Abstract
A diamine, 9,9-bis[4-(4-amino-2-trifluromethylphenoxy)phenyl]fluroene (I) containing the CF3 group, was prepared from 9,9-bis(4-hydroxyphenyl)fluorene and 2-chloro-5-nitrobenzotrifluoride. The imide-containing diacids (V a-j and VI a,b) were prepared by condensation reaction of amino acids, aromatic diamines, and trimellitic anhydride. Then, a series of soluble fluorinated polyamides (VII a-e) and poly(amide imide)s (VIII a-j and X a,b) were synthesized from diamine (I) with various aromatic diacids II a-h and the imide-containing diacids (V a-j and VI a,b) via direct polycondensation with triphenyl phosphate and pyridine. All polymers showed excellent solubility in amide-type solvents such as N-dimethylforamide and can also be dissolved in pyridine, m-cresol, and tetrahydrofuran. Polymers afford transparent and tough films by solvent casting. The glass transition temperature of these polymers were in the range of 278–366°C, and the poly(amide imide)s had better thermal stability than polyamides. In comparison with the isomeric IX a-d, VIII a-d showed a lighter color with lower b* (yellowness index) values than the corresponding IX series.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Aromatic polyamides have been noted for their high thermal and chemical resistance, as well as their high-strength and high-modulus as fibers [1, 2]. Rigid rod-like aromatic polymers are usually difficult to process due to their high softening temperatures and their insoluble nature in most organic solvents [3–6]. Current or prior attempts at the solubilization and processing of rigid-chain polymers have been made through synthetic modification by the introduction of flexible linkages [7, 8], molecular asymmetry [9], or bulky side groups [10] into the backbone. Aromatic polyimides are well-known for their excellent thermal stabilities, electric insulation properties, and chemical resistance. However, applications are limited due to their high softening temperatures and insoluble nature in most organic solvents [11, 12]. To overcome these drawbacks, modifications of polyimide structures are often used.
One of the methods is by using copolymerization to synthesize copolymer to improve processability, such as poly(amide imide)s (PAI) [9–15]. Aromatic PAI possess desirable characteristics with the merits of the polyamides and polyimides such as high thermal stability, good mechanical properties, and easy processability [12, 13].
PAI can be synthesized from various aromatic monomers containing anhydride, carboxylic acid, and aromatic diamine by condensation. These polymers usually are synthesized through three main routes, a, b, and c, illustrated as follows. Route a goes through the amide–imide-forming reaction, in which trimellitic anhydride (TMA) reacts either with diisocyanate to produce PAI [14–16] or with thionyl chloride to synthesize TMA chloride and then reacts with diamine to produce PAI [17]. Route b is through imide-forming reaction from medium of amide-containing monomer, for example, polycondensation of amide-containing diamine with dianhydride to obtain PAI [18, 19]. Route c goes through the amide-forming reaction from imide-containing monomers such as dicarboxylic acids or diamines [20]. Therefore, aromatic PAI can be prepared from imide-containing monomers and aromatic diamines or dicarboxylic acids by polycondensation like prepared polyamide. Route c generally involves use of TMA as a major component to synthesize PAI, where a specific diamine or amino acid first reacts with TMA to synthesize a diimide–dicarboxylic acid (DIDA) or diacid, and then DIDA is reacted with various aromatic diamines to synthesize a series of alternative co-poly(amide-imide)s. Route c was a convenient method to prepare in the laboratory to probe into molecular structure and property. Our laboratory coworkers synthesized various imide-containing diacids [21, 22] used for the preparation of PAIs by the direct polycondensation of diacids with a new aromatic diamine.
In this study, diamine 4,4′-bis(4-amino-2-trifluoromethylphenoxy)biphenyl(I) [23] was reacted with various diacids (II a-e) and the imide-containing diacids (V a-j) by direct polycondensation to form polyamides (VII) and PAIs (VIII and X). Solubility, optical properties, tensile properties, and thermal properties of the resultant polymers will be investigated. It shows that the position of diamine I will influence the optical properties of polymers. PAI VIII, based on I in the amide segments, is much lighter in color than the IX series, with I in the imide segment.
Experimental
Materials
Diamine (I) was synthesized from 9,9-bis(4-hydroxyphenyl)fluorene (Acros) and 2-chloro-5-nitrobenzotrifluoride (Acros). Aromatic dicarboxylic acids, tetrephthalic acid (II a, TCI), 4,4′-biphenyldicarboxylic acid (II b, TCI), 2,6-naphthalenedicarboxylic acid (II c, TCI), 4,4′-sulfonyldibenzoic acid (II d, NEW Japan Chemical), and 2,2-bis(4-carboxyphenyl)-1,1,3,3,-hexafluoropropane (II e, Chriskev) were used as received. Aromatic diamine: 4,4′-oxydianiline (III a, Wakayama), 4,4′-bis(4-aminophenoxy)benzene (III b, TCI), 1,3-bis(4-aminophenoxy)benzene (III c, Chriskev), 4,4′-bis(4-aminophenoxy) biphenyl (III d, Chriskev), 4,4′-diaminodiphenyl sulfide (III e, TCI), 1,4-bisamnio-2-methylbenzene (III f, TCI), 1,4-bisamnio-2,5-dimethylbenzene (III g, TCI), 1,4-bisamnio-2,5-dichlorobenzene (III h, TCI), 1,4-bisamnio-2,3,5,6-tetramethylbenzene (III i, TCI), and 3,3′-dimethyl-4,4′-diamonobiphenyl (III j, TCI) were used as received. Amino acids: 4-aminobenzoic acid (VI a, TCI), 3-aminobenzoic acid (VI b, from TCI) were also used without previous purification. Solvents including N-methyl-2-pyrrolidone (NMP, Fluka), pyridine (Py, Wako), and triphenyl phosphite (TPP, TCI) were all used as received.
Synthesis of diamine (I)
9,9-Bis(4-hydroxyphenyl)fluorene (8.75 g, 25 mmol) was first dissolved in 20 ml of DMAc in a 100-ml flask with stirring, and 2-chloro-5-nitrobenzotrifluoride (11.13 g, 50 mmol) and potassium carbonate (6.7 g, 48 mmol) were added into it in one portion, and the mixture was heated at 110°C for 12 h. The obtained mixture was poured into methanol/water (100 ml, volume ratio 2:1) to gain a light yellow solid, which was collected, washed with water, and dried under vacuum. The crude product was recrystallized from DMF/methanol to gain fine crystals dinitro compound (I′) 18.01 g (yield 92%). To a suspension solution of the purified I′ (18.01 g, 25 mmol) and 10% Pd/C (0.18 g) in ethanol (160 ml ml), hydrazine monohydrate (4.6 ml) was added drop-by-drop to the stirred mixture at 80°C. After complete addition, the mixture was heated at the reflux temperature. The reaction solution was filtered to remove Pd/C, and the filtrate was then distilled to remove the partial solvent to obtain fine, white crystals (I) 18.25 g (yield 83%) [melting point: 241–242°C (Lit [24] 239–240°C)].
Synthesis of DIDAs (V a-j and VI a,b)
Synthesis of DIDA is described as a typical procedure [24, 25]. Diamine (1 mol) and TMA (2 mol) were dissolved in DMAc or NMP, and toluene was added distilled off azeotropically to condense. Synthesis of V g was performed as follows: 2,5-dimethyl-p-phenylene-diamine (4.09 g, 30 mmol) was dissolved in 80 ml of NMP. TMA (11.55 g, 60 mmol) was added while stirring at 60°C. Toluene (30 ml) was added, and the mixture was heated under reflux for 3 h until 1.1 ml of water was distilled off azeotropically. After complete removal of water, the residual toluene was then distilled off under reduced pressure. After cooling, the precipitated white solid (V g) was isolated by filtration, washed several times with hot water and dried in a vacuum to gain 13.10 g of white powder (yield 90%, melting point 440–441°C) [by differential scanning calorimeter, (DSC)]. Elem. anal. calcd. for C26H16N2O8 (484.09): C 64.47 %, H 3.33 %, N 5.78 %. Found: C 64.44%, H 3.56 %, N 5.53 %.
Other DIDAs were synthesized in an analogous procedure.
Synthesis of polyamides (VII)
Synthesis of polyamide VII h is described as a typical procedure. A mixture of diamine I (0.664 g, 1 mmol), diacid II a (0.165 g, 1 mmol), CaCl2 (0.06 g), pyridine (0.4 ml), TPP (0.4 ml), and NMP (2 ml) were heated at 100°C for 3 h, while stirred. At the end of the reaction, the obtained polymer solution was trickled into stirred methanol. The yellow stringy polymer was washed thoroughly with hot water and methanol, collected by filtration, and dried at 100°C under reduced pressure. Inherent viscosity of the polyamide VII h was 0.72 dl/g (yield: 0.812 g), measured with a polymer concentration of 0.5 g/dl in DMAc at 30°C. Other polyamides were synthesized in similar methods.
Synthesis of PAIs (VIII, IX, X)
Synthesis of PAI VIII a is described as a typical procedure. A mixture of diamine I (0.534 g, 0.8 mmol), diacid V a (0.438 g, 0.8 mmol), CaCl2 (0.15 g), Py (0.6 ml), TPP (0.6 ml), and NMP (2 ml) were heated at 100°C for 3 h, while stirred. The viscosity of reaction solutions increased after 1 h, and additional amount of NMP (1 ml) was added to the reaction mixture. At the end of the reaction, the obtained polymer solution was trickled into stirred methanol. The yellow stringy polymer was washed thoroughly with hot water and methanol, collected by filtration and dried at 100°C under reduced pressure (yield: 0.964 g). The inherent viscosity of PAI VIII a was 0.56 dl/g. All other PAIs were synthesized in similar methods.
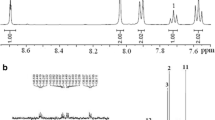
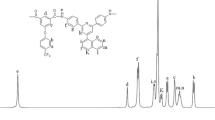
Infrared (IR, film) of VIII f: 3342, 1604 (N-H stretching of amide group), 1679 (C=O stretching), 1322 (C-N stretching), 1779 and 1724 (typical imide carbonyl asymmetrical and symmetrical stretch), 1378 (C-N stretch), 1133 and 730 (imide ring deformation), and some of peaks in the range of 1100–1300 cm-1 (C-O and C-F stretching). 1H nuclear magnetic resonance (NMR) (DMSO-d 6, δ, ppm): 8.54 (s, 2H, Hj), 8.44 (d, 2H, Hk), 8.29 (s, 2H, Ha), 8.11 (d, 2H, Hl), 8.05 (d, 2H, Hb), 7.93 (d, 2H, Hi), 7.52 (d, 4H, Hm), 7.48 (s, 2H, Hf), 7.41 (t, 2H, Hh), 7.34 (t, 2H, Hg), 7.24 (d, 4H, Hn), 7.17 (d, 4H, He), 7.12 (d, 2H, Hc), 6.95 (d, 4H, Hd). 13C NMR (DMSO-d 6, δ, ppm): 166.6 (C19), 166.4 (C30,31), 163.7 (C7), 156.0 (C12), 155.4 (C4), 150.5 (C29), 149.8 (C22), 141.0 (C1), 139.6 (C17), 139.5 (C10), 134.6 (C25), 134.4 (C24), 134.1 (C26), 131.9 (C21), 129.4 (C9), 129.2 (C27), 128.1 (C13), 127.9 (C16), 127.3 (C20), 126.0 (C15), 124.3 (C14), 123.7 (C23), 123.1 (C18, quartet, 1 J C-F=271 Hz), 122.1 (C6), 120.9 (C15), 120.7 (C5), 120.0 (C3, quartet, 2 J C-F=31 Hz), 119.1 (C8), 118.7 (C2), 118.1 (C28), 63.9 (C11).
Film preparation
A solution of polymer was made by dissolving about 0.5 g of the polyamide or polyamide–imide in 5 ml of DMAc to afford an approximate 10wt% solution. The homogeneous solution was poured into a 9-cm diameter glass culture dish, which was placed in 110°C oven overnight to allow the solvent to evaporate and let a solid film form. The solution was then heated to 200°C and held at that temperature for 1 h. By soaking in water, polymer films were self-stripped off from the glass surface. The polymer films were further dried in vacuum at 120°C for 8 h.
Measurements
IR spectra were recorded on a Horiba Fourier Transform Infrared Spectrometer FT-720. Elemental analyses were run in a Perkin-Elmer Model 2400 CHN analyzer. 1H and 13C spectra were recorded on a Bruker AV-500 FT-NMR spectrometer. Inherent viscosities were determined at 0.5 g/dl concentration using a Cannon-Fenske viscometer at 30°C. Thermogravimetry analysis (TGA) was conducted with a TA Instruments TGA 2050. Experiments were carried out on 8- to 10-mg film samples heated in flowing nitrogen or air (90 cm3/min) at a heating rate of 20°C/min. DSC traces were measured on TA Instruments DSC 2010 at the rate of 15°C/min in flowing nitrogen (40 cm3/min). Glass transition temperatures were read as the midpoint of the heat capacity jump and were taken from the second heating scan after a quick cooling down from 400°C. Mechanical properties of the films were measured with an Instron model 1130 tensile tester with a 5-kg load cell at a crosshead speed of 5 cm/min on strips approximately 40–50 μm thick and 0.5 cm wide with a 2-cm gauge length. An average of at least five individual determinations was used. Color intensity of the polymers was evaluated by a Macbeth Color-eye colorimeter. Measurements were performed with films, using an observational angle of 10° and a CIE (Commission International de l’Eclairage)-D illuminant. A CIE LAB color difference equation was used. Ultraviolet–visible (UV–vis) spectra of the polymer films were recorded on a Shimadzu UV-1601 UV–vis spectrophotometer.
Results and discussion
Synthesis of polymers
The CF3-containing diamine I was prepared in two steps according to the literature [26] as shown in Scheme 1. An intermediate dinitro compound I′ was synthesized initially by added potassium carbonate into DMAc, which contained dissolved 9,9-bis(4-hydroxyphenyl)fluorene and 2-chloro-5-nitrobenzotrifluoride. Diamine I was readily obtained in high yields by the catalytic reduction of I′ with hydrazine hydrate and Pd/C catalyst in refluxing ethanol.
The imide-contaning diacids (V a-j and VI a,b) were obtained by the procedure from TMA and the corresponding diamine III a-j or amino acids IV a,b. The polyamides (VII a-e) and PAIs (VIII a-j and X a,b) were synthesized using the polycondensation procedure from diamine I and dicarboxylic acids II a-e, or from I and imide-containing V a-j or VI a,b (Scheme 2). To understand the influence of the position of diamine I in PAI on the physical properties, isomeric PAIs IX a-d, which has diamine I in the imide segments, were synthesized by V i and aromatic diamines III a-d (Scheme 3).
The synthesis conditions and inherent viscosities of all the synthesized polymers are summarized in Table 1. Inherent viscosities for VII a-e range from 0.32–0.72 dl/g. For VIII a-j, viscosities range from 0.56–1.20 dl/g, and for X a,b, viscosities were 0.79–0.92 dl/g. These values indicate moderate to high molecular weight polymer. In general, the molecular weight of the polymers obtained from the phosphorylation reaction is highly dependent on the reaction concentration. It has been consistently observed that the higher the monomer concentrations, the higher the final inherent viscosity that provided no precipitation or gelation of the product from the reaction medium took place. Inherent viscosities of the polymers could be obtained by using a higher initial reaction concentration and add an additional amount of NMP to the highly viscous reaction medium before the formation of swollen gel.
The composition and structure of polymers were characterized by elemental analysis, IR spectra, and NMR spectra. The result of the elemental analysis is listed in Table 2, which shows that the polymer has slight moisture absorption in the range of 0.54–3.74% because of its amide group. The observed values were corrected by eliminating the amount of absorbed water, and the corrected values were in good agreement with the calculated ones. A typical IR spectrum is shown in Fig. 1. The IR spectrum of VIII f shows characteristic absorptions for the imide ring at 1,779 and 1,724 cm-1 due to the asymmetrical and symmetrical carbonyl stretching vibration and the amide ring at 3,342 and 1,679 cm-1. The typical NMR spectra of polymer are shown in Fig. 2. The aromatic protons of VIII f showed in the region of 6.9–8.6 ppm and the proton of amide group at10.8 ppm. Hn and Hd shifted to a higher field due to the electron-donating property of the ether group, and Hj, Hk, and Hl close to the imide ring appeared at the farthest downfield. In 13C NMR spectrum, there were 30 peaks for VIII f. Though C30 and C31 were overlapping, the number of carbons was still consistent with the structure. Carbon C19 and C30,31 were carbonyl groups, the former was an amide group, and the latter was the imide group, therefore they evidenced in the downfield. Furthermore, the splitting of the 13C signals caused by couplings between carbon and fluorine also could be observed in the spectrum. The magnitudes of the one-bond and two-bond carbon–fluorine couplings 1 J CF and 2 J CF are 271 and 31 Hz, respectively. The above cited results show evidences that these polymers have been synthesized successfully.
Properties of polymers
The qualitative solubility of all the polymers is shown in Table 3. All polyamides (VII a-e) and PAI (VIII a-j, X a,b) showed good solubility in dimethyl sulfoxide and DMF, and also could be dissoluble in THF and m-cresol except VII b. VII b derived from diacids with more rigid structure such as naphthalene was insoluble. In this study, most of the polymers were soluble due to the fact that diamine containing a bulky fluorene cardo group and the trifluoromethyl groups exhibit a steric hindrance, which prevents close chain-packing and allows the solvent molecules to diffuse into the polymer chains.
All of the polymers were cast into transparent and flexible films from polymer solutions of DMAc except VII b. The results are summarized in Table 4. Polyamide (VII) films have tensile strengths of 78–91 MPa, elongations to break of 8–14%, and initial moduli of 2.0–2.2 GPa. Poly(amide imide)s of VIII series have tensile strength of 89–124 MPa, initial moduli of 2.0–2.7 GPa, and elongations to break of 8–23%. VII a,c, VIII a,b,e, and VIII g showed a clear yield point on their stress–strain curve and they have strengths at a yield of 86–111 MPa, reflecting that these polymers have good toughness. In general, the incorporation of para-substituted phenylene units leads to an increase in tensile properties. However, some of these polymers show lower initial moduli and tensile strength. The presence of a bulky fluorene cardo group and the trifluoromethyl group, which lowers the interchain and intrachain interactions in polymers and disturbs the coplanarity of aromatic units reduce the packing efficiency and crystalline.
Thermal property of all the polymers was evaluated by TGA and DSC measurements. The results are summarized in Table 5. Quenching from an elevated temperature of about 400°C to room temperature in air gave predominantly amorphous samples so that the glass transition temperatures (T g) of all the polymers could be easily revealed in the subsequent DSC scans. The T g values of polyamides (VII a-e) and PAIs (VIII a-j, X a,b) were in the range of 291–312°C and 278–366°C, which followed the decreasing order of the chain flexibility and steric hindrance of the polymer backbones. In general, incorporation of less symmetric m-phenylene unit leads to a decrease in T g such as VIII c lower than VIII b. T g of VIII i was higher because its structure rotated with greater difficulty as a result of the tetramethylphenyl.
The thermal stability of the polymers was characterized by TGA. The temperatures at 10% weight loss (T 10) in nitrogen and air atmospheres were determined from the original thermograms. The T 10 values of polyamides (VII) and PAIs (VIII and X) stayed in the range of 472–555°C in nitrogen and 465–538°C in air. In general, the T 10 values in nitrogen are larger than in air. However, VII d showed higher T 10 in air than in nitrogen due to the antioxidating actions of the sulfonyl group. The structure also affect thermal stability. VIII b showed higher T 10 values than its isomer VIII c. This can be attributed to the polymer chains of the para-structure VIII b that were packing tighter than the metastructure VIII c. Moreover, the char yield of all polymers at 800°C was above 55%. These indicated that all these polymers possess excellent thermal stability.
The color coordinates of these polymers are given in Table 6. In this case, we based our judgment of the degree of yellowness on the b* value. Color intensities of the polymers are determined by the polymer structure. If the monomer has color or colored by-products are not eliminated fully, the polymers can show color. The change transfer complex (CTC) formation between polymer chains through steric hindrance would also influence the color intensities of the polymer. Generally, the aromatic polyamide could be colorless when they are synthesized by the monomers with a light color. PAIs still show color (yellow or brown) because of their conjugated aromatic structures and/or the intermolecular and intramolecular CTC formation.
Table 6 shows that polyamides (VII) with a b* values (a yellowness index) range from 8.8–19.4. The imide-containing PAIs (VIII, IX, and X) were shown as deep-colored (yellow) with larger b* values than the VII series due to the intermolecular CTC formation. Comparing monoimide-contaning X with diimide-contaning VIII and IX, it was quite obvious that the coloration would be deep as the imide group increased. In addition, comparing the b* value of polymers VIII with their analogous IX, the b* values of polymers VIII is apparently lower than those of corresponding polymers IX. The color comparisons are summarized as shown in Fig. 3. The polymer structure of VIII has a lower intermolecular CTC effect than the isomeric IX, leading to the idea that the polymers of VIII show lighter colors than the polymers of IX. As the ether chain increased, the lighter colored polymer films were obtained such as VIII b(IX b), which showed lower b* values than VIII a(IX a). Concerning the structure, the para-structure of X a showed a lighter color than the metastructure of X b. The metastructure can reduce the electron-conjugation on the imide ring and these results were attributed to the reduction of the intermolecular CTC effect.
Moreover, the color intensities of the polymers could also be elucidated from the cutoff wavenumber (λ 0) observed in the UV–vis absorption spectra. As λ 0 is close to ultraviolet regions (200–400 nm), the color of polymers is lighter. In other words, as λ 0 is closer to the visible region (400–700 nm), the color of the polymer is deep. The λ 0 of VII a-e in the range of 348–375 nm is lower than 380 nm, indicating that the VII series was colorless and had high transmittance. Though theλ 0 values of the VIII and IX series are higher than 400 nm, the results conformed to the b* values. The VIII series showed lower λ 0 values than the IX series. This exhibited that the VIII series was indeed lighter-colored and had higher transparency than IX series did.
To summarize, the light-colored polymers stem from the structures of diamine. Diamine (I) with the strong electronegativity of the pendent CF3group and the fluorene cardo group are effective in preventing the CTC formation between the polymer chains through steric hindrance to form light-colored polymers. In addition, the position of the fluorine groups also have an effect on coloration, the VIII series showed lighter color than the IX series.
Conclusions
A series of high molecular weight polyamides (VII) and PAIs (VIII and X) were successfully obtained by direct polycondensation reaction of the CF3-containing diamine (I) and various diacids. The synthesized polymers exhibited better solubility than common polyamides and PAIs because of the presence of the CF3 group in the polymer chains. Most of the polymer films displayed high tensile strength together with good thermal stability. In comparison with the isomeric IX, PAIs VIII based on I in the amide segments exhibited less color together with good thermal properties.
References
Cassidy PE (1980) Thermally stable polymers. Marcel Dekker, New York
Seymour RB, Carraher CE (1981) Polym chem: an introduction. Marcel Dekker, New York
Kricheldrof HR, Schmidt B (1992) Macromolecules 25:5471
Kricheldrof HR, Burger R (1994) J Polym Sci [A1] 32:355
Fled WA, Ramalingam B, Harria FW (1983) J Polym Sci [A1] 21:319
Yang HH (1989) Aromatic high-strength fibers. Wiley, New York
Imai Y, Malder NN, Kakimoto M (1989) J Polym Sci [A1] 22:2189
Bruma M, Mercer F, Fitch J, Cassidy P (1995) J Appl Polym Sci 56:527
Jeong HJ, Oishi Y, Kakimoto M, Imai Y (1991) J Polym Sci [A1] 2:39
Oishi Y, Ishida M, Kakimoto M, Imai Y, Kurosaki T (1992) J Polym Sci [A1] 30:1027
Liou GS, Kakimoto M, Imai Y (1993) J Polym Sci [A1] 31:3265
Song CE (1979) J Polym Sci D 11:161
Imai Y, Kakimoto NN, Kakimoto M (1995) J Polym Sci [A1] 33:2209
Ghosh MK, Mittal KL (eds) (1996) Polyimides: fundamentals and applications. Marcel Dekker, New York
R Buch P, R Reddy AV (2005) Polymer 46:5524
Hitachi Chemical Co (1967) French Patent 1,473,600
Kakimoto M, Akiyama R, Negi YS, Imai Y (1988) J Polym Sci [A1] 26:99
Imai Y, Malder NN, Kakimoto M (1985) J Polym Sci [A1] 23:2077
Dezern JF (1988) J Polym Sci [A1] 26:2157
Bower GM, Frost LW. (1988) J Polym Sci Part A: Polym Chem 26:2157
Yang CP, Chen RS, Chen KH, Chen YP (2002) J Chin Chem Soc 49:927
Yang CP, Hsiao SH, Lin JH (1992) Macromol Chem 193:1299
Yang CP, Chen WT, Lin JH (1994) J Polym Sci Part A: Polym Chem 32:435
Xie K, Shang SY, Liu JG., He MH, Yang SY (2001) J Polym Sci Part A: Polym Chem 39:2581
Yang CP, Lin, J H (1995) Polym Int 38:335
Yang CP, Chiang HC (2004) Colloid Polym Sci 282:1347
Acknowledgement
The authors are grateful to the National Science Council of the Republic of China for the support of this work (Grant NSC 93-2216-E-036-003).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yang, CP., Su, YY. & Hsu, MY. Syntheses and properties of fluorinated polyamides and poly(amide imide)s based on 9,9-bis[4-(4-amino-2-trifluromethylphenoxy)phenyl]fluroene, aromatic dicarboxylic acids, and various monotrimellitimides and bistrimellitimides. Colloid Polym Sci 284, 990–1000 (2006). https://doi.org/10.1007/s00396-005-1440-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-005-1440-0