Abstract
A series of new cholesteric side-chain liquid crystalline polymers were prepared containing cholesteric monomer and nonmesogenic chiral monomer. All polymers were synthesized by graft polymerization using polymethylhydrosiloxane as backbone. The mesomorphic properties were investigated by differential scanning calorimetry, polarizing optical microscopy and X-ray diffraction measurements, and temperature-changing solidistic optical rotation. The chemical structures of the monomers and polymers obtained were confirmed by Fourier transform infrared and proton nuclear magnetic resonance spectra. M1 showed cholesteric phase during the heating and the cooling cycle. Polymer P1 were chiral smectic A phase, whereas P2–P7 were cholesteric phase. Experimental results demonstrated that nonmesogetic chiral moity offered the possibility of application because of its lower glass-transition temperature, and the glass-transition temperatures and isotropization temperatures reduced, and the ranges of the mesophase temperature changed abruptly at first and then smoothly with increasing the content of chiral agent.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
It is well known that the cholesteric liquid crystalline has outstanding optical properties: It selectively reflects visible light and exhibits brilliant colors if the pitch of the cholesteric helix coincide with the wavelength of visible light with the material [1]. Because of the angular dependence of the reflection conditions, different colors are seen depending on the observation angle. This occurs in a band of wavelengths Δλ=PΔn, where P = 2d is the pitch, d the spatial period, and Δn the birefringence [2]. Cholesterics have been subjected to different applications such as optical filters for selective reflection and large optical rotation, thermal imaging, flat-panel displays, laser, or paint technologies [3–9].
It is common knowledge from low molar mass liquid crystals (LC) that the presence of a chiral center in the molecules are arranged in a helicoidal structure along an axis perpendicular to their longitudinal axis. Based on this, chiral LC can be achieved by mainly three methods as follows: by directly inducing cholesteric materials and monomer into the polymer backbone; by photoreacting when suitable reactive groups are present; and by inducing chiral materials and nematic LC moiety into the polymer backbone [10–13].
Recently, many novel side-chain chiral LC materials have been reported [14–23] Therefore, it would be necessary to synthesize various kinds of side-chain chiral LC polymers (LCPs) to explore their potential applications. Furthermore, at the same time, the synthesis of lower T g cholesteric polymers were the aim of research, which offered the ability of application [24]. T g value is highly sensitive to the polymer backbone and is profoundly influenced by many secondary forces. Our team has done much research in the field of cholesteric LCPs [21–23]. In addition, the lower T g cholesteric polymers had been obtained with the long carbochain and nonmesogenic chiral group [22, 25]. In the present study, the biphenyl and flexible carbochain were introduced into the polysiloxane polymer chain at the same time. On one hand, the LC properties will be strengthened by containing biphenyl; on the other hand, the lower T g cholesteric polymers will be obtained by introducing flexible carbochain and polymer chain. Basing on the aims, a new side-chain chiral LC were synthesized deriving from two new monomers as follows: cholesterical LC monomer-(Cholest-5-en-3-yl(3β) 5-{[4-(4′-allyloxy) phenyl] phenyloxycarbonyl}) pentanoate (M1) and chiral nonmesogenic monomer-(menthyl 5-{[4-(4′-allyloxy) phenyl] phenyloxycarbonyl}) pentanoate (M2). The aim of this work is to research the effect of the chiral moiety on the phase behavior of polymers. The mesomorphic properties of the monomer and polymers obtained were characterized by differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), polarizing optical microscopy (POM), and X-ray diffraction (XRD); and at the same time, solid optical activities were measured by temperature-changing polarimeter. The influence of the contents of the chiral agent on the phase behavior of the polymers and the relations between the change of polymers solid optical activity with the optical texture are discussed.
Experimental
Materials
3-Bromopropene, 4-4′-biphenyldiol, hexanedioic acid, menthol, and cholesterol were purchased from Beijing Chemical. Polymethylhydrosiloxane (PMHS) was obtained from Jilin Chemical Industry and used without further purification. Chloroplatnic acid was obtained from Shenyang Chemical. Tetrahydrofuran was purified by distillation over sodium hydrogen, and pyridine was purified by distillation over potassium hydroxide.
Characterization
Specific rotation was performed with the Perkin Elmer 341 polarimeter. Phase-transition temperatures and thermodynamic parameters were determined by using a Netzsch DSC 204 (Netzsch, Germany) with a liquid nitrogen cooling system. The heating and cooling rates were 10 °C/min. A Leica DMRX (Leica, Germany) polarizing optical microscope equipped with a Linkam THMSE-600 (Linkam, England) hot stage was used to observe phase-transition temperatures and analyze LC properties for the monomers and polymers through observation of optical textures. XRD measurements were performed with a nickel-filtered \( {\text{Cu}} - {\text{K}}_{\alpha } \) (λ = 0.1542 nm) radiation with a DMAX-3A Rigaku (Rigaku, Japan) powder diffractometer. IR spectra were measured on a Perkin Elmer spectrum one Fourier transformed infrared (FTIR) spectrometer (Perkin Elmer Instruments, USA). Proton nuclear magnetic resonance (1H NMR) spectra (300 MHz) were recorded on a Varian WH-90PFT spectrometer. Temperature-changing rotation activities were measured on a Perkin Elmer Model 341 polarimeter.
Synthesis of the monomers
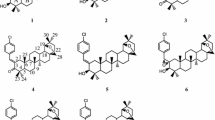
The synthesis of the olefinic monomers are shown in Scheme 1.
The synthesis of M1:
Potassium hydroxide (56.0 g, 1.00 mol) and potassium iodide (0.6 g, 0.03 mol) were dissolved in 150 ml of water to obtain an aqueous solution and then added into a mixture of 4,4′-biphenyldiol (93.0 g, 0.5 mol) and 400 ml of ethanol. 3-Bromopropene (50.0 g, 0.41 mol) was added dropwise into the mixture and stirred at room temperature for 2 h and then refluxed for 24 h. The cold reaction mixture was precipitated into the hydrochloric acid aqueous solution; the resulting precipitate was washed by water and isolated by filtration and was then recrystallized from ethanol and dried overnight at 85 °C under a vacuum to obtain 4-allyloxy-4′-biphenylol as a white sheet powder in 80% yield; mp, 174 °C.
Hexanedioic chloride(91.5 g, 0.5 mol) and 100 ml of dried tetrahydrofuran were mixed in a three-necked flask; then the mixture was added with cholesterol(22.6 g, 0.1 mol), 150 ml of dried tetrahydrofuran, and 20 ml of dried pyridine in drops while stirring. Furthermore, the mixture was then refluxed for 36 h. The cold reaction mixture was precipitated into water. The resulting precipitate was washed by hot water and isolated by filtration and was then recrystallized from ethanol and dried overnight at 85 °C under a vacuum to obtain Cholest-5-{[4′-(4-allyloxyphenyl} phenoxycarbonyl] pentanoic acid as a yellow powder in 60% yield; mp, 131 °C.
At last, esterified 4-allyloxy-4′-biphenylol with Cholest 5-[4′-(4-allyloxyphenyl) phenoxycarbonyl] and recrystallized from acetone to obtained the target product as a yellow grainy solid in 70% yield; mp, 124 °C.
-
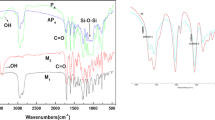
IR (KBr, cm−1): 3,072 (=C–H), 2,951–2,867 (–CH3, –CH2–), 1,749, 1,728 (C=O), 1,606, 1,511 (Ar–), 1,275, 1,168(C–O–C).
-
1H NMR(CDCl3): 0.68–1.99, 3.57(m, 26H, -chol-), 2.33–2.31(d, 2H, –COCH 2–), 4.57–4.59(d, 2H, CH2=CHCH 2O), 5.29–5.36(m, 2H, CH 2=C-), 5.42–5.46(m, 1H, CH2=CH–), 6.97–7.54(m, 8H, ArH).
Synthesis of M2
It was prepared according to the similar method of M1; mp, 74 °C, and the yield is 70%.
-
IR (KBr, cm−1): 3,076(=C–H), 2,954–2,871 (–CH3, –CH2–), 1,749, 1,726 (C=O), 1,607, 1,513 (Ar–), 1,228, 1,132(C–O–C).
-
1H NMR(CDCl3): 0.76–1.83, 3.57(m, 3H, -menthol-), 2.37–2.61(m, 4H, –COCH 2–), 4.57–4.59(d, 2H, CH2=CHCH 2O), 5.29–5.32(m, 2H, CH 2=C-), 5.45–5.46 (m, 1H, CH2=CH–), 6.97–7.54(m, 8H, ArH).
Synthesis of the polymers
The synthetic route of polymers is outlined in Scheme 1. The mesogenic monomer and chiral agent reacted with Si–H of PMHS to form polymers in the presence of a Pt catalyst. All polymers synthesized are listed in Table 1. The monomers M1 and M2 and PMHS were dissolved in dried, freshly distilled toluene. The mixture was heated to 65 °C under nitrogen and anhydrous conditions, and then a proper amount of THF solution of hexchloroplatinate hydrate catalyst was injected with a syringe. The hydrosilication reaction, following the track of Si–H stretch intensity up to its disappearance, would be completed within 30 h as indicated by IR. Furthermore, it is then precipitated with methanol. The products were dried in a vacuum at room temperature.
IR(KBr, cm−1): 2,925–2,851 (CH3– and –CH2–), 1,750, 1,730(C=O), 1,606–1,490 (Ar), 1,273 (C–C), 1,200–1,000 (Si–O–Si).
Results and discussion
Synthesis
The novel monomers, a cholesteric liquid crystalline (M1) and a chiral agent (M2), have been synthesized. The reaction pathways to M1 and M2 are shown in Scheme 1. The chemical structures of the two monomers were characterized by FTIR and 1H NMR spectroscopy. The FTIR of M1 and M2, respectively, showed characteristic bands at 1,749–1,720 cm−1 originating from ester C=O stretching, 1,648–1,645 cm−1 because of olefinic C=C stretching, and 1,605–1,450 cm−1 corresponding to aromatic C=C stretching. The 1H NMR spectra of M1 and M2 showed a multiplet at 6.97–7.54 ppm, 5.29–5.46 ppm, 4.57–4.59 ppm, and 0.68–1.99, 3.57 ppm, corresponding to aromatic, vinyl, methyleneoxy, carbonyl methylene and cholestyl, and menthyl menthylene protons. The new series of polymers P1–P7 were prepared by a one-step hydrosilation reaction between Si–H groups of PMHS and olefinic C=C of cholesteric monomer and chiral agent in toluene, using hexachloroplatinate as a catalyst at 60 °C. The yields and polymerizations of P1–P7 are summarized in Table 1. The FTIR spectra of P1–P7 showed the complete disappearance of the Si–H stretching band at 2,166 cm−1. Characteristic absorption bands appeared at 1,732 cm−1, 1,605–1,450 cm−1, and 1,200–1,000 cm−1, attaching to the stretching of ester C=O, aromatic, and Si–O–Si, respectively. It can be concluded that the chemical structures of the obtained monomers and polymers are consistent with our expectation.
Thermal properties
It is necessary to measure the phase-transition of monomers and polymers for many research works. The DSC measurement is a proper choice. The chain segment movement temperature from calm to moving was usually taken by the glass-transition temperature, and the LC phase temperature from emerging to disappearing was taken by the clearing points, which are important parameters for characterization and application or not.
The polymers phase-transition temperatures and corresponding enthalpy changes are summarized in Table 2; DSC curves of P1, P3, and P5 were shown in Fig. 1. A melting transition and a cholesteric phase to isotropic phase transition for M1 appeared at 124.0 and 202.5 °C. For the chiral polymers P1–P7, a glass-transition temperature and a cholesteric to isotropic-transition temperature appeared on the DSC curves. As seen from the data in Table 2, the T g value decreased first and then increased with the content of chiral moiety. It is well known that the influential factors of T g are summarized as the property of polymer backbone, the rigidity of mesogenic unit, and the flexible spacer length. In addition, for the copolymer system, the effect of the copolymer component should be regarded. The molecule structure and composition of M1 and M2 are homologous except for the chiral moiety; thus, when the change trend of T g are analyzed, the content and structure of menthol should be considered. The dilute effect might be expected to decrease the glass-transition temperature, and the sterically hindered effect might be expected to increase the glass transition.
Taking the two effects into account, T g is given by [26]:
Where T g and T go are the glass-transition temperatures of copolymer and homopolymer, K x is constant, and ρ x is the content of the chiral agent. At the beginning, when the menthyls were brought into the polymer, which included a large, sterically hindered effect, it acted as a diluting agent, and Eq. 1 is adopted. Furthermore, when the content of the menthyl reached a certain degree, the effect between the menthyls started to reinforce, and the sterically hindered effect played a main role, and Eq. 2 will be put into use. Figure 2 shows the effect of the concentration of menthyl units on the phase-transition temperature of P1–P7. It can be seen that the T g values of P1–P5 reduced from 45.7 to 32.5 °C, which is the influence of the dilution of menthyl. T g values of P6 and P7 rose again from 53.6 to 53.9 °C; it can be concluded that the sterically hindered effect is gradually predominant when the content of menthyl reaches a certain critical value. Similar to T g, menthyl may influence the clearing point (T i) of polymers in two ways. Firstly, menthyl may act as diluter and may lead to a downward shift in the clearing point as increasing proportions are added to a LC polymer; secondly, the sterically hindered effect between molecules required additional energy to distort the polymer backbone from the anisotropic state when heating to the isotropic state and led to the forward shift in the clearing point with increasing proportions of chiral agent. In this study, the non-LC chiral agent is induced into the polymer backbone; thus, the first factor is predominant. It can be seen that the T i value reduced with the increasing content of the chiral agent. According to Table 2, T i values of P1–P7 decreased from 192.4 to 71.4 °C. In addition, P1–P5 displayed wide mesophase temperature ranges (ΔT) from 145.7 to 77.8 °C. ΔT values of polymers decreased with the increasing content of the chiral agent.
Thermogrametry can explain well about the stability of the polymers. It can show clearly the point and the degree of the polymer decomposition. The representative TGA curves and data is shown in Fig. 3 and Table 2, respectively. It can be seen that temperatures at which 5% weight loss occurred (T d) were greater than 300 °C for P1–P7 and revealed that the synthesized polymers have a high thermal stability.
Optical properties
The optical textures of monomers and polymers were characterized by POM with hot stage under nitrogen atmosphere. The POM observation results showed that M1 exhibited enantiotropic cholesteric phase. When M1 was heated to 77.9 °C, the sample began to melt into a typical cholesteric oily texture, and then a broken focal-conic texture appeared, and the texture disappeared at 202.5 °C. When the isotropic state was cooled to 172.8 °C, the bright focal-conic texture appeared again, as shown in Fig. 4a and b. This can be seen in selective reflection.
It is well known that the helical pitch is an important parameter in connection with structures and optical properties of the cholesteric phase. The pitch and the reflection wavelength depend on the molecular structure, such as the polymers backbone, the length of mesomorphic core, flexible spacer length, and intermolecular force and so on. In the polymers, the cholesteryl and menthyl interact and strengthen the helicity of the polymer with increasing the contents of menthyl.
The polymer P1 showed a bright color and a stepped-partical texture, which is the typical texture of a smectic LCP. The polymers P2–P7 all showed cholesteric typical Grandjean texture, and P3–P5 can be seen in selective reflection. Although the LC properties of P6 and P7 are also excellent, its shallow scopes of LC phase will restrict it from being applied.
To research the relation between the optical activity and cholesteric properties, the optical activities were measured at different temperatures in heating and cooling cycles. The representative photomicrographs and optical activities curve are shown in Figs. 5 and 6, respectively. When the curves were compared with the relevant photomicrographs of polymers, it can be seen that the color and texture changed when the optical activities changed abruptly. From the curve, it can be seen that the optical activities changed smoothly at first and then decreased abruptly in the heating cycle, which is because of intermolecular force, and increased smoothly in the cooling cycle, which suggested the stable LC phase of the polymers.
X-ray diffraction
Although the LC phase type of the polymers can be concluded preliminarily by DSC and POM, it is necessary to measure them by XRD analysis and give additional information about their structure parameters. XRD patterns of representative polymers are shown in Fig. 7.
In general, a smectic, nematic, cholesteric structure has a broad peak associated with lateral packing at 2θ ≈ 16–21° in a wide-angle XRD curve. A sharp and strong peak at a low angle (1° < 2θ < 6°) in a small-angle X-ray scattering curve can be observed for smectic structures, but it cannot be seen for nematic and cholesteric structures. An XRD pattern of the quenched polymer P1 films showed sharp reflection peaks at 2θ of 4.37, 4.91, and 6.11(d = 20.19, 17.97, 14.44 Å), which were corresponding to the smectic layer spacing. The d value is lower than the molecular length of the fully stretched mesomorphic units that were calculated to 30.18 Å. The results are due to the molecule of cholesteryl that were more inclined to slantwise arrangement to relieve the obstruction between the molecule. In addition, the films of P2–P7 only showed broad peaks at 2θ of 20.03–20.46(d = 4.43–4.34 Å). Combining the polarized microscopy with XRD measurements may reveal that polymer P1 was a chiral smectic A phase, and P2–P7 were cholesteric phases.
Conclusion
In this study, a series of lower glass-transition temperature LCPs were obtained by introducing chiral nonmesogenic moity, which offered the possibility of application. The obtained polymers P1–P5 showed wide mesophase temperature ranges and high thermal stability. The polymers containing more than 30% of chiral units showed excellent LC properties, but its shallow ranges of mesophase temperature restricted its application. For P1–P7, the LC phase of homopolymers and copolymers changed from SA to cholesteric phase; the glass-transition temperatures and the isotropization temperatures reduced, and the ranges of the mesophase temperature changed abruptly first and then smoothly with the increase in content of the chiral agent.
References
Belayev SV, Schadt MI, Funfschiling J, Malimoneko NV, Schmitt K (1990) Jpn J Appl Phys 29:634
Bunning TJ, Kreuzer FH (1995) Trends Polym Sci 3:318
Broer DJ, Lub J (1995) Mol G N Nature 378:467
Kreuzer FH et al (1991) Mol Cryst Liq Cryst 199:345
Yang DK, West JL, Chien LC, Doane JW (1994) J Appl Phys 76:1331
Jacobs SD et al (1988) J Opt Soc Am B 5:1962
Kricheldorf HR, Sun SJ, Chen CP (1997) J Polym Sci Part A: Polym Chem 35:1611
Peter PM (1998) Nature 391:745
Sapich B, Stumpe J, Kricheldorf HR (1998) Macromolecules 31:1016
Broer DJ, Finkelman H, Kondo K (1988) Makromol Chem 189:185
Broer DJ, Mol GN, Challa G (1989) Makromol Chem 190:19
Barney PL, Dubois JC, Friedrich C, Noel C (1986) Polym Bull 15:341
Hsiech CJ, Wu SH, Hsiue GH, Hsu CSJ (1994) Polym Sci Part A: Polym Chem 32:1077
Pfeuffer T, Strohriegl P (1999) Macromol Chem Phys 200:2480
Dierking I, Kosbar LL, Held GA (1998) Liq Cryst 24:387
Hsu CS, Percec VJ (1989) Polym Sci Part A: Polym Chem 27:453
Wu YH, Lu YH, Hsu CS (1995) J Macromol Sci Pure Appl Chem 32:1471
Stohr A, Strohriegl P (1998) Macromol Chem Phys 199:751
Espinosa MA, Cadiz V, Galia (2001) J Polym Sci Part A: Polym Chem 39:2847
Finkelmann HS, Kim T, Munoz A, Taheri B (2001) Adv Mater 13:1069
Hu JS, Zhang BY, Sun K, Li QY (2003) Liq Cryst 30:1267
Zhi JG, Zhang BY, Zang BL, Shi GH (2002) J Appl Polym Sci 85:2155
Zhang BY, Meng FB, He XZ, Lin D (2005) Liq Cryst 32:1161
Deepa P, Sona C, Jayakannan M (2006) J Polym Sci Part A: Polym Chem 44:5557
Hu JS, Zhang BY, Pan W (2005) Liq Cryst 32:441
He XZ, Zhang BY, Meng FB, Ling JR (2005) J Appl Polym Sci 96:4
Acknowledgment
The authors are grateful to the National Natural Science Fundamental Committee of China, the Hi-tech Research and development program (863) of China, the National Basic Research Priorities Program (973) of China, and the Science and Technology Research Major Project of Ministry of Education of China for the financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, Y., Zhang, By., He, Xz. et al. Side-chain cholesteric liquid crystalline polymers containing menthol and cholesterol—synthesis and characterization. Colloid Polym Sci 285, 1077–1084 (2007). https://doi.org/10.1007/s00396-007-1654-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-007-1654-4