Abstract
Grafted copolymer of Plantago psyllium mucilage and acrylonitrile (Psy–g–PAN) has been synthesized in the presence of nitrogen using a ceric ion–nitric acid redox system. The solid removal efficiency of this copolymer was tested with tannery effluent. The suitable pH, optimum dose of polymer and contact time for the maximum removal of suspended (SS) and dissolved solids (TDS) are reported. The optimum dose was found to be 1.2 mg l−1. The suitable pH values, at which a maximum SS removal of about 89% and TDS removal of about 27% occurred, were found to be 7.0 and 9.2 for SS and TDS, respectively. The optimum treatment duration was 3 h. The analysis of X-ray diffraction patterns of Psy–g–PAN and solid waste from effluent before and after treatment suggests the interaction of the solid waste with the Psy–g–PAN copolymer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the development of the tanning industry, water plays a vital role as the industry consumes large quantities. The wastewater, after processing rawhide/skin into finishing leather, is highly alkaline and contains decomposing organic matter, sulphide and organic nitrogen with a high amount of other toxic chemicals. Without proper treatment the discharge of tannery effluent to the environment can cause serious and long-lasting consequences. One approach to remove the suspended solids (SS) and the dissolved solids (TDS) from a wide variety of wastewaters is the adsorption technique, which is more effective and simpler to operate than other presently available methods. The use of natural organic polymers for this purpose has become increasingly essential, in light of their effectiveness in extremely low concentrations, biodegradability, inert behavior to pH changes and easy availability from reproducible agricultural resources. Among the disadvantages of the natural organic polymers are shear instability, uncontrolled biodegradability and varying inefficiency. On the other hand synthetic polymers do not suffer from these drawbacks and are very good treatment agents but are nonbiodegradable and expensive.
Many attempts have been made to contribute the best properties of both by grafting synthetic polymers onto the backbone of natural polymers [1, 2]. Acrylamide-grafted natural polymers, such as amylopectin, guargum and xantham gum [1], starch [1, 3, 4], sodium alginate [5, 6], psyllium mucilage [7], find extensive application as flocculants. Acrylonitrile-grafted natural polymers have also been used as wastewater treatment agents [8, 9]. Cellulose-grafted coplymers were prepared by the fibres of the Kenaf plant (Hibiscus cannabinus) with polyacrylonitrile (PAN) and were use for removal of Zn(II) and Cr(III) ions [10]. Cellulose-grafted PAN was also utilized for removal of metal ions from the effluent of paper mills and textile industries [11]. We have recently reported the synthesis of Plantago psyllium (Psy) grafted-PAN [12] and its use for the treatment of textile effluent [9]. In the present communication, the use of Psy–g–PAN as a treatment agent for SS and TDS removal from tannery wastewater is reported.
Materials and methods
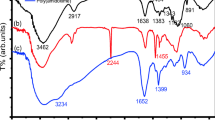
Psy mucilage was obtained from its husk (Sidhpur Sat-Isabgol Factory, Gujrat, India) and was used after purification. It was purified by precipitation from aqueous solution with alcohol and finally washed with acetone. Acrylonitrile, ceric ammonium nitrate (Merck Chemical Co., extra pure) and nitric acid (BDH, Analar grade), were used as received. The Fourier transform IR (FTIR) spectrum of purified Psy mucilage was recorded using a Brucker-Vector-22 spectrometer.
The Tannery effluent (wastewater) was collected from a tannery situated at Jajmau, Kanpur, India, where vegetable and chrome tanning processes are used. The pH of the wastewater samples and of the mucilage solution in water was measured using a CP 931 microprocessor pH meter. The conductivity of the wastewater sample was measured by a Century CC 631 microprocessor conductivity meter and chemical oxygen demand (COD) [13] by the usual standard methods. The buffer solutions, prepared by using ready-made buffer tablets (E. Merck), were used for maintaining the pH of the wastewater sample.
The Psy–g–PAN was synthesized by the method given by Fanta et al. [14]. The graft copolymers were synthesized by grafting acrylonitrile onto purified Psy. The details of the synthesis are given in our previous publication [12]. The total monomer conversion was calculated by the standard equation [15].
A copolymer sample with about 86% grafting was used in this study. The batch experiments were conducted for adsorption studies [8]. The SS and TDS were calculated by the standard equation [16, 17, 18].
The adsorption efficiency of the copolymer sample was studied by carrying out the experiments at three pH values: 4.0, 7.0 and 9.2. X-ray diffraction (XRD) patterns of powder samples of grafted copolymer, solid waste and flocs were obtained at ambient conditions using an Iso-Debyflux-2002 X-ray diffractometer (Rich and Scifert) with a Cu Kα radiation source.
Results and discussion
Characterization of Psy–g–PAN
The FTIR spectrum of Psy–g–PAN gives characteristic peaks of –OH between 3,609 and 3,288 cm−1, –C=O between 1,662 and 1,647 cm−1, the ether linkage at 1,455–1,400 cm−1 and nitrile at 2,357 cm−1. The intrinsic viscosity of the copolymer could not be determined owing to its insolubility in many polar, nonpolar and mixed solvents.
The tannery effluent had a pH of 8.37, a conductivity of 5.73 mS, a COD of 2815 mg l−1, TDS of 3796 mg l−1, SS of 415 mg l−1 and a turbidity of 70 NTU. The pH of the tannery effluent after adding Psy–g–PAN was found to be 8.33.
Adsorption studies
Determination of optimum dose of Psy–g–PAN
The effect of Psy–g–PAN dose on solid removal from tannery effluent is presented in Fig. 1. It shows the plots of the percentage removal of SS and dissolved solids TDS versus copolymer dose. It is apparent from the plots that a Psy–g–PAN dose of 1.2 mg l−1 produced the maximum percentage removal of TDS and SS. With an increase in the copolymer dose from 0.4 mg l−1 to the optimal value, the percentage SS and TDS removal also increased but a further increase in the dose caused a decreasing trend in solid removal. This behaviour could be explained by the fact that the optimal dose of flocculant in suspension causes larger amounts of SS to aggregate and settle. However, Chan and Chiang [2] suggest that an above optimal amount of flocculant in suspension would cause the aggregated particle to redisperse in the suspension and would also reduce particle settling.
Effect of contact time
The flocculation efficiency of the mucilage with varying contact time is shown in Fig. 2. It shows the plots of percentage removal of the solids (SS and TDS) and contact time using different polymer doses. The maximum TDS (26.34%) and SS (76.63%) removal occurred after 5 and 3 h, respectively, using the optimal dose. In the absence of copolymer, the percentage removal of SS and TDS was found to be less than 1% even after 24 h.
Percentage removal of suspended solid versus contact time with polymer dose 0.4 mg l−1 (closed squares), 0.8 mg l−1 (closed circles), 1.2 mg l−1 (closed triangles), 1.6 mg l−1 (closed diamonds). Percentage removal of total dissolved solid versus contact time with polymer dose 0.4 mg l−1 (open squares), 0.8 mg l−1 (open circles), 1.2 mg l−1 (open triangles), 1.6 mg l−1 (open diamonds)
The maximum solid removal occurred only after a particular duration, i.e., the optimal treatment time. After this duration, the reverse trend was seen. The SS removal always improves with time but the results obtained in the present study did not follow this statement. Therefore, the most plausible reasons for the reverse trend may be due to the destabilization of the aggregated particles after a long duration [9, 18], and the change in surface chemistry of the edge face with time that changes the adsorption of polymer with time [19].
Effect of pH
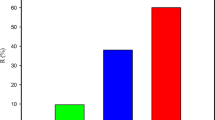
The adsorption efficiency of grafted copolymer at three pH values (4.0, 7.0 and 9.2) is shown in Fig. 3. The plots show that the maximum SS removal was 89% at neutral pH (pH 7.0), though appreciable SS removal was seen at acidic and alkaline pH as well. The treatment duration was 3 h. On the other hand, in the case of TDS removal, the maximum removal (26.93%) was seen at alkaline pH (9.2) and in acidic and neutral pH it was 11.01 and 21.68%, respectively, and it requires only 1-h treatment time. The optimal polymer dose was 1.2 mg l−1.
The changes in the adsorption efficiency of the copolymer occur with varying pH. It may be suggested that lowering of the pH and an increase in the pH cause strong ion association, but only at the sites not involved in the hydrogen bonding. At neutral pH, the hydrogen bonding between neighbouring hydroxyls and between surface adsorbed water and surface hydroxyls was disrupted by electrolyte adsorption resulting in an increase in the percentage of solid removal. The lowest percentage removal obtained at acidic pH is probably due to the utilization of H+ ions in oxidation of proteinacious matter rather than in the oxidation of metallic ions present in tannery effluent. The appreciable percentage removal of SS at alkaline pH may be due to the precipitation of metal ions, like chromium and sodium, in the form of their hydroxides, thus increasing the adsorption efficiency of the copolymer [19, 20]. The presence of these two metals has been confirmed by XRD. An appreciable percentage removal of TDS in the neutral-to-alkaline pH range may be due to the presence of such substances, which precipitate in this pH range.
The increase and decrease in the percentage solid removal is indeed the effect of pH. It does not seem to be the effect of dilution (owing to addition of buffer) because if it were, the percentage removal should be the same at all pH values. The maximum removal of SS and TDS at two different pH values suggested that the wastewater sample treatment process would require a two-step procedure. Since the effluent samples were alkaline in nature, the TDS removal was the first step and in the second step SS was removed by lowering the pH slightly to neutral.
Although the XRD patterns do not give any specific evidence for the mechanism of flocculation, they may be used as supportive evidence. The comparison of XRD patterns observed for the copolymer and solid waste before and after treatment at room temperature from 2θ=10–90° (the error range of 2 θ is 0.01–0.31) showed changes. The diffraction pattern of the copolymer showed an amorphous nature, whereas the patterns for solid waste showed a crystalline nature but the 2θ (diffraction angle) and the d values (diffracting intensities) observed in the XRD pattern for solid waste before treatment are changed altogether in the pattern for treated waste (Fig. 4). This constitutes primary evidence that a different crystal type was formed [21]. The change in the 2θ angle and d values indicates the change in the nature of the crystalline waste material in wastewater during the adsorption process. This may be due to interactions between the functional groups of copolymer and the contents of the tannery waste [8, 9].
Conclusion
A new grafted copolymer, Psy–g–PAN, was synthesized by ceric ion initiation and was used as a treatment agent for tannery effluent. The optimum polymer dose for maximum solids removal was 1.2 mg l−1and the most suitable pHs for SS and TDS removal were neutral and alkaline, respectively. Therefore the overall wastewater treatment process involves two steps. XRD patterns were used to suggest the primary chemical interaction between the polymer and solid waste. All the results obtained after treatment process indicate that Psy–g–PAN copolymer is as good an adsorbent as activated carbon in controlling water pollution.
References
Singh RP, Karmarkar GP, Rath SK, Pandey SR, Tripathy T, Panda J, Kanan K, Jain SK, Lan NT (2000) Polym Eng Sci 40:46
Chan WC, Chiang CY (1995) J Appl Polym Sci 58:1721
Khalil MI, Farag S (1998) J Appl Polym Sci 69:45
Karmakar GP, Singh RP (1998) Colloids Surf 133:119
Tripathi T, Pandey SR, Bhagat RP, Singh RP (2001) Eur Polym J 37:125
Rajani S, Agarwal M, Padma SV, Mishra A (2002) Asian Textile J 11:48
Agarwal M, Rajani S, Mishra A (2002) J Polym Res 9:69
Kang DW, Choi RC, Kweon DK (1999) J Appl Polym Sci 73:469
Rajani S, Agarwal M, Mishra A (2002) Water Qual Res J Can 37:371
Eromosele IC, Bayero SS (2000) Bioresour Technol 71:279
Warner RR, Rerai EJ (1997) J Appl Polym Sci 65:1471
Rajani S, Gupta RK, Mishra A (2003) Colloid Polym Sci 281:187
Eaton AD, Clesceri LS, Greenberg AE (1995) Standard methods for examination of water and effluent, 19th edn. APHA, Washington, DC
Fanta GF, Burr RC, Russel CR, Rist CE (1969). J Polym Sci 13:133
Athawale VD, Rathi SC (1999) J Macromol Sci Rev Macromol Chem Phys 39:445
Huck PM (1997) Scavenging and flocculation of metal bearing effluent using polyelectrolyte. Environmental Protection Service, Burlington, Canada
Jha A, Agarwal S, Mishra A, Rai JSP (2001) Iran Polym J 10:85
Agarwal M, Rajani S, Mishra A (2001) Macromol Mater Eng 286:560
Hocking HB, Klichuk KA, Lowen S (1999) J Macromol Sci Rev Macromol Chem Phys C 39:177
Ayumi I, Teruyuki V, Jiaro A, Toshiyoki T, Koji M (2000) Water Res 34:751
Huang L, Allen E, Tonelli AE (1999) Polymer 40:3211
Acknowledgements
We are grateful to the University Grants Commission, India, and the Council for Scientific and Industrial Research, India, for financial support. We are also thankful to Tapan Routh, Incharge, Zonal Laboratory Kanpur, National Environmental Engineering Research Institute, for his valuable help during this study by providing some of the research facilities.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mishra, A., Yadav, A., Agarwal, M. et al. Polyacrylonitrile-grafted Plantago psyllium mucilage for the removal of suspended and dissolved solids from tannery effluent. Colloid Polym Sci 282, 300–303 (2004). https://doi.org/10.1007/s00396-003-0895-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00396-003-0895-0