Abstract
A polymer, poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMDM), was accumulated on a silica microparticle by surface-initiated atom transfer radical polymerization (ATRP). The hydrodynamic diameter of the polymer-grafted silica particle (PMDM-SiP) dispersed in water was observed to decrease above 24 °C, which could be ascribed to a coil-globule transition of PMDM chains. This is in accordance with the increase in turbidity of an aqueous solution of free PMDM, which was produced parallel in liquid phase at the ATRP, above 23 °C. The lower critical solution temperature (LCST), defined as the temperature at which the absorbance reached the optical density of 0.30, was 27.6 °C for free PMDM. An introduction of zwitterionic vinyl monomer, carboxymethylbetaine (CMB), into both a polymer brush and a free polymer (P(MDM-r-CMB)) raised the LCST. In addition, the hydrodynamic diameter (D h) of the polymer-modified particles was not affected by the presence of NaBr nor lysozyme at 15 °C, whereas it was largely affected at 35 °C due to the decrease in hydrophilicity of the polymer graft chain above the LCST. In contrast, bovine serum albumin did not affect the D h value of the polymer-modified particles both below and above the LCST. The usefulness of the PMDM brush having CMB as a comonomer in the biomedical field was suggested.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Modification of solid surface with a functional soft moiety drastically enlarges usefulness of the materials. The surface modification with synthetic polymer chains is categorized into two classes. The first one is a “polymer layer,” that is, a polymer chain fixed to the solid substrate at multiple points. In the procedures to construct the polymer layer, both covalent binding and chemical and physical adsorption of polymer chains are included [1–4].
The other class of surface modification with polymer chains is a “polymer brush” in which one end of the polymer chain is chemically or physically fixed to the solid substrate. For constructing polymer brushes, the surface-initiated polymerization (“grafting-from” method) [5–7] and the grafting of preformed polymers on the surface of solid materials via covalent bond or physical adsorption (“grafting-to” method) have been examined [8–13].
Meanwhile, a suppression of nonspecific binding of biomolecules to solid materials is very important for biomedical applications of the materials. For example, polymer brushes composed of a zwitterionic monomer residue such as 2-methacryloyloxyethyl phosphorylcholine (MPC, phosphobetaine), 3-sulfo-N,N-dimethyl-N-(2-methacryloyloxyethyl)propanaminium inner salt (SPB, sulfopropylbetaine), and 1-carboxy-N,N-dimethyl-N-(2-methacryloyloxyethyl)methanaminium inner salt (CMB, carboxymethylbetaine) (PMPC, PSPB, and PCMB, respectively) on a gold surface and a glass substrate are highly resistant against nonspecific protein adsorption and cell adhesion [8, 14, 15].
Silica nano- and microparticles have been focused as a potential carrier in the field of medical imaging system, drug delivery, and targeting systems. Especially, fluorescent-encapsulated and metal-encapsulated (such as Gd and Au) silica particles for the CT and MR imaging have been reported previously [16–18]. Generally, the interaction of biomolecules with the surface of silica particles used in the medical field must be suppressed or regulated, and the introduction of additional function such as thermal and pH responsiveness to the surface of silica particles would lead to further application.
In this report, a temperature-responsive polymer brush-modified silica particle has been prepared by the surface-initiated atom transfer radical polymerization (SI-ATRP). 2-(2-Methoxyethoxy)ethyl methacrylate (MDM) has been chosen as a basic monomer for temperature-responsive polymers [19–22] because of easiness to pursue ATRP. For the ATRP of MDM, it is not necessary to use high-cost multi-dentate ligands such as N,N,N′,N″,N″-pentamethyldiethylenetriamine (PMDETA) and tris[2-(dimethylamino)ethyl]amine (Me6TREN). These ligands have often been used for the preparation of poly-N-isopropylacrylamide (PNIPAm), one of the most well-known temperature-responsive polymers, using ATRP [23–25]. Furthermore, a zwitterionic monomer, carboxymethylbetaine (CMB), has been introduced as a comonomer in the temperature-responsive polymer brush to modulate its responsiveness.
Experimental
Materials
MDM (Scheme 1a) from Tokyo Chemical Industry, Tokyo, Japan, was purified from hexane-0.1 M NaOH aqueous solution. CMB monohydrate (commercial name, GLBT; Scheme 1b) was kindly donated from Osaka Organic Chemical Industry, Osaka, Japan [26, 27]. 2,2′-Azobisisobutyronitrile (AIBN) from Wako Pure Chemicals, Osaka, was recrystallized in methanol. Bovine serum albumin (BSA) and lysozyme from egg white (EWL) were obtained from Sigma. Silica microparticle (Seahoster KE-P30; diameter, 270–350 nm) was kindly donated from Nihon Shokubai, Co., Osaka, Japan. 3-(2-Bromo-2-isobutyryloxy)propyltriethoxysilane (BPE; Scheme 1c) was synthesized from propen-1-ol by a two-step reaction, as described elsewhere [28]. Silicon wafers [N(100), having 0.001–0.003 Ω cm resistivity and 0.525 ± 0.025 mm thickness] from Furuya Metal Co., Ltd. (Tokyo, Japan) were cut into the most suitable size (20 × 10 mm2). Other reagents used were commercially available. All aqueous solutions were prepared with ultrapure water (18 MΩ cm, Millipore System).
Preparation of initiator-coated silica microparticles via silane coupling
At first, silica microparticles (SiP, 400 mg), which had been washed and ultrafiltrated (Millipore; membrane JG, pore size, 0.20 μm) several times with ethanol (EtOH), were dispersed in EtOH (30 mL) in a reaction vial. BPE (423.5 mg), 20 % aqueous ammonia solution (4.6 mL), and EtOH (15 mL) were added to the vial and the reaction solution was slowly stirred at room temperature (Scheme 2) [29]. After 18 h, the dispersion of microparticle was washed with EtOH several times using an ultrafiltration apparatus. The initiator-coated microparticle (SiP-BPE) was dispersed in EtOH and stored in a sample vial filled with Ar.
Accumulation of PMDM brush on silica microparticles
Poly(2-(2-methoxyethoxy)ethyl methacrylate) (PMDM) brush was introduced to the surface of the silica microparticle: The SiP-BPE (400 mg/32.6 mL EtOH) was incubated with MDM (7.4 mL, 40 mmol), EBiB (14.7 μL, 0.10 mmol), Cu(I)Br (29 mg, 0.20 mmol), and 2,2′-bipyridine (63.3 mg, 0.40 mmol) ([MDM]:[EBiB]:[CuBr]:[bipyridine] = 400:1:2:4) at 25 °C for 48 h in Ar (Scheme 2). The SiP-PMDM was separated from a homopolymer of MDM (Et-PMDM), which had been parallel produced in liquid phase, by ultrafiltration in EtOH. Et-PMDM in liquid phase was recovered, and after evaporation, dissolved in EtOH again and passed through a silica gel column to remove Cu salts. The Et-PMDM was purified by dialysis (Spectra/Por, Spectrum Laboratories; MWCO, 1000) in MeOH and condensed by evaporation. After dissolution in water and lyophilization, the Et-PMDM was characterized by GPC (column, Shodex OHpak SB-803HQ; Showa Denko, Tokyo, Japan; mobile phase, 0.1 M NaBr aqueous solution, chloroform, or methanol/ethanol (50:50) mixture; standard, pullulan (Showa Denko), PMMA (Showa Denko), or PEG (Sigma-Aldrich)). The similar procedures were adopted for preparation of the copolymer-modified SiPs (Scheme 3). In addition, the polymer brushes (PMDM and copolymers, P(MDM-r-CMB)s) were prepared on the surface of a silicon wafer in the same way as that on the glass surfaces too.
Surface density of the polymer brush on the glass substrate
The surface density (in chains/nm2) of the polymer brushes on a silicon wafer was determined by both the thickness of the brush determined by ellipsometry (M-2000U, J. A. Woollam Co., Inc., USA) and the molecular weight of the polymers produced simultaneously in liquid phase during surface grafting. The M n values of the graft and free polymers produced in the same batch were previously found to be nearly the same in both ATRP [30] and RAFT polymerization [31].
Measurements of hydrodynamic diameter and ζ potential of microparticles
Hydrodynamic diameter (D h) and ζ potential of various silica microparticles were determined by a ζ potential and particle size analyzer (ELSZ-2, Otsuka Electronics, Hirakata, Japan; laser, He–Ne, 632.8 nm). Dynamic light scattering (DLS) measurements were carried out in water unless mentioned. At the measurements of ζ potential, microparticles were dispersed in a 10-mM NaCl aqueous solution.
Determination of LCST
An aqueous solution of PMDM or P(MDM-r-CMB) (1 mg/mL) in a glass cell (light path 10 mm) with a PMMA cap was set in an observation chamber of a spectrophotometer (Lambda 19 UV/VIS/NIR Spectrometer, PerkinElmer). The turbidity (actually decadic absorbance) at 350 nm was followed at various temperatures (heating and cooling rates, 1 °C/3 min except the temperature range 5 °C above the lower critical solution temperature (LCST) (2 °C/3 min)). The temperature at which the absorbance of the polymer solution reached the optical density of 0.30 (transmittance 50 %) during the heating process was defined as LCST.
Results and discussion
Preparation of free polymers by ATRP
At the surface-initiated atom transfer radical polymerization (SI-ATRP) in the presence of a free ATRP initiator (EBiB in this work), the polymerization in the solution phase occurred parallel. It was previously indicated that the polymers obtained in the solution phase had similar M n and M w/M n values to those parallel constructed at the solid surface [30]. The GPC data indicated that the polymers obtained in this work had a satisfactorily narrow distribution of molecular weight (Table 1).
Temperature responsiveness of free polymers
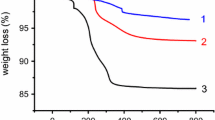
Temperature responsiveness of free homopolymer (PMDM) and copolymers (P(MDM-r-CMB)s) were examined at 350 nm (Fig. 1, Table 1). The figure and table showed that all the polymers indicated a distinct coil-globule transition, and the LCST value was raised with an increase in the content of CMB. Such a temperature responsiveness was ascribed to the dehydration of the 2-(2-methoxyethoxy)ethyl side group of the polymer with the increase in temperature, which drastically decreases the hydrophilicity of polymer chains and results in the coil-globule transition [20].
By the incorporation of a hydrophilic comonomer, dehydration of the side chain in PMDM would be hindered to some extent, resulting in the rise in LCST. It was previously observed that the incorporation of ω-methoxy(oligoethyleneoxy)ethyl methacrylate as a comonomer raised the LCST of PMDM [19]. We also reported that the LCST of poly(N-isopropylacrylamide) (PNIPAm) was raised by the incorporation of a hydrophilic comonomer such as acrylamide [31]. These results are in accordance with the present results. Furthermore, pH dependence of the LCST for polyallylamine grafted with NIPAm chains was clearly observed [32]. These results indicated the possibility to modulate the LCST of polymer materials by various factors.
There was no significant hysteresis of PMDM and P(MDM-r-CMB) in the absorbance vs. temperature profiles in water. When the solvent was changed from water to phosphate-buffered saline (PBS), the LCST decreased due to the decrease in activity of water which dissolved the polymer, and furthermore, the hysteresis appeared (Fig. 2). However, the tendency in the copolymer system was opposite to the ordinary hysteresis where the profile of decrease in absorbance shifted to the lower-temperature region, which is probably due to the entangled structure of the polymer chains within the globules, resulting in retardation of the re-dissolution of globules during the cooling process. In contrast, Fig. 2 shows that the decrease in absorbance in the cooling process in PBS occurred at a higher temperature than the increase in the heating process. Such an opposite tendency might be attributed to the perturbation of entanglement and aggregation by the polar CMB residues in the polymer.
Construction of a polymer brush onto a silica microparticle
We introduced ATRP initiator, BPE, on the surface of a silica microparticle, and subsequently, the ATRP was pursued from the initiating site on the surface of the particle. The hydrodynamic diameter (D h) of the silica particle was observed to increase at each modification step (Fig. 3, Table 2) (i.e., (i) introduction of ATRP initiator and (ii) grafting of PMDM brush or P(MDM-r-CMB) brush), which indicated that chemical modification of the particle surface was successfully pursued.
Polymer brushes were also constructed on silicon wafers by the same procedures as those for the modification of glass substrates, and the thicknesses of various polymer brushes determined by ellipsometry were 6.8, 3.2, and 3.8 nm for PMDM, copolymer 1, and copolymer 2, respectively.
The data by DLS (hydrodynamic diameter of the polymer-modified silica microparticles) and ellipsometry (thickness of polymer brushes on the silicon wafer) were contradictory with each other, even by taking account of the extension of hydrated polymer brush in the aqueous media in comparison with the shrunken brush in dry state. This is probably due to the slight aggregation of silica particles during SI-ATRP, though the polydispersity indices for the diameter of particles were satisfactorily small even after the ATRP.
Using Eq. (1), the surface density of the polymer brush on the silicon wafer was also determined.
where σ is the graft density (chains/nm2), ρ is density of the polymer (g/cm3), N A is the Avogadro number, d is the thickness of the polymer brush (nm), and M n is the number-averaged molar mass of bulk polymer [30, 33]. The ρ value for polymethylmethacrylate (PMMA, 1.318 g/cm3) [34] was used for calculation of the surface density of PMDM and copolymers. M n of the polymer brush was assumed to be equal to that of the polymer produced in liquid phase at the same time [30]. The surface densities of the brushes were 0.104, 0.162, and 0.082 chains/nm2 for SiP-PMDM, SiP-copolymer 1, and SiP-copolymer 2, respectively.
The ζ potential also changed upon modification with a polymer (Table 2), which suggests the presence of polymer brush on the surface of the particle. Previously, it was reported that the ζ potentials for glass plates modified with a brush of poly(methacrylic acid) (PolyMA) and that of poly[2-(dimethylamino)ethyl methacrylate] (PolyDMAEMA) prepared by the reversible addition-fragmentation chain transfer (RAFT) method were −30.2 and +35.3 mV, respectively, and that with a Poly(MA-r-DMAEMA) brush (feeding ratio, MA:DMAEMA = 1:1) was −6.8 mV [35]. Furthermore, the ζ potential of PolyCMB brush prepared by the ATRP method on a glass substrate was −4.9 mV [35]. The ζ potential of the bare silica particle (SiP) was largely negative (−31.3 mV, Table 2), which would affect the ζ potential of polymer-grafted SiP. Actually, the ζ potential of silica particle modified with zwitterionic PCMB brush was reported to be −18.4 mV [29]. Electrophoretic properties of a soft particle having a rigid core were theoretically analyzed in detail [36]. The present results could be interpreted as a core-shell model having a negatively charged rigid core and a nonionic soft shell. Therefore, the surface of both the polymer brush (PMDM) and the copolymer brush (P(MDM-r-CMB)) on the SiP might not be strongly charged at 25 °C [37].
Temperature responsiveness of surface-confined PMDM brush
The PMDM chain introduced on the surface of SiP indicated temperature responsiveness in a similar manner to the water-soluble polymers. Figure 4 indicates the hydrodynamic diameter (D h) of bare SiP, SiP-PMDM, and SiP-P(MDM-r-CMB) at various temperatures. The polymer-modified SiPs indicated temperature-responsive changes in D h values, whereas the bare SiP notably did not.
Figure 5 indicates the reproducibility of D h values during the heating-cooling processes. Repeated changes in temperature between 15 and 35 °C (SiP-PMDM) and between 20 and 40 °C (SiP-P(MDM-r-CMB)) induced the reproducible changes in the D h value of the polymer-modified SiPs, whereas the D h for the bare SiP notably did not change (data not shown).
Effect of additives on the colloid stability of microparticles
Furthermore, the D h value was affected by the dispersing media (Fig. 6). As for SiP, the D h value was increased by the addition of NaBr, NaBr + BSA, lysozyme, and NaBr + lysozyme at 15 and 35 °C. BSA alone did not affect the dispersion at both temperatures. As for PMDM-SiP, the D h value was not affected at 15 °C by the additives examined in this work. At 35 °C, however, NaBr, NaBr + BSA, lysozyme, and NaBr + lysozyme increased the D h value, whereas BSA alone did not. Therefore, the aggregation of PMDM-SiP in the presence of NaBr + BSA was mostly due to NaBr and not to BSA.
Lysozyme was extremely adsorptive to the SiP with and without graft chains. This is mostly due to the electrostatic attraction between positively charged lysozyme (pI 10.5) [38] and deprotonated silanol groups on the surface of SiP, which might diminish the electrostatic repulsion between both negatively charged SiPs and SiP-PMDMs.
The similar tendency was observed for SiP-P(MDM-r-CMB). As indicated in Fig. 4c, the P(MDM-r-CMB) graft chain on the SiP particle began to shrink around 35 °C and reached an equilibrated state around 40 °C. Figure 6b indicates that the surface of copolymer-modified SiP below LCST was anti-biofouling, whereas it turned to be adsorptive above LCST in a similar manner to the homopolymer (PMDM)-modified SiP. This is because the hydrophilic CMB residues in the shrunken copolymer brush (above the LCST) might be overwhelmed by the excess amount of dehydrated (apolar) MDM residues.
Recently, a zwitterionic monomer (SPB) was introduced into PNIPAm, and rheological interpretation of the specific ion effect on the LCST was pursued [39]. Thermoresponsive glycopolymers, which were prepared by combining RAFT polymerization, thiol-ene reaction, and subsequent immobilization onto solid supports, indicated thermoresponsive interaction with the lectin RCA120 [40]. Further development of stimuli-responsive polymer materials having additional functions ascribable to incorporated comonomers will be highly promising.
Conclusion
The PolyMDM brush could be easily constructed on a silica microparticle by the SI-ATRP. The introduced PMDM brush was hydrophilic and resistant against the nonspecific adsorption of proteins (BSA and lysozyme) on the surface below the LCST of the PMDM, but induced self-aggregation in the presence of salt and proteins above the LCST. Moreover, introduction of zwitterionic CMB residues to the brush as a comonomer raised the LCST, while the shrunken copolymer brush above the LCST provided a nonpolar surface in a similar way to the homopolymer (PMDM) brush, which may be appropriate for diverse biomedical applications.
References
Kitano H, Maehara Y, Matano M, Sugimura M, Shigemori K (1997) Interfacial recognition of sugar residues in block copolymers by lectin as studied by the multiple internal reflection fluorescence method. Langmuir 13:5041–5048
Kitano H, Fukui N, Ohhori K, Maehara Y, Kokado N, Yoshizumi A (1999) Temperature-responsiveness of A-B-A block telomers at solid-liquid interfaces. J Colloid Interf Sci 212:58–64
Chang Y, Chen S, Zheng Z, Jiang S (2006) Highly protein-resistant coatings from well-defined diblock copolymers containing sulfobetaines. Langmuir 22:2222–2226
Decher G (1997) Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science 277:1232–1237
Ohno K, Koh K, Tsujii Y, Fukuda T (2002) Synthesis of gold nanoparticles coated with well-defined, high-density polymer brushes by surface-initiated living radical polymerization. Macromol 35:8989–8993
Ohno K, Koh K, Tsujii Y, Fukuda T (2003) Fabrication of ordered arrays of gold nanoparticles coated with high-density polymer brushes. Angew Chem Int Ed 42:2751–2754
Edmondson S, Osborne VL, Huck WTS (2004) Polymer brushes via surface-initiated polymerizations. Chem Soc Rev 33:14–22
Kitano H, Kawasaki A, Kawasaki H, Morokoshi S (2005) Resistance of zwitterionic telomers accumulated on metal surfaces against nonspecific adsorption of proteins. J Colloid Interf Sci 282:340–348
Kitano H, Tachimoto K, Shinohara H (2004) Wrapping of single-walled carbon nanotubes with A-B-A block telomers. Macromol Chem Phys 205:2064–2071
Matsuura K, Ohno K, Kagaya S, Kitano H (2007) Carboxybetaine polymer-protected nanoparticles: high dispersion stability and resistance against non-specific adsorption of proteins. Macromol Chem Phys 208:862–873
Morokoshi S, Ohhori K, Mizukami K, Kitano H (2004) Sensing capabilities of colloidal gold modified with self-assembled monolayer of a glucose-carrying chain on a glass substrate. Langmuir 20:8897–8902
Kitano H, Anraku Y, Shinohara H (2006) Sensing capabilities of colloidal gold monolayer modified with a phenylboronic acid-carrying polymer brush. Biomacromolecules 7:1065–1071
Kitano H, Takahashi Y, Mizukami K, Matsuura K (2009) Kinetic study on the binding of lectin to mannose residues in a polymer brush. Colloids Surf B 70:91–97
Zhang Z, Chao T, Chen S, Jiang S (2006) Superlow fouling sulfobetaine and carboxybetaine polymers on glass slides. Langmuir 22:10072–10077
Kitano H, Suzuki H, Kondo T, Sasaki K, Iwanaga S, Nakamura M, Ohno K, Saruwatari Y (2011) Image printing on the surface of anti-biofouling zwitterionic polymer brushes by ion beam irradiation. Macromol Biosci 11:557–564
Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI (2008) Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2:889–896
Lee CS, Chang HH, Bae PK, Jung J, Chung BH (2013) Bifunctional nanoparticles constructed using one-pot encapsulation of a fluorescent polymer and magnetic (Fe3O4) nanoparticles in a silica shell. Macromol Biosci 13:321–331
Mignot A, Truillet C, Lux F, Sancey L, Louis C, Denat F, Boschetti F, Bocher L, Gloter A, Stephan O, Antoine R, Dugourd P, Luneau D, Novitchi G, Figueiredo LC, de Morais PC, Bonneviot L, Albela B, Ribot F, Van Lokeren L, Dechamps-Olivier I, Chuburu F, Lemercier G, Villiers C, Marche PN, Le Duc G, Roux S, Tillement O, Perriat P (2013) A top-down synthesis route to ultrasmall multifunctional Gd-based silica nanoparticles for theranostic applications. Chem A Eur J 19:6122–6136
Kitano H, Nakaji-Hirabayashi T, Gemmei-Ide M, Kyogoku M (2004) Effect of macrocycles on the temperature-responsiveness of poly(methoxydiethylene glycol methacrylate)-graft-PEG. Macromol Chem Phys 205:1651–1659
Maeda Y, Kubota T, Yamauchi H, Nakaji T, Kitano H (2007) Hydration changes of poly(2-(2-methoxyethoxy)ethyl methacrylate) during thermosensitive phase separation in water. Langmuir 23:11259–11265
Kitano H, Kago H, Matsuura K (2009) Temperature-responsive polymer brush constructed on a colloidal gold monolayer. J Colloid Interf Sci 331:343–350
Kitano H, Kondo T, Suzuki H, Ohno K (2010) Temperature-responsive polymer-brush constructed on a glass substrate by atom transfer radical polymerization. J Colloid Interf Sci 345:325–331
Ye J, Narin R (2009) Water-assisted atom transfer radical polymerization of N-isopropylacrylamide: nature of solvent and temperature. J Phys Chem B 113:676–681
Alli S, Alli A, Hazer B (2012) Hyperbranched homo and thermoresponsive graft copolymers by using ATRP-macromonomer initiators. J Appl Polym Sci 124:536–548
Cai Y, Liu Y-Y (2013) Amphiphilic unimolecular nanoparticles based on a hyperbranched polyacrylate and a PNIPAm shell: synthesis via ATRP and properties. Macromol Chem Phys 214:892–891
Okuda Y (2000) Japan Patent 3032155
Uchiyama Y, Okuda Y, Ninoi T, Mukaiyama T (2007) Japan Patent 3878315
Ohno K, Akashi T, Huang Y, Tsujii Y (2010) Surface-initiated living radical polymerization from narrowly size-distributed silica nanoparticles of diameters less than 100 nm. Macromol 43:8805–8812
Suzuki H, Murou M, Kitano H, Ohno K, Saruwatari Y (2011) Silica particles coated with zwitterionic polymer brush: formation of colloidal crystals and anti-biofouling properties in aqueous medium. Colloids Surf B 84:111–116
Ohno K, Morinaga T, Koh K, Tsujii Y, Fukda T (2005) Synthesis of monodisperse silica particles coated with well-defined, high-density polymer brushes by surface-initiated atom transfer radical polymerization. Macromol 38:2137–2142
Takeuchi S, Omodaka I, Hasegawa K, Maeda Y, Kitano H (1993) Temperature-responsive graft copolymers for immobilization of enzymes. Makromol Chem 194:1991–1999
Kitano H, Yan C, Nakamura K (1991) Microspheres prepared from temperature-sensitive graft polymers. Makromol Chem 192:2915–2923
Tsujii Y, Ejaz M, Sato K, Goto A, Fukuda T (2001) Mechanism and kinetics of RAFT-mediated graft polymerization of styrene on a solid surface. 1. Experimental evidence of surface radical migration. Macromolecules 34:8872–8878
Brandrup J, Immergut EH, Grulke EA, Grulke EA, Abe A, Bloch D (2003) Polymer handbook, 4th Ed; Wiley-Interscience
Kitano H, Kondo T, Kamada T, Iwanaga S, Nakamura M, Ohno K (2011) Anti-biofouling properties an amphoteric polymer brush constructed on a glass substrate. Colloids Surf B 88:455–462
Ohshima H (2010) Biophysical chemistry of biointerfaces. Wiley, Hoboken
Ohshima H, Makino K, Kato T, Fujimoto K, Kondo T, Kawaguchi H (1993) Electrophoretic mobility of latex particles covered with temperature-sensitive hydrogel layers. J Colloid Interface Sci 159:512–514
Yamakawa T, Imahori K (1979) Biochemistry data book, vol I. Tokyo Kagaku-Dojin, Tokyo
Obiweluozor FO, GhavamiNejad A, Hashmi S, Vatankhah-Varnoosfaderani M, Stadler FJ (2014) A NIPAM–zwitterion copolymer: rheological interpretation of the specific ion effect on the LCST. Macromol Chem Phys 215:1077–1091
von der Ehe C, Weber C, Wagner M, Czaplewska JA, Gottschaldt M, Schubert US (2014) Synthesis of thermoresponsive glycopolymers combining RAFT polymerization, thiol-ene reaction, and subsequent immobilization onto solid supports. Macromol Chem Phys 215:1306–1318
Acknowledgments
This research was supported by a Grant-in-Aid for Scientific Research (22350101 and 26188100) from the Japan Society for the Promotion of Science (JSPS) and a Grant-in-Aid for Innovative Areas (20106007) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Tokyo, Japan. T. N.-H. is grateful to the Japan Science & Technology Promotion Foundation (JST) for the tenure track program. The authors are indebted to Osaka Organic Chemical Industry for the kind gift of CMB.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nomura, K., Makino, H., Nakaji-Hirabayashi, T. et al. Temperature-responsive copolymer brush constructed on a silica microparticle by atom transfer radical polymerization. Colloid Polym Sci 293, 851–859 (2015). https://doi.org/10.1007/s00396-014-3476-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-014-3476-5











 ) SiP-copolymer 1. The data for SiP-copolymer 1 at 40 °C were also indicated in b (
) SiP-copolymer 1. The data for SiP-copolymer 1 at 40 °C were also indicated in b ( )
)