter polymer, PMAA. The reason was considered that Cu+ and 2, 2′-bipyridine (bpy) in ATRP initiation system were dominantly partitioned in the globules due to the interaction with PMAA, whereas the oil-soluble initiators such as AIBN could not enter the globules passing through the aqueous phase. For KPS, most of them were partitioned in the aqueous phase; thus, only MAA therein was polymerized. As for the drug loading and releasing, the H-bond interaction is also utilizable. As shown in Scheme 1, the drug molecules H-bond interact with polymers at lower pH. At higher pH, the H-bond will be destroyed; thereby, the particles will be disintegrated and the drug is released. Since both of PMAA and PVP are water-soluble and biocompatible, they will be easily metabolized soon after the drugs are released [18, 19]. In addition, the candidates of such drug are numerous [20–22]. For example, the potential antitumor drugs, e.g., doxorubicin and paclitaxel, can form H-bond with both PMAA and PVP.
Therefore, in order to investigate the effects of PVP on the pH sensitivity of PMAA, in this paper, the pH-sensitive polymer, PMAA, is selected as the template polymer, whilst VP is used as the monomer. The nanoparticles will be prepared by using ATRP initiator system. Meanwhile, the DDS behavior of these nanoparticles will be addressed, in addition to the effects of salts such as phosphate buffered solution (PBS), calcium chloride, etc.
Experimental
Materials
All the chemical reagents used in this paper were purchased from Aladdin Chemical Reagent Co. Ltd., Shanghai, China, except for 2,2′-azobis (2,4-dimethylvaleronitrile) (V-65) from the J&K Chemical Reagent Co. Ltd., Japan. Methacrylic acid (MAA) and N-vinyl pyrrolidone (N-VP) were purified by distillation under the reduced pressure. V-65, rifampicin (RFP), doxorubicin (DOX), and the ATRP initiator system of methyl 2-bromopropionate (MBP), 2,2′-bipyridine (bpy) except for CuBr, were used without further purification. CuBr was purified by stirring and successively washing with acetic acid and methanol, respectively, then dried for 24 h under vacuum oven. DDI water was used for all experiments.
Polymerization
All polymerizations were performed in a four-necked 100-ml flask equipped with a thermometer, a condenser (also outlet of N2), an inlet of N2 gas, and an inlet for charging the chemical reagents, which settled in a thermostat water bath with magnetic stirrer.
Poly (methacrylic acid)
The template polymer polymethacrylic acid (PMAA) was self-synthesized in ethanol at 70 °C by using 2,2′-azobis(2,4-dimethylvaleronitrile) (V-65) as initiator [23]. The formulated reagents except for V-65 were added into the flask and deoxygenated by N2 bubbling for 1 h at room temperature. After the temperature of water bath was reached at 70 °C, V-65 was added into the system. Polymerization was carried out for 8 h at nitrogen atmosphere. The polymer was precipitated by diethyl ether and dried at 60 °C for 12 h under vacuum. The viscosity average molecular weights of prepared PMAA, Mν were 30,000 and 48,000, respectively.
Poly (methacrylic acid)/poly (N-vinyl pyrrolidone) composite
For ATRP, the molar ratio of composition was set at N-VP/bpy/CuBr/MBP = 100:4:1:2 [17]. Formulated PMAA and N-VP were charged firstly into the flask, and pH was adjusted by adding the standard HCl or NaOH solution. Thereafter, the mixture of the above solution was deoxygenated by bubbling N2 for 2 h at the ambient temperature. The tin foil was employed to cover the polymerization system in order to shade the light. At the temperature of polymerization, bpy and CuBr were added at first. After all of CuBr dissolved, i.e., disappeared at the bottom of flask judging by naked eyes, MBP was charged. This moment was set as the start of polymerization.
Characterization
The conversion of N-VP was measured gravimetrically. Since the father polymer PMAA and daughter polymer PVP in ATRP polymerization system were not separable, as described in our previous paper [17], a special calculation approach was developed. For the same reason, the molecular weight of PVP was estimated by an available high-performance liquid chromatography (HPLC) (RID-10A Shimazu, Japan) equipped with a gel permeation column (TSK-GEL SW, Tosoh Co. Ltd., Japan). PBS was employed as the solvent and elution phase, and the speed of elution phase was 1 ml/min at 25 °C.
The hydrodynamic diameters were measured by the dynamic light scattering (DLS) (90 plus Particle Sizer/BI-MAS, Brookhaven, USA) by which the scattering light at 90゜was collected; thereby, the diameters were automatically calculated. The solution (or latex) was directly used for DLS measurements, and all measurements were performed at 25 °C. TEM (JEM2000EX, JEOL, Japan) images of nanoparticles were also taken, which the operations were described elsewhere [17].
The behavior of control release was estimated by the ultraviolet-visible light spectroscopy (UV-Vis) (UV-2450, Shimazu Co. Ltd., Japan). The drug loading was measured as following: Dry nanoparticles were redispersed in the aqueous solution of drug with formulated concentration and pH and then centrifuged at 8000 rmp/5 min. The concentration of drug in serum was measured by UV–Vis for the calculation of drug loading. The drug releasing was detected as following: Dry nanoparticles were redispersed in the aqueous solution of drug with formulated concentration and pH and then centrifuged at 8000 rmp/5 min. The concentration of drug in serum was measured by UV–Vis for the calculation of drug releasing. The equations for calculating the drug loading and releasing are shown as following:
Results and discussion
Concentrations of template PMAA and N-VP
As described in our previous paper [17], the concentrations of father polymer and the daughter monomer are two key factors for the preparation of stable latex by the template polymerization since they seriously affected the stability of polymer–monomer globules. In the polymer solution with a high concentration, the entanglement of chains takes place [24, 25], which, in turn, evokes the bridge effect among globules [15]. The bridge effect is one of the reasons for the coagulation of particles [15] and has been widely applied for the precipitation of nanoparticles [26]. Therefore, an appropriate concentration of polymer is the first premise for the preparation of stable latex. On the other hand, the high concentration of monomer impacts both of the solubility parameter of dispersion system and size of globules, especially in case of MAA that greatly affects the pH of system. The over-sized globules and unbalanced solubility parameters between polymer and dispersing solution are usually two factors for the failure of preparation of stable latex [15]. Therefore, in our previous paper, the concentrations of father polymer PVP and the daughter monomer MAA were determined by using conductance meter [17]. As a result, the concentration of PVP (K-30) should be lower than 10 wt%, whilst the concentration of MAA should be lower than 5 wt%. Of course, the concentration of PVP is related to its molecular weight. On the basis of these results, the concentration of father polymer PMAA was formulated to be lower than 5 wt% and the concentration of N-VP was lower than 10 wt%.
The number-averaged sizes of PMAA/N-VP particles prepared by ATRP-template polymerization are shown in Table 1. As shown in Table 1, at the same ratio of MAA/N-VP (run 1–5), the hydrodynamic diameter of particles decreased as pH increased. For example, as the ratio of MAA/N-VP was equal to 1:1.5 (3 wt% PMAA and 6 wt% N-VP), corresponding to the pH that increased from 3.84 to 4.70, the diameters are about 340, 80, 74, 63, and 32 nm. It confirmed that pH played a key factor to the formation of globules. The globule was created by the H-bond interaction of PMAA and N-VP, rather than the ionic interaction. The higher pH of system indicated the higher degree of dissociation of PMAA; thus, more H-bonds of PMAA/N-VP were destroyed. However, at pH 3.84, the particles were unstable due to the high ionic strength, whilst as pH > 4.70 the particles were not prepared because of the degradation of globules. On the other hand, in order to increase the solid content of latex, the other ratios of MAA/N-VP, i.e., 1:1.2 (4 wt% PMAA, 6 wt% N-VP) and 1:2 (3 wt% PMAA, 7.8 wt% N-VP), were tried. It was observed that the particle size was big (Table 1, run 6 and 7) and unstable. These results indicated that the solid content of 3 wt% PMAA and 6 wt% N-VP was the highest in this polymerization system.
The typical size distributions of particles at different pHs and TEM micrograph of particles in stable latex are shown in Fig. 1. According to DLS diagrams, it is clear that the number of particles with the bigger size was very small. Moreover, the peak of smaller size distribution moved to the smaller scale as pH increased. TEM micrograph indicated that the nanoparticles were successfully prepared.
Conversion of ATRP template miniemulsion polymerization
As we know, in the template polymerization, there exist two types of monomer molecules, i.e., monomers interacting with template polymers and the free monomers in the aqueous phase. The globule is formed by the former type of monomers. Since most of ATRP initiator is partitioned in the globules, the conversion of N-VP is dominantly determined by the partition of N-VP between the globules and the aqueous phase. There are mainly three factors affecting the partition of monomer, i.e., pH, the concentrations of monomers, and template polymer. In the previous section, the effects of pH on the stability and size of particles was described. In fact, as for the conversion of N-VP, it was observed that the conversion was increased with the decrease of pH. This result was normal since the low pH depressed the dissociation of PMAA, which was favorable to the H-bond interaction. However, as pH was lower than 4, the particles were unstable. Therefore, in this paper, pH 4.17 was selected and the conversion of N-VP vs. the polymerization time of ATRP template miniemulsion is shown in Fig. 2. It was observed that, with the highest solid content of PMAA (3 wt%) and N-VP (6 wt%), the highest conversion of N-VP was only 64 % in 9 h. This result was ascribed to the partition of N-VP and ATRP initiator in the globules. Besides, compared with the polymerization by using thermally degradable initiator such as 2,2′-azobis(isobutyl nitrile) (AIBN) and potassium persulfate (KPS), ATRP-template miniemulsion polymerization exhibited a shorter induction time. Therefore, these results further confirmed that most of ATRP initiators partitioned in the globules.
The effects of concentrations of template polymer and the monomer on the conversion of N-VP are shown in Fig. 3, respectively. As shown in Fig. 3a (N-VP, 6 wt%), when the concentration of PMAA (Mν = 30,000) was lower than 1.0 wt%, the conversion of N-VP was very low. When the concentration of PMAA increased from 1.0 to 3 wt%, the conversion of N-VP increased. These results indicated that the conversion of N-VP was greatly dependent to the concentration of template polymer. It is normal because the adsorbed amount of N-VP on PMAA increased with the increase of PMAA chains. However, the more important reason should be attributed to the partition of initiators. In fact, as the concentration of PMAA was less than 1.0 %, the insoluble MBP was obviously found to float on the top of aqueous phase. Finally, it should be remarked that when the concentration of PMAA was 4 % (N-VP, 6 wt%), the globules of PMAA/N-VP precipitated.
Figure 3b exhibits the effects of the concentration of N-VP on the conversion of N-VP (PMAA, 3 wt%). As expected, the conversion of N-VP increased as the concentration increased until 4 wt%. It indicated that the saturated adsorption of N-VP on PMAA was ca. 4 wt%. As the concentration increased from 4 to 6 wt%, the conversion of N-VP was just increased from ca. 60 to 64 %.
In conclusion, the optimal condition for the preparation of particles was considered as pH 4.2, 3 wt% of PMAA, and 6 wt% of N-VP.
Molecular weight of the daughter polymer PVP
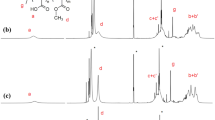
Since it was hard to separate the daughter polymer PVP and mother polymer PMAA in the aqueous solution, the mixture of two polymers was eluted simultaneously with a gel permeation chromatography (GPC) equipment. As shown in Fig. 4a, using PMAA with different molecular weights (Mν = 48,000 and 30,000) as the template polymers, the daughter polymers PVP can basically duplicate their father molecular weights by ATRP template miniemulsion polymerization. Certainly, the duplication is affected by the ratios of PMAA/N-VP because the amount of N-VP adsorbed on PMAA is determined by both of the concentration of PMAA and N-VP. Figure 4b exhibits the effects of concentrations of PMAA and N-VP on the duplication of molecular weight. It was observed that as the concentration of PMAA was low (Fig. 4b), the peak of elution time moved to the longer time, whilst the width of peak became narrow. For example, the peak of PMAA was at 10.7 min. However, when MAA/N-VP = 0.2/1 and 0.4/1, the main peak was located at 10.9 min accompanied with several small peaks with the longer elution time. This result indicated that a lot of PVP with the small molecular weight were prepared. However, when MAA/N-VP = 0.6/1, the curve of mixed polymers (peak at 10.8 min) almost overlapped with that of father polymer, PMAA. It indicated that the molecular weight of PMAA was completely duplicated. These results were likely ascribed to the partition of initiators. It should be expected that, at the lower concentration of PMAA, the adsorption of N-VP on PMAA chains should be saturated. However, the lower concentration of PMAA also implied that the number of globules should be small. Therefore, the more ATRP initiators were partitioned in one globules, which turned out a lot of PVP with the smaller molecular weight.
Figure 4c shows the effects of the concentration of N-VP on the duplication of molecular weight. It was observed that, as the concentration of N-VP increased from 3 to 6 wt%, the peak moved to the shorter elution time; meanwhile, the curve became broader. This result indicated that the molecular weight of daughter polymer PVP increased as the concentration of N-VP increased. Since the concentration of PMAA was constant, the number of globules and the partition of ATRP initiators inside of one globule were constant. Therefore, this result was attributed to the increase of N-VP in the globules. It is normal that the concentration of N-VP inside of globules increased as the concentration of N-VP increased.
pH–volume transition
Utilizing the property of pH–volume transition, PMAA is usually selected as the main composition of pH-sensitive carriers for control release of drug. However, the pH–volume transition of pure PMAA takes place at pH4.5, closed to the pKa of COOH group. Such pH is far from the pH in body where the drug carrier works. Therefore, many approaches have been applied to elevate the pH at which the volume transition occurs, for instance copolymerizing amide [8, 9, 27] or amine [6, 28] group containing monomers with MAA. As we know, using the amide-containing monomers such as acrylamide (AAm) [8, 9] and N-isopropyl acrylamide (NIPAM) [27], the so far highest pH of volume transition is about pH 5. Using the amine-containing monomers [6, 28], the pH of volume is adjustable in a large range of pH. However, the instability of carriers in the aqueous phase is always an obstacle for the bio-application due to the existence of zwitterions in the surface of carrier. Therefore, in this paper, a new approach was proposed to elevate the pH of volume transition, namely pairing a PMAA chain with a PVP chain of different length.
Figure 5 shows the pH–volume transition of PMAA/PVP particles prepared by ATRP polymerization. It should be remarked at first that, unlike those common pH-sensitive microgel (lightly cross-linked chains) [6, 8, 9, 27, 28] which the hydrodynamic diameter increases with the increase of pH, until the microgel is over-swollen at high pH (from which the diameter begins to decrease with the increase of pH), as shown in Fig. 5, the hydrodynamic diameter of uncross-linked PMAA/PVP particles directly decreased with the progress of swollen degree. This feature implies that the degradation of H-bonded pair of PMAA/PVP chains starts from the outside and gradually progress to the inside of particle. In other words, the microgel expands isotropically due to the constrained chains conducting the osmotic pressure towards the inside of microgel, whereas in the particle of uncross-linked PMAA/PVP hereof, as those in microgel, the outer H-bonds are suffered from the attacks of OH− at first, but the disbanded segments of PMAA and PVP are free due to the absence of chemically cross-linking bonds. These free segments with the dissociated COO− ions on the outer layer of the particle effectively relieves the osmotic pressure created by OH− in the aqueous phase and temporally protected the inside H-bonds. Such a feature was expected to give the more effectively release of drug directly evoked by the outside pH of particles, rather than the inside pH as microgels. It was also the speculation of design (Scheme 1).
As shown in Fig. 5, the pH sensitivity of particles is similar to that of particles copolymerized PMAA with N,N-dimethyl amino ethylene methacrylate (DMAEMA) [6], i.e., in a certain pH range, the particle precipitated, whereas beyond the range the particles expanded. For example, in the range of pH 3.8 to 7, the particles precipitated (Fig. 5a, MAA/N-VP = 1:1.5). When pH > 7, the diameter decreased as pH increased (particle expanded). Moreover, the pH range changed as the ratio of MAA/N-VP changed. For example, in Fig. 5a (Mν = 30,000), corresponding to the ratios of 1:0.75, 1:1, and 1:1.5, the pH ranges of precipitation were pH 2.5–5.0, 3.0–6.2, and 3.8–7.0. Similar result was also observed in the case of using PMAA (Mν = 48,000) as the template polymer. It indicated that pH response range could be moved to the higher pH by elongating the length of PVP. Furthermore, in the higher pH range, the particles expanded slowly as the increase of pH, whereas it sharply expanded in the lower pH range. However, such a difference disappeared in PBS. As shown in Fig. 6, there is no difference. Both of the particles (MAA/N-VP = 1:0.75 and 1:1) exhibited the same pH-sensitive behavior, namely that they precipitated in the same pH range (pH 2.5–5.5), and beyond the pH range, the particles were abruptly disbanded by a small variation of pH (pH 2.0–2.5 and 5.5–6.0). It is reminiscent of effects of ions on the pH sensitivity of microgel [6, 9].
In the practice, the effects of ions on the pH sensitivity of particles are inevitable, but the composition of PBS is complicated; thus, the simple salts such as NaCl and CaCl2 were selected. As shown in Fig. 7, the effects of Na+ and Ca2+ are significant. For example, in the NaCl solution, the particle size was much bigger than those in water and CaCl2 solution. Meanwhile, the pH of system was significantly increased as [CaCl2] increased, in contrast to the almost constant pH in NaCl solution. It indicated that NaCl just acted as a precipitant, whereas the dissociation of COOH was depressed in the presence of Ca2+. These results are quite different from those obtained in the microgels of P(MAA/AAm) [8] and P(MAA/DMAEMA) [6]. In those microgels, both of pH and diameter decreased as the ion concentration increased, irrespective of Na+ and Ca2+. Reminiscent of the action Ca2+ in the aqueous solution of gelatin [29], in this paper, the action of Ca2+ seemed similar. The aqueous solution of gelatin interacted with the groups of protein chains, thereby condensing the gelatin. In contrast, Na2HPO4 diluted the solution of gelatin [29]. Accordingly, it implies that the performance of PMAA/PVP in particles prepared by ATRP miniemulsion polymerization is something like the performance of gelatin. It interpreted the abnormal pH-sensitive behavior of particles in PBS (Fig. 6), though the reason was unclear so far.
The pH sensitivity of particles in CaCl2 solution was further investigated. As shown in Fig. 8, the hydrodynamic diameter exhibits more sensitive to pH at the higher concentration of CaCl2. For example, in 0.1 mol/L CaCl2, the diameter increased from 105 to 190 nm, whereas it varied from 100 to 150 nm in 0.01 mol/L CaCl2. It was coincident with the result that the pH increased with the increase of CaCl2 concentration (Fig. 7). This result further proved that Ca2+ could condense the particle.
Drug delivery by PMAA/PVP particles
As models of application, the particles of PMAA (Mν = 30,000)/PVP (MAA/N-VP = 1:1.5) were selected as nanocarriers for two types of drugs. One was doxorubicin (DOX), a good candidate for model anticancer drug experiments due to its consistent reported value of water solubility in acid and adriamycin metamorphism occurred in base. The other one was rifampicin (RFP) with very good solubility in PBS at basic condition. These two drugs, RFP and DOX, were able to be loaded in the particles, as shown in Scheme 1, by forming hydrogen bonds with PMAA and PVP.
It should be remarked that the loading of RFP was carried out at pH 3.6 in PBS. The reason was that the loading efficiency was the highest, about 60 %, at this pH value, whereas the loading of DOX was conducted at pH 7 and about 57 % DOX was loaded. It was reported that the structure of DOX was not stable in alkaline solution; thereby, the release of DOX was conducted in the acidic solution. For the release of RFP in PBS, as shown in Fig. 9, at pH 5.6, about 58 % of RFP was released, whereas at pH 6.0 about 95 % of RFP was released. The release profile was much closed to the profile of pH–volume transition of particles in PBS (Fig. 6).
The release profiles of DOX are also shown in Fig. 9. It is clear that the release of DOX was significantly impacted by CaCl2. In 0.01 mol/L CaCl2 solution, the release rate of DOX was slow in the range of pH 3.0–4.0, but abruptly accelerated in the range of pH 2.0–3.0. In contrast, the release rate was much higher in the pure water. This result indicated that Ca2+ played a role to seal the drug molecules in the particles. It was coincident with the above result that Ca2+ fastened the PMAA-PVP chains by the interactions.
Anyway, the release profiles indicated that the behavior of drug release by PMAA/PVP particles prepared in this paper was quite different from those of pH-sensitive microgels. Particles hereof sustained the release of drug in a large range of pH, no matter whether the particles precipitate or not. It was completely controlled by the pH of the surrounding medium.
Conclusions
PMAA/PVP nanoparticles were prepared by ATRP-template miniemulsion polymerization, where PMAA was used as template and PVP as the daughter polymer. pH was the predominant factor to prepare the stable emulsion. At pH < 3.8, the coagulum was obtained, whereas pH > 4.7 the transparent solution was prepared by ATRP-template miniemulsion polymerization. Therefore, the optimum condition for the preparation of stable latex was pH 4.2, 3 wt% PMAA, and 6 wt% N-VP. The averaged size of particles was around 50 nm.
GPC results indicated that the molecular weight of PMAA was duplicated by the daughter polymer PVP. Moreover, the molecular weight of PVP increased as the concentration of N-VP increased. Being noncross-linked, the pH–volume transition of PMAA/PVP was particular to the common pH-sensitive microgels, namely that the particles precipitated in a certain range of pH, whereas beyond the pH range the particles were stable and swollen as pH varied. Effects of CaCl2 and NaCl on the particles were quite different. NaCl acted as a pure precipitant, whereas CaCl2 played the cross-linking role.
DDS behaviors of PMAA/PVP were evaluated with two drugs, i.e., RFP and DOX. It was observed that, at pH 3.6, the loaded efficiency of RFP was about 60 %, and in a very narrow range of pH 5.6–6.0, there is about 95 % of RFP that was released in PBS. For DOX, at pH 7, the loaded efficiency was about 57 %. The releasing rate of DOX was affected by CaCl2. In the range of pH 3.0–4.0, the rate was slower than that in the pure water, but when pH < 3.0, the releasing rate was accelerated. All the above results indicated that, unlike the lightly cross-linked microgel wherein the cross-linked chains transferred the osmotic pressure of OH− from the outer chains into the inside chains, the noncross-linked PMAA/PVP particles were directly responded to the change of surrounding medium without the transfer of osmotic pressure.
References
You YZ, Kalebaila KK, Brock SL, Oupicky D (2008) Temperature-controlled uptake and release in PNIPAM-modified porous silica nanoparticles. Chem Mater 20:3354–3359
Li LY, He WD, Li J, Zhang BY, Pan TT, Sun XL, Ding ZL (2010) Shell-cross-linked micelles from PNIPAM- b-(PLL)2 Y-shaped miktoarm star copolymer as drug carriers. Biomacromolecules 11:1882–1890
Scherzinger C, Lindner P, Keerl M, Richtering W (2010) Cononsolvency of poly( N, N-diethylacrylamide) (PDEAAM) and poly(N-isopropylacrylamide) (PNIPAM) based microgels in water/methanol mixtures: copolymer vs core -shell microgel. Macromolecules 43:6829–6833
Nikdel M, Salami-Kalajahi M, Salami Hosseini M (2014) Synthesis of poly(2-hydroxyethyl methacrylate-coacrylic acid)-grafted graphene oxide nanosheets via reversible addition–fragmentation chain transfer polymerization. RSC Adv 4:16743–16750
Bauri K, Pant S, Roy SG, De P (2013) Dual pH and temperature responsive helical copolymer libraries with pendant chiral leucine moieties. Polym Chem 4:4052–4060
Ni HM, Kawaguchi H, Endo T (2007) Preparation of amphoteric microgels of poly(acrylamide/methacrylic acid/dimethylamino ethylene methacrylate) with a novel pH-volume transition. Macromolecules 40:6370–6376
Zhan K, Liu HL, Zhang H, Chen YL, Ni HM, Wu M, Sun DM, Chen Y (2014) A facile method for the immobilization of myoglobin on multi-walled carbon nanotubes: poly(methacrylic acid-co-acrylamide) nanocomposite and its application for direct bio-detection of H2O2. J Electroanal Chem 724:80–86
Ni HM, Kawaguchi H, Endo T (2007) Characteristics of pH-sensitive hydrogel microsphere of poly(acrylamide-co-methacrylic acid) with sharp pH–volume transition. Colloid Polym Sci 285:873–879
Ni HM, Kawaguchi H, Endo T (2007) Preparation of pH-sensitive hydrogel microspheres of poly(acrylamide-co-methacrylic acid) with sharp pH–volume transition. Colloid Polym Sci 285:819–826
Esser-Kahn AP, Odom SA, Sottos NR, White SR, Moore JS (2011) Triggered release from polymer capsules. Macromolecules 44:5539–5553
Nurkeeva ZS, Mun GA, Khutoryansiy VV, Zotov AA, Mangazbaeva RA (2000) Interpolymer complexes of poly(vinyl ether) of ethylene glycol with poly(carboxylic acids) in aqueous, alcohol and mixed solutions. Polymer 41:7647–7651
Okubo T, Okamoto J, Tsuchida A (2010) Convectio nal, sedimentary, and drying dissipative patterns of colloida l suspension s of polymer complexes of poly(acrylic acid) with poly(ethylene glycol) and poly(vinyl pyrrolidone). Colloid Polym Sci 288:189–197
Polowinski S (2002) Template polymerization and co-polymerisation. Prog Polym Sci 27:537–577
Ni HM, Ma GH, Nagai M, Omi S (2001) Effects of ethyl acetate on the soap-free emulsion polymerization of 4-vinylpyridine and styrene. I. Aspects of the mechanism. J Appl Polym Sci 82:2679–2691
Ni HM, Ma GH, Nagai M, Omi S (2001) Mechanism of soap-free emulsion polymerization of styrene and 4-vinylpyridine: characteristics of reaction in the monomer phase, aqueous phase, and their interface. Macromolecules 34:6577–6585
Ni HM, Kawaguchi H (2004) Mechanism of preparing monodisperse poly(acrylamide/methacrylic acid) microspheres in ethanol. I. J Polym Sci A Polym Chem 42:2823–2832
Yang CC, Meng D, Zhan K, Chen YL, Zhang H, Wu M, Ni HM (2014) ATRP-template dispersion polymerization of methacrylic acid/PVP. Chin J Polym Sci 32:476–487
McFarlane NL, Wagner NJ, Kaler EW, Lynch ML (2010) Poly( ethylene oxide) ( PEO) and poly( vinyl pyrolidone) ( PVP) induce different changes in the colloid stability of nanoparticles. Langmuir 26:13823–13830
Liu X, Sun K, Wu Z, Lu J, Song B, Tong W, Shi X, Chen H (2012) Facile synthesis of thermally stable poly(N-vinylpyrrolidone)-modified gold surfaces by surface-initiated atom transfer radical polymerization. Langmuir 28:9451–9459
Burke PA, Pun SH, Reineke TM (2013) Advancing polymeric delivery systems amidst a nucleic acid therapy renaissance. ACS Macro Lett 2:928–934
Kazarian SG, Chan KLA (2003) “Chemical photography” of drug release. Macromolecules 36:9866–9872
Duncan R (2006) Polymer conjugates as anticancer nanomedicines. Nat Rev Cancer 6:688–701
Zhan K, Zhang H, Li M, Chen YL, Chen GX, Liu JX, Wu M, Ni HM (2014) Charges of soluble amphiphiles and particles: random and diblock copolymerizations of MAA/AAm, MAA/St, and MAA/4VP in ethanol. Colloid Polym Sci 292:1553–1565
Ji GD, Ni HM, Wang C, Xue Q, Liao YT (1996) Concentration dependence of crystalline poly(ethylene terephthalate) prepared by freeze-extracting solutions. Macromolecules 29:2691–2693
Sun B, Lu Y, Ni HM, Wang C (1998) Highly crystallizable poly(ethylene terephthalate) prepared by freeze-extracting solutions. Polymer 39:159–165
Wu M, He Q, Shao QF, Wang YG, Zuo F, Ni HM (2011) Preparation and characterization of monodispersed microfloccules of TiO2 nanoparticles with immobilized multienzymes. ACS Appl Mater Interfaces 3:3300–3307
Bartlett RL II, Panitch A (2012) Thermosensitive nanoparticles with ph-triggered degradation and release of anti-inflammatory cell-penetrating peptides. Biomacromolecules 13:2578–2584
Dong J, Zhang RC, Wu H, Zhang XW, Yang H, Zhu SQ, Wang GJ (2014) Polymer nanoparticles for controlled release stimulated by visible light and pH. Macromol Rapid Commun 35:1255–1259
Zhang H, Zhan K, Chen YL, Chen GX, Zhou XM, Liu JX, Wu M, Ni HM (2014) Three dimension Liesegang rings of calcium hydrophosphate in gelatin. J Sol-Gel Sci Technol 71:597–605
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, G., Liu, J., Yang, Y. et al. Preparation of pH-sensitive nanoparticles of poly (methacrylic acid) (PMAA)/poly (vinyl pyrrolidone) (PVP) by ATRP-template miniemulsion polymerization in the aqueous solution. Colloid Polym Sci 293, 2035–2044 (2015). https://doi.org/10.1007/s00396-015-3554-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-015-3554-3