Abstract
Silver nanoparticles were prepared in aqueous AgNO3 solution by using hydroquinone and sodium citrate as reducing agents with neutral polymers poly(vinylpyrrolidone) and poly(vinyl alcohol) as stabilizers. The rate of particle formation was determined with a diode array UV-Vis spectrophotometer. The effects of the polymer concentration on the reaction rate, the size, and the size distribution of the particles formed were studied by transmission electron microscopy. Both the reaction rate and the size of silver nanoparticles decreased with increasing polymer concentration in the range 0.07–0.50 w/v%.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The preparation of noble metal nanoparticles has been the subject of numerous publications. The particles were mainly generated by reduction and stabilized by various methods [1, 2, 3, 4, 5, 6, 7, 8]. Further publications describe the combination of noble metal nanoparticles with supports and the binding of the particles to the surfaces [9, 10, 11]. The literature describing the preparation of silver nanoparticles is especially broad, since classical colloid methods [12, 13] are combined with modern nanotechnology leading to many procedures for particle preparation, control of particle size, and surface modification [14, 15, 16, 17, 18].
When, for instance, Na-oleate is used as stabilizer [19] the surfactants are adsorbed on the surface of Ag nanoparticles and form a protective surfactant layer. Hydrophilic polymers are excellent stabilizers for Pd nanoparticles when the palladium ions are reduced by hydrazine or NaBH4 at room temperature [20]. Neutral polymers, such as poly(vinylpyrrolidone) (PVP) and poly(vinyl alcohol) (PVA), protect the particles by steric stabilization, thereby preventing their aggregation [17, 21]. Henglein used various polymers for stabilizing Ag nanoparticles [16]. The narrowest size distribution and the best optical characteristics were obtained with poly(ethylene imine) as stabilizer.
Huang et al. [21] showed by XPS measurements that the carbonyl group of PVP plays a role in the photochemical reduction of Ag+ ions. When the PVP concentration was increased, the rate of reduction increased. Mayer et al. [22] stabilized Ag particles by cationic polyelectrolytes. They studied the effect of silver precursors formed in the course of the procedure (e.g., AgCl and Ag2O) on the shape of Ag nanoparticles. Kim et al. [23] chose various silver salts as starting material and examined the effect of the chemical quality of the initial precursor on the rate of metal nanoparticle formation. They found that in the presence of AgBF4, AgPF6, and AgClO4 the initial fast reaction rate was reduced after about 10 min, whereas in the case of AgNO3, the reaction rate was slower but constant. They assumed that the difference is the result of strong interactions between silver and nitrate ions. Kéki et al. used dendrimers for the stabilization of silver particles [24], a method allowing the preparation of particles with an extremely narrow size distribution. The particle size was controlled by the functional group and dendrimer size.
The kinetics of particle growth, with special regard to the effect of the chemical structure and the concentration of the polymer stabilizers, is rarely discussed in the literature. Our aim was to control the growth of Ag nanoparticles and to determine the kinetic constants of the process from UV-Vis absorption spectroscopic experiments.
Materials and methods
Materials used for the preparation of silver sols were AgNO3 (Reanal, 99.9%), sodium citrate dihydrate (Aldrich, 99+%), hydroquinone (Aldrich, 99%), poly(vinylpyrrolidone) (Fluka, M w=40,000), and poly(vinyl alcohol) (Reanal, M w=72,000).
The reduction of sols was monitored by UV-Vis spectrophotometry (Ocean Optics Chem2000-UV-VIS spectrophotometer) at wavelengths (λ) of 200–800 nm. Metallic silver has a characteristic absorbance maximum at about 420 nm [16]. The first stage of sol formation was monitored by filling the solution without the reducing agent in a 1-cm cuvette (total volume 2.8 cm3; 1.2 cm3 of 10 mM Na-citrate solution and 0.4 cm3 of 10 mM AgNO3 solution, and the polymer concentration was 0.7–5 g L−1). The absorbance spectrum was recorded every minute over 40 min, starting at the moment of the addition of the reducing agent (0.2 cm3 of 1 mM hydroquinone). In the course of the reduction, the reaction mixture was stirred with a micromagnetic stirrer.
Transmission electron micrographs (TEMs) were recorded with a Philips CM-10 transmission electron microscope with an accelerating voltage of 100 kV. The microscope was equipped with a Megaview II digital camera. The size distribution of the particles was determined by using UTHSCSA Image Tool 2.00 software.
Results and discussion
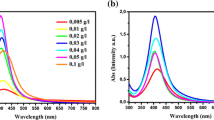
The reduction of Ag+ ions was studied in aqueous medium without polymers and in systems containing PVA. The absorbance spectra of the Ag sol stabilized with 0.7 g L−1 PVA solution recorded every minute over 20 min is shown in Fig. 1a. The shape of the spectra gives preliminary information about the size and the size distribution of the silver particles [25]. The initial rate of reduction was high, but particle formation slowed down after a reaction time of about 20 min. At a PVA concentration of 0.7 g L−1, after 20 min, the absorbance value at the wavelength of maximal absorbance exceeded 1.6. At higher polymer concentrations (e.g., in the silver sol containing 3 g L−1 polymer), the absorbance was about 1.5 at the same reaction time; thus, in the sol containing less stabilizer, more Ag+ ions were reduced within the same time period. The polymer chains surrounding the Ag+ and Ag0 particles sterically inhibit particle nucleation and growth. The wavelength of maximal absorbance by silver (λ=410–420 nm) was not changed significantly in the course of reduction. The nanosol containing 0.7 g L−1 polymer had the highest absorbance (1.7), a value slightly exceeding that of the polymer-free sol (Fig. 1b). The absorbance peak of the sol containing 3 g L−1 polymer was unsymmetrical: a shoulder at longer wavelengths indicates the presence of aggregated particles [21].
The absorbance spectra of Ag nanosols containing PVP as stabilizer (Fig. 2) revealed that increasing concentration of this polymer had a more marked negative effect on the formation rate of Ag particles than had PVA. Increasing the PVP concentration decreased the rate of particle formation. At reaction times exceeding 30 min, however, there were no longer significant differences between maximal absorbance values. At PVP concentrations of 1.9 and 5 g L−1 the spectra were unsymmetrical. Again, shoulders indicated the presence of larger aggregates of particles.
The rate of Ag+ reduction was measured by determining the time dependence of absorbance at various concentrations (Fig. 3). Evidently new particles were not formed after the first 20 min of the reaction: the process of nucleation may be regarded as finished, since the absorbance remained nearly constant. The rate of nucleation decreased as a result of the addition of the polymer, because the polymer chains present in the solution interfere with particle formation.
PVA-containing Ag sols were formed faster than in the presence of PVP. For example, particle formation was finished in about 16 min at the highest amount of PVA, but needed about 26 min in the presence of PVP. Particle formation seems to be more sterically hindered by pyrrolidone than by vinyl alcohol.
In order to study the rate of the Ag+ reduction, straight lines were fitted to the initial sections of the kinetic curves. The apparent rate values calculated from the slopes of the straight lines (ΔA/Δt) are listed in Table 1. Obviously, relatively high concentrations of sterically stabilizing polymers decreased the rate of nucleation.
The absorbance spectra of sols prepared without stabilizers were recorded daily (Fig. 4). The absorbance increased with time, and the wavelength at maximum absorbance gradually shifted towards higher values. Wavelengths at maximum absorbance were normalized by the following relationship: Δλ max/λ max0=f(t), where λ max0 is the wavelength of maximal absorbance of the spectra recorded after 40 min and Δλ max is the wavelength shift relative to this value. The shift towards longer wavelengths indicates increasing particle size (i.e., aggregation). Average particle sizes based on electron micrographs are listed in Table 1. In the absence of polymer stabilization, aggregated particles with an average diameter of 9.3 nm were observed (Fig. 5). The sol was quite polydisperse. In the presence of stabilizers, the particle size decreased and the size distribution became more monodisperse. As the polymer concentration was increased, the size of Ag particles decreased, although aggregated particles were also observed at 3 g L−1 PVA and 5 g L−1 PVP, so that higher average particle sizes (8.4 and 5.0 nm) were observed. This aggregation was also indicated by the absorbance spectra. On transmission electron micrographs of sols stabilized by PVA, polymer chains were clearly seen to line up 5–20 Ag particles per chain. At higher polymer concentrations the number of Ag particles per chain was still higher.
Conclusions
The formation and the stability of silver nanoparticles were studied in the presence of two stabilizers, namely, PVP and PVA. Reduction of silver ions was monitored by recording the UV-Vis absorbance spectrum over 40 min. The absorbance–time functions allow an easy overview of the formation, stability, and aggregation of Ag nanoparticles.
The size and the size distribution of the particles formed were determined by electron microscopy. In agreement with the spectrophotometric studies, higher polymer concentrations led to smaller particle sizes. The average particle size was similar in the case of both polymers.
Comparison of the stabilizing effects of the two neutral polymers reveals that PVP polymer chains provide a more effective steric stabilization and reduced the growth rate of the Ag nanoparticles.
References
Jana NR, Wang ZL, Pal T (2000) Langmuir 16:2457
Brust M, Kiely CJ (2002) Colloids Surf A 202:175
Vorobyova SA, Sobal NS, Lesnikovich AI (2001) Colloids Surf A 176:273
Han MY, Quek CH (2000) Langmuir 16:362
Papp S, Dékány I (2001) Colloid Polym Sci 279:449
Chen C-W, Akashi M (1997) Langmuir 13:6465
Wang Y, Toshima N (1997) J Phys Chem B 101:5301
Esumi K, Matsuhisa K, Torigoe K (1995) Langmuir 11:3285
Aihara N, Torigoe K, Esumi K (1998) Langmuir 14:4945
Patakfalvi R, Oszkó A, Dékány I (2003) Colloids Surf A 220:45
Papp S, Szűcs A, Dékány I (2001) Solid State Ionics 141–142:169
Lee PC, Miesel D (1982) J Phys Chem 86:3391
Creighton JA, Blatchford CG, Albrecht MG (1979) Trans Faraday Soc 75:790
Quaroni L, Chumanov G (1999) J Am Chem Soc 121:10642
Rodríguez-Gattorno G, Díaz D, Rendón L, Hernández-Segura GO (2002) J Phys Chem B 106:2482
Henglein A (1998) Chem Mater 10:444
Kapoor S (1998) Langmuir 14:1021
Bell WC, Myrick ML (2001) J Colloid Interface Sci 242:300
Wang W, Efrima S, Regev O (1998) Langmuir 14:602
Papp S, Dékány I (2003) Colloid Polym Sci 281:727
Huang HH, Ni XP, Loy GL, Chew CH, Tan KL, Loh FC, Deng JF, Xu GQ (1996) Langmuir 12:909
Mayer ABR, Hausner SH, Mark JE (2000) Polym J 32:15
Kim HS, Ryu JH, Jose B, Lee BG, Ahn BS, Kang YS (2001) Langmuir 17:5817
Kéki S, Török J, Deák G, Daróczi L, Zsuga M (2000) J Colloid Interface Sci 229:550
Kim D-W, Shin S-I, Oh S-G (2003) Preparation and stabilization of silver colloids in aqueous surfactant solutions. In: Mittal KL, Shah DO (eds) Surfactant sciences series, vol 109. Marcel Dekker, New York, pp 255–268
Acknowledgements
The authors wish to express their thanks to National Scientific Research Fund, OTKA T13 034430 for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patakfalvi, R., Virányi, Z. & Dékány, I. Kinetics of silver nanoparticle growth in aqueous polymer solutions. Colloid Polym Sci 283, 299–305 (2004). https://doi.org/10.1007/s00396-004-1138-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-004-1138-8