Abstract
Composite latex particles consisting of epoxidised natural rubber (ENR) and poly(methyl methacrylate) (PMMA) were synthesised to obtain interpenetrating polymer networks. Among the ENR latices having 9 to 36 mol% epoxide, prepared by in situ reaction using performic acid, the ENR latex with 25 mol% epoxide was selected for prevulcanisation by sulphur or γ-radiation system. The swelling ratios of sheets cast from the sulphur-prevulcanised ENR (SPENR) latices decreased with increasing prevulcanisation time while those cast from the γ-radiation-prevulcanised ENR latices were also inversely proportional to the irradiation dose. By applying the phase transfer/bulk polymerisation/transmission electron microscopy (TEM) technique, a homogeneous network structure in each of the SPENR particles and also a relative dense network near the surface in γ-radiation (RV) ENR particle were noticed. When 10 to 30 wt% of MMA swollen in ENR particles was polymerised, the mesh structure was observed in each particle. The dense network near the RVENR particle surface might be used as additional evidence that the degree of epoxidation and, hence, the presence of swollen n-butyl acrylate in the outer zone were higher than in the internal region.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Natural rubber (NR) latex, consisting of cis-1,4-polyisoprene and a small amount of non-rubber substances such as proteins, fatty acids and inorganic salts, must be prevulcanised before use to enhance the physical properties of the rubber products [1, 2]. The prevulcanisation of NR latex, a process in which chemical cross-link takes place inside the particle, is generally performed by using sulphur, peroxide or γ-radiation system [3, 4]. Although vulcanisation of NR latex by using high-energy radiation has many advantages, its very high dose deteriorates properties of the rubber due to the main chain degradation. To reduce the radiation dose, sensitisers such as n-butyl acrylate and halogenated hydrocarbon are required [5, 6]. In the case of sulphur prevulcanisation (SP), the cross-linking reaction in the latex proceeds much more rapidly than that in the dry rubber [4]. It has been proposed that the vulcanising ingredients, either dissolved in the aqueous serum or forming reactive species, are transported into the rubber particle before cross-linking [7]. The microscopy techniques, such as atomic force microscopy (AFM) [8] and transmission electron microscopy (TEM) [9, 10], have been used in the elucidation of chemical mechanism of latex prevulcanisation. Evidence from AFM of the surface of SPNR latex film implied that the rate of cross-linking is much greater than the rate of diffusion; therefore, an unvulcanised core surrounded by a highly cross-linked shell of rubber molecules is obtained. However, the interparticle links and/or postvulcanisation in the film resulted in an inaccurate interpretation of the actual cross-linking in latex particles. The phase transfer/bulk polymerisation/TEM technique has been successfully applied to elucidate the internal morphology of prevulcanised NR and skim latex particles [11–13]. The technique involves the titration of negatively charged latex particle with an aqueous solution of cationic surfactant such as benzyldimethyl hexadecylammonium chloride (BHAC) in the presence of a non-water-miscible monomer, e.g. styrene, until reaching the endpoint. Due to the neutralisation, the latex particles completely transfer from the aqueous phase into the organic phase. The monomer containing swollen rubber particles was then polymerised. After sectioning the specimen and staining with OsO4, the rubber particles embedded in polystyrene (PS) was studied under TEM. By using this process, the freeze-drying of latex normally required for specimen preparation is omitted and, hence, the disturbance of rubber particle structure is minimised. The internal morphology of prevulcanised NR latex particles under TEM showed that the sulphur and γ-radiation systems caused homogeneous cross-link inside each particle while a non-uniform network, a dense network near the surface of the particle, was noticed in the sample employing peroxide.
Besides prevulcanisation, the improvement of hardness and strength of the film by combination of NR with other polymers having high glass transition temperature (T g) such as PS or poly(methyl methacrylate) (PMMA) has been of great interest [14, 15]. However, the transparent PMMA grafted on NR, even with low grafting level, provided the crack film and phase separation. Various blends of NR and PMMA in the form of interpenetrating polymer networks (IPNs) were, therefore, studied. Full IPNs, a combination of two polymer networks, and semi-IPNs, composed of only one cross-linked polymer of NR and PMMA, have been prepared in both dry and latex forms. The complex morphologies including the cellular and core-shell structures can be obtained from latex-IPNs prepared by two-stage emulsion polymerisation method [16]. When PMMA was used as the second polymer, a combination of elastic properties of NR, hardness and transparency of PMMA was obtained. However, the film forming was poor when the PMMA content was increased from 10 to 30% [17]. It was explained that hydrophilic PMMA tended to be localised around NR particle.
In order to increase the miscibility of NR and PMMA, an increase in polarity of NR by using epoxidation reaction was essential and it was of our interest to study this process. Epoxidised NR (ENR) with 1 to 90 mol% epoxide could be prepared in the form of solution or latex [18]. Previous studies of sulphur vulcanisation in solid ENR showed that vulcanisation of ENR 50 (ENR having 50 mol% epoxide) by using only sulphur was faster than that of NR due to the isolated double bond in ENR inhibiting the formation of intramolecular sulphide linkage [19]. With reference to the radiation vulcanisation, the UV curing of blends consisting of ENR, cycloaliphatic diepoxide and glycidyl methacrylate induced by cationic photoinitiator was investigated by real time Fourier-transform infrared [20]. Results from γ-radiation (RV) of ENR using trimethylolpropane triacrylate as an acrylic cross-linking agent [21] indicated that the irradiation induced not only the cross-linking and oxidation at double bonds but also the ring-opening reactions resulting in inter- or intrachain aliphatic ether cross-links, furans and hydroxyls.
Although ENR should be cross-linked before use as a raw material of the rubber products, the structure of prevulcanised ENR latex particle has never been visualised. It was our goal to firstly synthesise ENR latex and to use the non-cross-linked ENR 25 latex (25 mol% epoxide) for preparation of semi-IPNs, in which PMMA was cross-linked (called semi-II). Secondly, the prevulcanisation of ENR latex, using sulphur or γ-radiation system, was systematically studied before preparation of semi-I with non-cross-link and fully IPNs with cross-linked PMMA. The internal morphology of the prevulcanised ENR and latex-IPNs particles, examined by using the phase transfer/bulk polymerisation/TEM technique, was lastly used to indicate the site of epoxidation taking place in latex particle.
Experimental
Latex and reagents
Commercial high ammonia (HA)-preserved NR latex concentrate (Rayong Bangkok Rubber, Thailand) [60% dry rubber content (DRC)] was filtered through a 250 mesh aluminium screen before use. A laser particle size analyser (Mastersizer 2000, Malvern) was employed for the determination of particle size of latex in 1% aqueous solution of nonylphenylpoly(ethylene glycol) (Nonidet P40) (Fluka, Biochemika), a medium used for preparation of ENR latex. The average value of each sample was calculated from three measurements.
The monomers, i.e. styrene (Fluka, Purum), MMA (Fluka, Purum) and n-butyl acrylate (Fluka, Purum), were purified by passing through a column packed with neutral aluminium oxide and basic aluminium oxide. The purified monomers were stored in a refrigerator at 4°C until use. Other reagents were used as received.
Synthesis and characterisation of ENR latex
HA-NR latex (100 g) was diluted to 30% DRC with distilled water (100 g) and Nonidet P40 (3 g). After gentle stirring in a glass reactor at room temperature for 1 h, the mixture was neutralised by 85% formic acid (Carlo Erba, RPE) and then acidified with an excess of the acid (9.7 ml). After raising to 40°C, a 30% aqueous solution of hydrogen peroxide (BDH, AR) (72.8 ml) was added within 15–20 min and the reaction was kept at 50°C for a period varying from 4 to 24 h. Aliquots were taken at various time intervals and immediately precipitated in methanol, washed with water and dried under vacuum at room temperature until a constant weight was obtained. At the end of the reaction, the pH of the latex was adjusted to 10 by using 10% NH4OH. The particle size and size distribution of ENR latex were then determined by using the particle size analyser. An optical microscope (Cover-018, Olympus) was also applied for direct observation of the ENR latex particles.
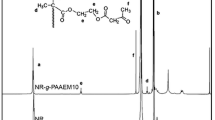
A differential scanning calorimeter (DSC7, Perkin Elmer) measured from −100 to +25°C at the first heating scan at a heating rate of 10°C/min was used to determine T g of ENR latex film while its epoxide content was calculated from the spectrum of proton-nuclear magnetic resonance spectroscopy (1H NMR, DPX400, Bruker) using CDCl3 as a medium [22].
when I 2.7 and I 5.1 are integrated area of peaks at chemical shifts 2.7 and 5.1 ppm, respectively.
Prevulcanisation of ENR
The prevulcanisation of ENR 25 was carried out by using sulphur or γ-ray system according to the methods previously used for the NR latex [11, 12]. In this case, the dose from Co-60 γ-ray source (Gamma Cell 220) at 25°C was varied from 4, 7, 10, 14, 26 to 32 kGy. The dried rubber sheets, cast from aliquot (∼5 g) of latices prevulcanised by sulphur at different time intervals or irradiated with various doses, were cut into a square piece of known weight (∼0.2 g) and their cross-link density was then determined from the swelling ratio of the rubber after immersing in toluene (40 ml) until equilibrium swelling as previously described [11]. The sample in the form of latex was kept in a dark cabinet at room temperature before using in the next step.
Preparation of semi- or fully IPNs of ENR/PMMA latex
A redox initiator system, consisting of tert-butyl hydroperoxide (t-BuHP) (Fluka, Purum) and tetraethylene pentamine (TEPA) (Fluka, GC), was used for polymerisation of MMA in semi- or fully IPNs of ENR/PMMA latex [15].
The prevulcanised ENR latices, having 10% DRC (100 g) and 2% NH4OH solution (Fluka, Purum) (47 g), were charged into a glass reactor under nitrogen atmosphere. A known amount of MMA and oleic acid (Fluka, GC) (0.5 g) [and 1 mol% of divinylbenzene (DVB) (Fluka, GC), a cross-linker of PMMA] were then slowly added into the latex followed by t-BuHP (0.3 g). When raising the temperature at 50°C, TEPA (1.17 g of 10% w/v solution) was finally added and the polymerisation was carried out for 24 h under continuous stirring.
Preparation of the sample for morphological study
The phase transfer technique was applied to transfer prevulcanised ENR or IPNs latex particles [6% total solid content (TSC)] (15 g) from the aqueous phase into the styrene (Fluka, Purum) (30 g) by titration with an aqueous solution of 0.0121 M BHAC (Fluka, Purum). The remaining trace of water in styrene containing swollen rubber particles was eliminated by gently centrifuging as previously mentioned [11, 12]. The upper monomer phase isolated and mixed with 0.6 wt% of benzoyl peroxide (BPO, Sigma, Microscope) was then poured into a Teflon-coated steel mould (10.4×10.4×2.6 cm3) and polymerised at 70°C in an oven (400 UM, Memmert) for 16 h.
Specimen of particles incorporating in the PS matrix was finely trimmed and sectioned under an ultramicrotome (Ultracut R, Leica). Micrograph of particles embedding in PS after being stained with 1% w/v aqueous solution of OsO4 vapour (Electron Microscopy Sciences) was obtained using TEM (H-300, Hitachi).
Results and discussions
Particle size and size distribution of latex particles
Particle sizes of NR in water and 1% aqueous solution of Nonidet P40 were measured by using the laser particle size analyser. The data obtained are shown in Fig. 1.
A single broad size distribution indicating the polydispersity of NR latex particles was observed in both media and their average particle size of about 0.7 μm agreed well with the typical reported data [23]. In the case of ENR, Nonidet P40 was added to sterically stabilise NR latex during the use of formic acid. The average particle size and size distribution of ENR 25 (12 h of reaction time) in the Nonidet P40 medium are also shown in Fig. 1. A broad size distribution of ENR particle, having an average size of 7 μm, was noticed. This might result from the effect of added acid decreasing the thickness of electrical double layer around the particle [7, 24] and facilitating the particles’ aggregation as also observed under optical microscopy. However, the latex was stable until use due to the steric stabilisation caused by the presence of Nonidet P40.
Determination of epoxide content of ENR latex
The mole percent epoxide of ENR latex film was determined by using 1H NMR and DSC techniques. The 1H NMR spectrum of the film cast from ENR latex, prepared within reaction time of 20 h, is shown in Fig. 2.
From the spectrum, the characteristic peaks of ENR at about 2.7 and 5.2 ppm, respectively, to oxirane and olefinic methylene hydrogens were observed. While the peak at 3.5 ppm indicated the presence of Nonidet P40, the evidence of a ring opening structure at 3.4 and/or 3.9 ppm could not be detected [25]. The mole percent epoxide of ENR was calculated from the ratio of the integrated areas of the remaining olefinic and appearance of epoxy methine protons [26]. The epoxide contents of ENR latex are plotted vs the reaction time as shown in Fig. 3.
It was observed that the mole percent epoxide of ENR linearly increased with longer reaction time. The ENR with 25 mol% epoxide (reaction time of 12 h) was then selected for the study of prevulcanisation due to the fact that the commercial ENR 25 has been used in many applications [18]. Furthermore, to obtain high mole percent epoxide, the high concentration of ingredients and/or long reaction time used might result in rapid coagulation of the latex [27].
DSC was then applied to determine T g of films cast from the ENR latices synthesised at different reaction times. Their thermograms are shown in Fig. 4.
It was observed that the T g of ENR latex films shifted from −60 to −37°C when the reaction times were increased from 4 to 24 h, i.e. the mole percent epoxide was varied from 9 to 35%. This was due to the fact that the insertion of oxygen atoms into polyisoprene molecules lowered the freedom of rotation of the NR backbone. Furthermore, it should be pointed out that all thermograms exhibited a single T g peak whose peak width was less than 10°C reflecting that the epoxidation reaction homogeneously took place in latex as previously reported [24]. When T g and the percent mole of epoxide at all ENR latices were plotted, the linear relationship was noticed. The increase of the level of epoxidation in ENR certainly increased its polarity and, hence, the T g due to intermolecular interaction between polar groups in ENR chains.
Size distribution of cross-linked ENR latex particles
Before preparation of latex-IPNs based on ENR, it was necessary to study the vulcanisation of ENR latex. The two systems, i.e. sulphur and γ-radiation, providing a uniform network structure inside a particle were applied for the cross-linking of ENR latex. Particle size distribution curves of SPENR and RVENR latices in an aqueous solution of Nonidet P40 determined by using a laser particle size analyser are shown in Fig. 5.
A single broad size distribution of SPENR latex was found in the curve while RVENR latex showed a bimodal distribution. The average size of SPENR was slightly less than that of ENR but it was not significantly different from that of the large peak of RVENR latex. The smaller size of cross-linked ENR latex particle might result from the small contraction of hydrated Nonidet P40 around particle surface due to the effect of ingredients added during prevulcanisation. The less hydrophilic portion of the added molecules moved to the particle surface resulting in the decrease of the amount of water in the hydrated layer of ENR. Both KOH and sodium dodecyl sulphate used in SPENR might also cause a decrease of hydrodynamic volume of rubber particle possibly by the alteration of arrangement of the indigenous surfactants on the latex particle [28]. Similar principle could be used in the explanation of RVENR latex particle size because the sensitisers, i.e. CCl4 and n-butyl acrylate, were added in this system. Because the average size of the small peak detected in RVENR latex was close to that of NR, it could be assumed that the presence of nonpolar solvents effectively de-aggregated ENR particles. In addition, γ-ray could greatly increase the particles’ motion and partly destroyed the aggregates [6] which occurred by using high irradiation dose (>7 kGy), whereas, using low irradiation dose, the small peak was not observed. Another possible effect of radiation involved the destruction of the coil of protein molecules [13].
Swelling ratio of prevulcanised ENR
The percent swelling ratios, used to approximately determine the cross-linking density, of rubber sheets cast from the SPENR latices synthesised for various prevulcanisation times are shown in Fig. 6. In order to understand the effect of epoxide groups, the swelling ratios of SPNR latex sheets were also measured and values obtained are plotted vs prevulcanisation times as also displayed in Fig. 6.
Although the similar data, i.e. the swelling ratios decreased with increasing prevulcanisation times, were observed in both curves, it could be noticed that the swelling ratios of SPENR were lower than those of SPNR. Our results agreed well with the previous works described for solid rubber which indicated that sulphur reacted with the double bonds of NR and triggered the cross-linking or cyclisation. Whereas, epoxide groups in ENR were randomly distributed and, hence, double bonds were more isolated than in the NR chains [19]. Therefore, the cyclisation, which required a neighbouring double bond, did not take place in ENR as schematically shown in Fig. 7.
However, the reaction in latex in this study might be more complicated than that formerly reported in the solid phase due to the unclear sites of epoxidation reaction in each particle and the added reagents during the reactions. The percent swelling ratios of rubber sheets cast from RVENR latices irradiated with various doses were then determined. Results are shown in Fig. 8.
It was observed that the percent swelling ratios of the RVENR latex sheets decreased with increasing irradiation dose. By using doses from 4 to 10 kGy, the percent swelling ratios were about 850–600% which indicated a moderate cross-linking density of latex sheets. The constant value of swelling ratio of about 500% was observed when irradiated with 14 kGy, i.e. full cross-linking took place as previously noticed in the case of RVNR [12].
Morphologies of SPENR and RVENR latex particles
With the application of phase transfer/bulk polymerisation/TEM technique, TEM micrographs of SPENR latex particles were prevulcanised for 5 h at which the swelling ratio was constant. These micrographs embedded in PS matrix are presented in Fig. 9a,b with two magnifications of ×30 and ×60K, respectively.
All micrographs revealed the two-phase morphology of cross-linked ENR particles (dark) dispersed in the PS matrix (light). The natural polydispersity in size of SPENR particles was clearly observed and their elliptical shape is considered to be an artefact of the sectioning process as already mentioned in our earlier works [11]. The uniform mesh structure, in which the dark threads of cross-linked rubber are separated by unstained PS, was noticed. During radical polymerisation of styrene swollen in SPENR, long-chain PS caused phase separation and forced the rubber chains to form a thread-like structure. Due to the homogeneous network in all particles, it was believed that the relative rate of diffusion of the vulcanising reagents in the rubber phase was faster than the vulcanisation as observed in SPNR [11]. However, it should also be pointed out from the values of solubility parameter (δ) that the δ of isoprene (NR) of 8.2 (cal/cm3)1/2 which is not much different from that of styrene’s 8.7 (cal/cm3)1/2 [29] was responsible for uniform absorption of styrene into SPNR. In the case of SPENR particle, although it was doubtful that the epoxidation took place non-uniformly in each particle [30], the hypothesis that the epoxidation reaction took place mainly at the outer zone of the particle could not be confirmed by its morphology under TEM. This was possibly due to the fact that the sulphur linkage can occur at any double bond and the small difference between δ of ENR 25 or of NR and δ of styrene. Hence, the equilibrium diffusion of styrene could provide the homogeneous rubber network morphology in SPENR particles.
In the morphological study of RVENR latex irradiated with 14 kGy, the TEM micrographs with magnifications of 30 and 60K are shown in Fig. 10a,b, respectively.
As expected, the two-phase morphology of RVENR particles (dark) dispersed in PS (light) was observed. Inside each particle, although the uniform mesh structure was noticed, it was important to indicate the observable dark layer surrounding each RVENR particle which was relatively undetermined in SPENR. The dark layer around RVENR particle was possibly derived from protein-lipid complex, the indigenous surfactants of NR latex particle. However, other possible reasons mainly related to the heterogeneity of epoxidation reaction within each particle could be also considered, as schematically displayed in Fig. 11. It existed some swollen regions, before irradiation, by the sensitisers, i.e. CCl4 and n-butyl acrylate, in the ENR particle. Assuming the degree of rubber epoxidation was high in the outer region of particle, the high probability of concentration of epoxy groups on the near surface of ENR particle might be responsible for the preferable swelling of the added n-butyl acrylate. It should be emphasised that δ of ENR 50 [8.9 (cal/cm3)1/2] was very close to that of n-butyl acrylate [9.0 (cal/cm3)1/2] [29]. However, in this step, the TEM micrographs in Fig. 10 could not be clearly regarded as the strong evidence to confirm the heterogeneity of epoxidation in the RVENR particles because the styrene monomer can greatly swell both NR and ENR.
Morphology of semi-I and fully IPNs latex particles based on RVENR/PMMA
In both semi-I and fully IPNs of RVENR and PMMA or cross-linked PMMA, the heterogeneity of epoxidation and cross-linking in RVENR and the compatibility of both polymers were the factors affecting the degree of phase separation in each composite particle. Before studying the internal morphology of semi-I and fully IPN particles, it should be realised that MMA [δ=9.5(cal/cm3)1/2] prefers to locate at the highly epoxidised region [δ of ENR 50=8.9 (cal/cm3)1/2] whereas the styrene used in the phase transfer/bulk polymerisation process might homogeneously diffuse into the particle. TEM micrographs of semi-I having PMMA concentrations of 10, 20 and 30% are shown in Fig. 12a–d.
The micrographs of composite latex particles in Fig. 12 were generally similar to those of the RVENR samples. The PMMA phase in each composite could not be directly observed under TEM due to the fact that PS and PMMA were presented in the light phase after applying OsO4. The mesh structure, therefore, showed dark threads of cross-linked rubber separated by the unstained PS and/or PMMA. It was noticed that the network in each particle was not homogeneous, i.e. the network near the particle surface was dense. This might be used as an additional evidence that the high cross-linking took place in the outer zone of particle where the degree of epoxidation and the presence of swollen n-butyl acrylate should be higher than in the central region as previously described. The non-uniform mesh structure was clearly observed when PMMA content was 30% as shown in Fig. 12c. The large size of occlusions observed in Fig. 12d also supported the assumption of low cross-link density in the core of particle greatly swollen by MMA and styrene, and would result in bursting. Because a coherent film would not be achieved if the particles were highly cross-linked near their surface, the crack films cast from semi-I and fully IPN latices were observed as expected. The crack composite films were found in all compositions of PMMA while a smooth film of RVENR could be prepared at room temperature. TEM micrographs of fully IPNs at different PMMA concentrations are presented in Fig. 13a–d.
It was also observed that the size of RVENR particles dispersed in PS phase was polydisperse. The non-uniform mesh structure inside each composite latex particle was noticed as seen in the case of semi-I. It could be, therefore, believed that the presence of the small amount of DVB did not significantly affect the particle morphology, although the cross-linking of the second polymer normally influences the viscosity and compatibility of the two polymer components. It was of interest to emphasise the light cross-link in the central zone of particle by the occlusions with large size in Fig. 13d.
Conclusions
The sulphur or γ-radiation prevulcanisation of selected ENR latex having 25 mol% epoxide (SPENR or RVENR) was examined by percent swelling ratios of the latex films and particle morphologies. The results of swelling ratios of SPENR films compared to those of SPNR indicated that the sulphur vulcanisation of ENR was more effective than that of NR. By applying the phase transfer/bulk polymerisation/TEM technique, the dark layer surrounding each RVENR particle was observed. This layer was possibly derived from protein-lipid complex and/or related to the heterogeneity of epoxidation reaction within each particle. A dense network near the particle surface of semi-I and fully IPNs based on RVENR and PMMA could be used to support the degree of epoxidation and, hence, the presence of swollen n-butyl acrylate in the outer zone was higher than in the central core. The appearance of large occlusions in IPNs with 30% PMMA and of the crack film also correlated well with the above assumption. Further study on peroxide prevulcanisation of ENR latex is still ongoing.
References
Lu G, Li ZF, Li SD, Xie J (2002) J Appl Polym Sci 85:1736
Lee DY, Subramaniam N, Fellows CM, Gilbert RG (2002) J Polym Sci A Polym Chem 40:809
Varkey JT, Rao SS, Thomas S (1996) J Appl Polym Sci 62:2169
Claramma NM, Mathew NM (1997) J Appl Polym Sci 65:1913
Makuuchi K, Hagiwara M (1984) J Appl Polym Sci 29:965
Hill DJT, O’Donnell JH, Perera MSC, Pomery PJ, Smetser P (1995) J Appl Polym Sci 57:1155
Porter M, Rawi R, Rahim SA (1992) J Nat Rubber Res 7:85
Ho CC, Khew MC (1999) Langmuir 15:6208
Hamzah S, Gomez JB, Rama RPS (1987) J Nat Rubber Res 2:118
Cook S, Cudby PEF, Davies RT, Morris MD (1997) Rubber Chem Technol 70:549
Tangboriboonrat P, Lerthititrakul C (2002) Colloid Polym Sci 280:1097
Tangboriboonrat P, Tiyapiboonchaiya C (1999) J Appl Polym Sci 71:133
Tangboriboonrat P, Tanunchai T, Tiyapiboonchaiya C (1999) Plast Rubber Compos 25:357
Hourston DJ, Romaine J (1989) Eur Polym J 25:695
Hourston DJ, Romaine J (1990) J Appl Polym Sci 39:1587
Sperling LH, Mishra V (1996) Polym Adv Technol 7:197
Schneider M, Pith T, Lambla MJ (1996) J Appl Polym Sci 62:273
Hashim AS, Kohjiya S (1993) Kautsch Gummi Kunstst 46:208
Gelling IR, Morrison NJ (1985) Rubber Chem Technol 58:243
Kumar RN, Kong WC, Abubakar A (1999) J Coat Technol 71:79
Ratnam CT, Nasir M, Baharin A, Zaman K (2000) Polym Int 49:1693
Tangboriboonrat P, Rakdee C (2000) Plast Rubber Compos 29:258
Blackley DC (1987) Latices. In: Mark HF, Bikaks NM, Overberger CG (eds) Encyclopedia of polymer science and engineering, vol 8, 2nd edn. Wiley, p 647
Perera MCS, Elix JA, Bradbury JH (1988) J Polym Sci A Polym Chem 26:637
Burfield DR, Lim KL, Law KS, Ng S (1984) Polymer 25:995
Sanguansap K, Suteewong T, Saendee P, Buranabunya U, Tangboriboonrat P (2005) Polymer 46:1373
Vernekar SP, Sabne MB, Patil SD, Patil AS, Idage SB, Avadhani CV, Sivaram S (1992) J Appl Polym Sci 44:2107
Blackley DC, Aisah AAN, Twaits R (1979) Plastics and rubber: materials and applications, May:77
Brandrup J, Immergut EH (1975) Polymer handbook, 2nd edn. Wiley-Interscience, New York
Lua CM, Gomez JB, Subramanium A (1985) Proceedings of the international rubber conference, Kuala Lumpur, Malaysia, p 525
Acknowledgements
The authors gratefully acknowledge the Thailand Research Fund (TRF) for the financial support. Special thanks go to the Office of Atomic Energy for Peace (Thailand) for allowing us to use the 60Co γ-ray source.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Saendee, P., Tangboriboonrat, P. Latex interpenetrating polymer networks of epoxidised natural rubber/poly(methyl methacrylate): an insight into the mechanism of epoxidation. Colloid Polym Sci 284, 634–643 (2006). https://doi.org/10.1007/s00396-005-1437-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-005-1437-8