Abstract
Remote ischemic conditioning (RIC) protects against acute ischemia–reperfusion injury and may also have beneficial effects in patients with stable cardiovascular disease. We investigated the effect of long-term RIC treatment in patients with chronic ischaemic heart failure (CIHF). In a parallel group study, 22 patients with compensated CIHF and 21 matched control subjects without heart failure or ischemic heart disease were evaluated by cardiac magnetic resonance imaging, cardiopulmonary exercise testing, skeletal muscle function testing, blood pressure measurement and blood sampling before and after 28 ± 4 days of once daily RIC treatment. RIC was conducted as four cycles of 5 min upper arm ischemia followed by 5 min of reperfusion. RIC did not affect left ventricular ejection fraction (LVEF) or global longitudinal strain (GLS) in patients with CIHF (p = 0.63 and p = 0.11) or matched controls (p = 0.32 and p = 0.20). RIC improved GLS in the subgroup of patients with CIHF and with NT-proBNP plasma levels above the geometric mean of 372 ng/l (p = 0.04). RIC did not affect peak workload or oxygen uptake in either patients with CIHF (p = 0.26 and p = 0.59) or matched controls (p = 0.61 and p = 0.10). However, RIC improved skeletal muscle power in both groups (p = 0.02 for both). In patients with CIHF, RIC lowered systolic blood pressure (p < 0.01) and reduced NT-proBNP plasma levels (p = 0.02). Our findings suggest that long-term RIC treatment does not improve LVEF but increases skeletal muscle function and reduces blood pressure and NT-proBNP in patients with compensated CIHF. This should be investigated in a randomized sham-controlled trial.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While advances in the treatment of acute myocardial infarction have translated into improved survival [33], prevention of evolving post-infarction heart failure remains a major clinical challenge with a 1-year mortality of nearly 50% [7]. Chronic ischemic heart failure (CIHF) evolves due to loss of ventricular function from ischemic myocardial tissue injury [19] and induces a vicious cycle by activating compensatory neurohormonal and inflammatory mechanisms [20], which leads to adaptive metabolic and remodeling processes [19] and a progressive decline of cardiac function.
Remote ischemic conditioning (RIC) by alternating brief episodes of limb ischemia and reperfusion is an evolving therapeutic strategy to achieve protection against acute ischemia–reperfusion injury in the heart [18] and several other organs [34]. RIC has been shown to reduce myocardial injury in patients with ST-segment elevation myocardial infarction [6] as well as in patients undergoing elective percutaneous coronary intervention [21], which may translate into improved long-term clinical outcome [9, 36]. The majority of these studies have utilized RIC as single-occasion treatment in settings of acute ischemia–reperfusion. Recently, attention has enlarged on the potential beneficial effects of repeated RIC application and the effect of RIC in patients with stable cardiovascular conditions [18]. Experimental studies have suggested that repetitive daily RIC administration may confer additional beneficial effects through anti-remodeling processes [41, 42] and improvement of endothelial and microcirculatory function [24, 26]. Furthermore, a clinical pilot study suggests that a single application of RIC may improve exercise capacity in a fraction of patients with heart failure and reduced ejection fraction [29]. We hypothesized that long-term, repetitive RIC application has potential clinical implication for patients suffering from stable ischemic heart disease. The aim of the present study was to investigate whether daily RIC application for 4 weeks improves left ventricular function, cardiopulmonary exercise capacity, skeletal muscle function, blood pressure and serology markers of heart failure in patients with stable compensated CIHF and matched controls without heart failure or ischemic heart disease.
Methods
Patients
We recruited patients with compensated CIHF from the outpatient heart failure clinic at Department of Cardiology, Aarhus University Hospital, Denmark and Department of Cardiology, Viborg Region Hospital, Viborg, Denmark. At the time of study enrollment, all patients were clinically stable and on optimal heart failure treatment. Criteria for inclusion were: (I) age ≥ 18 years, (II) left ventricular ejection fraction (LVEF) ≤ 45%, and (III) New York Heart Association functional class I–III. Criteria for exclusion were: (I) permanent atrial fibrillation, (II) diabetes mellitus, (III) peripheral neuropathy, (IV) current dialysis treatment, (V) contraindication to cardiac magnetic resonance (CMR) imaging examination (e.g., metal implants including implantable cardiac device and pacemaker), (VI) hospitalization for cardiovascular disease within 30 days, and (VII) strenuous exercise within 3 days prior to each study visit.
As control group, we recruited subjects without heart failure and ischemic heart disease matched on age and gender. Ischemic heart disease was ruled out based on cardiac 82Rubidium positron emission tomography/computed tomography imaging or coronary angiography. Matched control subjects were recruited from Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark, Department of Cardiology, Viborg Region Hospital, Viborg, Denmark and Department of Nuclear Medicine, Aarhus University Hospital, Aarhus, Denmark. Criteria for inclusion were: (I) age ≥ 18 years, and (II) LVEF > 45%. Criteria for exclusion were similar as for patients with CIHF.
Due to a pre-specified sub-study analysis evaluating the effect of RIC on platelet function and fibrinolysis [31], all participants were instructed in avoidance of non-steroidal anti-inflammatory drugs for at least 7 days prior to study initiation and during the entire study period. In addition, participants in the control group were instructed in pausing, if relevant, aspirin treatment for at least 5 days prior to each study visit. From ethical considerations, the latter was not applied for patients with CIHF.
Design
We conducted a prospective, outcome-assessor blinded, parallel group study based on paired observations before and after study intervention. All participants underwent two study visits with 28 ± 4 days apart. The ± 4-day variation was allowed for logistical reasons. At each study visit, participants underwent blood sampling, CMR examination, and leg extension power and cardiopulmonary exercise testing. In addition, patients with CIHF were asked to fulfill a Minnesota Living with Heart Failure Questionnaire (MLWHFQ). The technical staff and participants were blinded to baseline data at the follow-up visit, and similar instructions were given at both study visits. Outcomes were measured consecutively and analyzed following study completion.
In between study visits, participants were instructed in application of RIC once daily. RIC was conducted on the same arm throughout the entire study period as 4 cycles of 5-min upper arm ischemia followed by 5 min of reperfusion using the commercially available automatic AutoRIC device (CellAegis Devices Inc., Toronto, Canada). All participants were instructed in fulfilling a checklist to assess for RIC application compliance, and to follow normal routine with no alteration in medication intake, physical activity or dietary habits.
Cardiac magnetic resonance imaging
All CMR examinations were performed with a Philips Achieva dStream 1.5 T scanner (Philips Healthcare, Best, The Netherlands) with a 60-cm bore or a Siemens Skyra 3T scanner (Siemens Healthcare, Erlangen, Germany) with a 70-cm bore depending on the body size of the person investigated. Similar, anterior and posterior coil or a single-element anterior coil combined with a posterior coil array was used depending on the body size of the person investigated. The same CMR scanner and coils were used at both study visits. Cine-CMR examinations were conducted electrocardiogram-triggered and performed during breath-hold, and forward stroke volume was measured by velocity-encoded phase contrast measurement in the ascending aorta as previously described [16].
All CMR examinations were conducted blinded to the participants and observers in regard to study data, and data analysis was done outcome-assessor blinded in regard to both clinical and study data. The software system Segment (Medviso AB, Lund, Sweden) was used for CMR data analysis [17]. Data for LVEF and left ventricular end-diastolic volume were derived from short-axis views on which the epi- and endocardial border were automatically traced during end-diastole and end-systole. Manuel correction was done in case of obvious misalignment of the cardiac contour. The slice containing > 50% visible myocardium circumference constituted the basal slice. Myocardial trabeculae were included as part of the myocardium and papillary muscles as part of the cavity. LVEF was calculated as [(end-diastolic volume − end-systolic volume)/end-diastolic volume) × 100%]. Additional feature tracking cine-CMR data analyses were done for evaluation of peak global longitudinal strain (GLS). Data for GLS were derived from two- and four-chamber views on which the epi- and endocardial border was outlined in the end-diastole. Subsequently, an automatic computation was triggered in which the applied software algorithm automatically outlined the border throughout the cardiac cycle. The tracking contour was visually validated.
Cardiopulmonary exercise testing
Evaluation of peak cardiopulmonary exercise capacity was done using a staged exercise ergometer bicycle test (Lode Corival Ergometer, Groningen, The Netherlands) by experienced physiotherapists with exercise data collected breath-by-breath using the commercially available software Jaeger MasterScreen CPX System (CareFusion, San Diego, USA) and JLAB software package (Jefferson Lab, Newport News, USA). Prior to each test, body mass and height were measured, and the gas-analyzing system was calibrated with a defined gas mixture. All participants underwent an identical staged test protocol starting at 25 W and with increments of 2.5 W every 10 s. Test duration of 8–12 min was targeted to ensure steady state and to prevent prematurely test ending due to muscle fatigue. Endpoints of cardiopulmonary exercise testing were peak workload (W/kg) and peak oxygen consumption (ml/kg/min).
Leg extension power rig testing
Evaluation of skeletal muscle function was assessed as leg extension power using the leg extensor power rig (Medical Engineering Unit, Nottingham University, Nottingham, UK) by experienced physiotherapists. The leg extension power test has been described elsewhere in detail [5]. Briefly, we conducted the test on dominant leg and the same leg was used at both study visits. While sitting in an upright position with arms folded across the chest, participants were verbally instructed in extension the leg as quickly and powerful as possible against a foot pedal with applied weight. Verbal encouragement was given to ensure maximal performance. Measurements were repeated every 30 s until the test was followed by two tests with similar or less power, or a maximum of ten pushes were reached. Endpoint was peak leg extension power (W/kg).
Minnesota Living with Heart Failure Quality-of-Life Questionnaire
The impact of CIHF on quality of life was measured using the MLWHFQ, which is a standardized self-assessment measuring instrument for evaluating the therapeutic response to a given intervention in patients with heart failure [37] and allows for a comprehensive evaluation of the disease-specific quality of life in response to treatment.
All patients with CIHF were given similar instructions for filling out the questionnaire at both study visits. Fulfilling of the questionnaire was done unsupervised from any person involved in the study.
Blood samples
At the beginning of each study visit, blood samples were drawn from the cubital vein and collected in vials containing anticoagulants. Blood for N-terminal pro-brain natriuretic peptide (NT-proBNP) measurements underwent routine analysis at the Department of Clinical Biochemistry, Aarhus University Hospital, Aarhus, Denmark.
Blood pressure
Resting blood pressure and heart rate were measured on the same arm on both study visits. Following a resting period of 5 min, the blood pressure and heart rate was measured twice and mean value was calculated.
Statistics
Unpaired data were tested for normal distribution and equality of variance, and paired data were tested for normal distribution and equality and normality of distribution prior to statistical analysis. Logarithm transformation was performed when appropriate. Categorical variables were compared using χ 2 test or Fisher’s exact test. Paired continuous variables were analyzed using paired Student’s t test for parametric data or Wilcoxon signed-rank test for non-parametric data despite logarithm transformation, and unpaired continuously variables using unpaired Student’s t test. We performed additional simple and multiple linear regression analyses to test for relationship between responses of long-term RIC treatment on cardiovascular endpoints.
Statistical analyses were conducted using STATA/SE 13 (StataCorp, College Station, USA). Data are presented as number (%), or mean ± standard deviation [95% confidence interval (CI)] for parametric data, geometric mean (95% CI) for logarithm transformed data or median (interquartile range) for non-parametric data. Statistical significance was set as two-sided p value of < 0.05.
The primary study endpoint was change in LVEF. The coefficient of variability of LVEF using CMR imaging has been found to be 7% in patients with heart failure [14]. To be able to detect clinical relevant changes based on the impact of angiotensin-converting enzyme inhibitors and beta-blockers on LVEF in chronic heart failure studies [10, 13], power calculations revealed that 20 patients with CIHF were needed to detect an absolute change in LVEF of 3% with a power of 90% at a two-sided significance level of 5%.
Ethics
Data were collected according to the study protocol at the Department of Cardiology and Centre of Research in Rehabilitation (CORIR), Aarhus University Hospital, Aarhus, Denmark, The MR Centre, Aarhus University, Aarhus, Denmark, and Department of Cardiology, Viborg Region Hospital, Viborg, Denmark. The study was carried out in accordance with the Declaration of Helsinki (2008) of the World Medical Association, and was approved by The Central Denmark Region Committees on Health Research Ethics and The Danish Data Protection Agency. The study was registered with clinicaltrials.gov (Identifier: NCT02248441). All participants gave written informed consent prior to inclusion.
Results
A total of 22 patients with CIHF and 21 matched control subjects were included in the study (Fig. 1). Descriptive baseline characteristics are shown in Table 1.
Cardiac magnetic resonance imaging
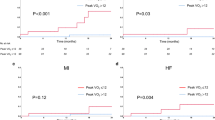
Data for CMR were available for all patients with CIHF and for 20 matched control subjects and are shown in Table 2. Overall, RIC did not affect LVEF or GLS in patients with CIHF (p = 0.63 and p = 0.11) or matched control subjects (p = 0.32 and p = 0.20). While RIC improved GLS in the subgroup of patients with CIHF and NT-proBNP plasma levels above the geometric mean of 372 ng/l (p = 0.04), we found no relationship between baseline GLS and the response of long-term RIC treatment on GLS in a simple linear regression analysis (p = 0.39). In the same subgroup of patients, we found no relationship between the responses of long-term RIC treatment on cardiovascular endpoints, i.e., GLS, peak oxygen uptake, systolic blood pressure and NT-proBNP, in multiple linear regression analyses (p = NS for all).
Cardiopulmonary exercise capacity and skeletal muscle function
Data for cardiopulmonary exercise testing were available for 21 patients with CIHF and for all matched control subjects (Table 2). Twenty (95%) patients with CIHF achieved a respiratory exchange ratio ≥ 1.10 at both study visits and hence representative data for the true cardiopulmonary exercise capacity. Similarly, in matched control subjects, 19 (90%) achieved a respiratory exchange ratio ≥ 1.10 at both study visits. Long-term RIC treatment did not affect peak cardiopulmonary exercise capacity in patients with CIHF or matched control subjects.
Skeletal muscle function data were available for all patients with CIHF and matched control subjects. Long-term RIC treatment increased skeletal muscle power in patients with CIHF (2.3 ± 0.8 W/kg vs. 2.5 ± 0.8 W/kg following long-term RIC treatment; 95% CI 0.02–0.21, p = 0.02), equivalent to a 9% increase. Similarly, long-term RIC treatment increased skeletal muscle power in matched control subjects (2.4 ± 0.8 W/kg vs. 2.6 ± 0.8 W/kg following long-term RIC treatment; 95% CI 0.04–0.38, p = 0.02), equivalent to an 8% increase.
Disease-related quality of life
Data for MLWHFQ score were available for all patients with CIHF. Long-term RIC treatment improved self-assessed disease-related quality-of-life borderline significantly (15 ± 10 points vs. 12 ± 9 points following long-term RIC treatment; 95% CI − 5.42 to 0.24, p = 0.07).
Serology markers of heart failure
Data for NT-proBNP were available for all patients with CIHF and matched control subjects. Long-term RIC treatment decreased plasma levels of NT-proBNP in patients with CIHF [372 (95% CI 256–540) ng/l vs. 315 (95% CI 224–443) ng/l following long-term RIC treatment, p = 0.02]. In contrast, long-term RIC treatment did not affect plasma levels of NT-proBNP in matched control subjects [67 (95% CI 50–88) ng/l vs. 71 (95% CI 50–100) ng/l following long-term RIC treatment, p = 0.53)].
Blood pressure
Data for resting blood pressure were available for all patients with CIHF and matched control subjects (Table 2). Among patients with CIHF, long-term RIC treatment decreased systolic blood pressure and non-statistical significantly diastolic blood pressure (p < 0.01 and p = 0.19). Similar, long-term RIC treatment tended to decrease systolic blood pressure and diastolic blood pressure in matched control subjects (p = 0.09 and p = 0.10).
Effect modification by baseline N-terminal pro-brain natriuretic peptide plasma level on outcome
Patients with CIHF were grouped according to the geometric mean of 372 ng/l of baseline NT-proBNP plasma levels (Table 3). We found no effect by long-term RIC treatment on LVEF or peak cardiopulmonary exercise capacity irrespective of baseline NT-proBNP plasma levels. The beneficial effect of long-term RIC treatment on NT-proBNP plasma levels was restricted to patients with CIHF and baseline NT-proBNP plasma levels above the geometric mean (p = 0.03). Among these patient, long-term RIC treatment improved GLS (p = 0.04). In contrast, the beneficial effect of long-term RIC treatment on skeletal muscle power was restricted to patients with baseline NT-proBNP plasma levels below the geometric mean (p < 0.001).
Discussion
The main findings of the present study were that although long-term RIC treatment did not affect LVEF per se in patients with CIHF, long-term RIC treatment reduced NT-proBNP plasma levels and systolic blood pressure, and improved left ventricular function in terms of GLS in patients with highest NT-proBNP plasma levels. Moreover, long-term RIC treatment improved skeletal muscle power in both patients with CIHF and matched control subjects without heart failure and ischemic heart disease. These findings suggest that RIC may not only improve myocardial contractile function in most severely compromised patients with CIHF but also add a general improvement of skeletal muscular function even in a compensated state of heart failure.
Previous findings indicate that repetitive application of RIC does not result in tachyphylaxis of the cardioprotective from RIC against subsequent acute I/R injury in terms of infarct size reduction [32]. In addition, experimental proof-of-principle studies have demonstrated that long-term, repetitive application of RIC following the acute phase of a myocardial infarction confers myocardial anti-remodeling effects [41, 42] and may improve survival [41]. Under stable conditions, Jones et al. demonstrated that repetitive daily RIC administration for 7 days improve peripheral endothelial and microcirculatory function assessed by non-invasive ultrasound-derived flow-mediated vasodilation and laser Doppler flowmetry-derived cutaneous vascular conductance, respectively [24]. More recently, the same authors demonstrated that improvement of endothelial function could be maintained for several weeks with three times per week application of RIC [25]. In addition, twice daily RIC application for 7 days enhanced echocardiography-assessed coronary flow reserve derived from coronary blood flow velocities [26], which may reflect improvement of the coronary microcirculatory in response to RIC. We extended previous findings by demonstrating that long-term RIC application for 4 weeks seems to have beneficial hemodynamic effects by decreasing systolic blood pressure, and hence, afterload, and to improve myocardial contractile function in those patients with compensated CIHF, who have the most extensive elevated NT-proBNP levels. Endogenously derived substances assumed causally involved in cardioprotection by RIC are vasodilative by nature, e.g., adenosine, nitric oxide and bradykinin [18]. Release of such substances in connection to RIC application may explain the blood pressure-lowering effect from RIC as demonstrated in the present study.
NT-proBNP is a cardiac polypeptide secreted from the ventricles in response to ventricular volume expansion and pressure overload [15]. It is a frequently used surrogate marker in clinical trials on patients with heart failure, as increasing levels are associated with worse outcome in patients with heart failure [28]. In addition, meta-analysis has demonstrated that brain natriuretic peptide-guided heart failure treatment lowers mortality in patients with heart failure and reduced LVEF [11]. In the present study, the decrease in NT-proBNP, therefore, likely indicates a beneficial effect of RIC, and the finding is consistent with a recent published study evaluating the effect of RIC in patients with mild ischemic heart failure [8]. Of importance, we demonstrated an increase in GLS measured by CMR in the subgroup with the highest levels of NT-proBNP. Traditionally, LVEF has been used to estimate systolic function and is a predictor of prognosis as well as pivotal in the planning of treatment in patients with heart failure. However, GLS is increasingly used and importantly has been demonstrated to be a superior predictor of prognosis compared to LVEF [38]. In addition, GLS is associated with wall stress and correlates well with NT-proBNP in patients with chronic heart failure [12]. The underlying reason for the changes in both NT-proBNP and GLS is likely related and may be due to alleviation in myocardial wall stress caused by reduction in afterload, as demonstrated by the decrease in blood pressure. Our findings suggests that the effect of RIC on myocardial contractile function may be of particular benefit in the most severely compromised heart failure patients even though they were clinically compensated and on optimal heart failure treatment. In contrast, RIC increased skeletal muscle power only in the subgroup of patients with the lowest levels of NT-proBNP. Thus, patients with CIHF irrespectively of NT-proBNP levels seem to benefit from long-term RIC treatment although partly in terms of different clinical endpoints. Intriguingly, our findings suggest that RIC may improve quality of life in both patients with normal and elevated NT-proBNP levels.
In heart failure, increased sympathetic activity correlates with a poor prognosis and is a target of established anticongestive treatment [40]. Recently, it was shown that 6 weeks of RIC improves cardiac dysautonomia evaluated by heart rate variability in patients with heart failure [8]. The finding supports that repeated RIC increases the parasympathetic nervous system and promotes withdrawal of sympathetic nervous activity, and hence, rebalances the cardiac dysautonomia that evolves in heart failure. These effects of RIC may be the cause of the observed reduction in NT-proBNP plasma levels. However, this remains speculative, as we did not evaluate heart rate variability in the present study.
During the progression of heart failure a decrease in muscle function is observed and overt cardiac cachexia may develop [23]. Such changes correlate with a poor prognosis and are related to a low-grade inflammation and release of pro-inflammatory cytokines [1]. Hence, approaches to circumvent this maleficent process or even reverse it are warranted. In the present study, we were able to demonstrate an increase in muscle power following RIC treatment using the leg extensor power rig testing, which has been found to correlate with functional performance and risk for mobility limitations in elderly persons [4]. The increase in muscle power from RIC may be due to the previous mentioned effect on the autonomic nervous system, anti-inflammatory effect of RIC [27, 32, 35] and partly by activation in nitric oxide pathways and improvement in metabolism by attenuation of adenosine triphosphate depletion [22]. Our findings are consistent with those from a previous study on short-term RIC application in healthy volunteers [39]. Importantly, we extended previous findings by demonstrating that the effect of RIC on skeletal muscle strength is likely caused by direct effects on the skeletal musculature that do not presuppose cardiac effects. However, this remains speculative and needs to be further investigated.
Despite an increase in muscle power in the present study and recent observed effect of RIC on 6-min walking testing and cardiopulmonary exercise testing [8], we were not able to find any effect on cardiopulmonary exercise capacity in the present study. The latter is in agreement with recent observations on young healthy volunteers [3], and with previous findings by McDonald and colleagues on the acute effects of a single application of RIC in patients with heart failure [29]. However, in the present study, the patients may be restricted by joint pain, leg fatigue or other factors limiting the value of exercise testing [2]. On the other hand, the study by McDonald and colleagues suggests that a fraction of patients with heart failure may be preconditioned per se and thus have no additional effect from RIC on exercise capacity [29].
Limitations
The present study is based on paired observations before and after intervention and not a randomized controlled design. Thus, we cannot exclude bias from potential non-RIC-related effects. In particular, this might be relevant when evaluating skeletal muscle function and self-assessed disease-specific quality of life. In addition, the study design did not allow exploration of any causal relations of the effect of long-term RIC treatment. Consequently, the study should be considered exploratory, and our results need to be further investigated in a randomized sham-controlled study and causality should be sought. Patients were treated for 4 weeks but the treatment time may have been too short to induce cardiac remodeling effects and left ventricular function improvements in terms of LVEF changes. Treatment with angiotensin-converting enzyme inhibitor or a beta-blocker induces cardiac remodeling after approximately 3 months of treatment [10, 13]. Nevertheless, the present study was able to demonstrate signs of improvements in heart failure in terms of reduced NT-proBNP and increased GLS. Even so, we found no relation between responses from long-term RIC treatment on GLS, peak oxygen uptake, systolic blood pressure or NT-proBNP plasma levels by multivariate analyses. However, due to sample size, results from multivariate analyses should be interpreted with caution.
We recruited patients with CIHF in NYHA class I–II only. Consequently, the results of the present study do not apply on patients with CIHF and more advanced heart failure, which may also benefit from long-term RIC treatment. In addition, we acknowledge that normal LVEF is by definition > 50% according to the present European guidelines [30]. However, due to the large variance of echocardiography-derived LVEF assessment, we decided on a cut-off inclusion criterion at LVEF ≤ 45% to avoid the risk of including patients with LVEF > 50%.
RIC compliance is an important potential confounder in this study. Participants were continuously reminded of RIC application throughout the entire study period and fulfilled a checklist hereof, implying excellent RIC compliance. While we have no documentation of the true RIC compliance, potential lack of compliance cannot explain the observed beneficial effects of long-term RIC treatment. For GLS analysis using feature tracking cine-CMR evaluation we relied only on two- and four-chamber views. Including three-chamber views might have allowed for a more comprehensive assessment of GLS. Twenty-four-hour blood pressure monitoring would have enabled a more comprehensive evaluation of the effect of long-term RIC treatment on blood pressure. We did not assess for any persistent effect from RIC treatment on blood pressure. Finally, we did not assess if the included patients or control subjects were already preconditioned and whether this could explain the lack of effect of long-term RIC treatment on myocardial contractile function and exercise capacity as observed in the present study.
Conclusion
Our findings suggest that long-term RIC treatment does not improve LVEF but increases skeletal muscle function and reduces blood pressure and NT-proBNP in patients with compensated CIHF. The clinical implications of our exploratory study should be further investigated in a randomized sham-controlled trial.
Abbreviations
- CIHF:
-
Chronic ischemic heart failure
- GLS:
-
Global longitudinal strain
- CMR:
-
Cardiac magnetic resonance
- LVEF:
-
Left ventricular ejection fraction
- MLWHFQ:
-
Minnesota Living with Heart Failure Questionnaire
- NT-proBNP:
-
N-terminal pro-brain natriuretic peptide
- RIC:
-
Remote ischemic conditioning
References
Anker SD, Sharma R (2002) The syndrome of cardiac cachexia. Int J Cardiol 85:51–66. doi:10.1016/S0167-5273(02)00233-4
Arena R, Myers J, Williams MA, Gulati M, Kligfield P, Balady GJ, Collins E, Fletcher G, American Heart Association Committee on Exercise R, Prevention of the Council on Clinical C, American Heart Association Council on Cardiovascular N (2007) Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation 116:329–343. doi:10.1161/CIRCULATIONAHA.106.184461
Banks L, Wells GD, Clarizia NA, Jean-St-Michel E, McKillop AL, Redington AN, McCrindle BW (2016) Short-term remote ischemic preconditioning is not associated with improved blood pressure and exercise capacity in young adults. Appl Physiol Nutr Metab 41:903–906. doi:10.1139/apnm-2016-0024
Bassey EJ, Fiatarone MA, O’Neill EF, Kelly M, Evans WJ, Lipsitz LA (1992) Leg extensor power and functional performance in very old men and women. Clin Sci (Lond) 82:321–327. doi:10.1042/cs0820321
Bassey EJ, Short AH (1990) A new method for measuring power output in a single leg extension: feasibility, reliability and validity. Eur J Appl Physiol 60:385–390. doi:10.1007/BF00713504
Botker HE, Kharbanda R, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Andersen NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HT, Redington AN, Nielsen TT (2010) Remote ischaemic conditioning before hospital admission, as a complement to angioplasty, and effect on myocardial salvage in patients with acute myocardial infarction: a randomised trial. Lancet 375:727–734. doi:10.1016/S0140-6736(09)62001-8
Chen J, Hsieh AF, Dharmarajan K, Masoudi FA, Krumholz HM (2013) National trends in heart failure hospitalization after acute myocardial infarction for Medicare beneficiaries: 1998–2010. Circulation 128:2577–2584. doi:10.1161/CIRCULATIONAHA.113.003668
Chen L, Zhou Q, Jin H, Zhu K, Zhi H, Chen Z, Ma G (2017) Effects of remote ischaemic conditioning on heart rate variability and cardiac function in patients with mild ischaemic heart failure. Heart Lung Circ. doi:10.1016/j.hlc.2017.03.164
Davies WR, Brown AJ, Watson W, McCormick LM, West NE, Dutka DP, Hoole SP (2013) Remote ischemic preconditioning improves outcome at 6 years after elective percutaneous coronary intervention: the CRISP stent trial long-term follow-up. Circ Cardiovasc interv 6:246–251. doi:10.1161/CIRCINTERVENTIONS.112.000184
Doughty RN, Whalley GA, Walsh HA, Gamble GD, Lopez-Sendon J, Sharpe N, Investigators CES (2004) Effects of carvedilol on left ventricular remodeling after acute myocardial infarction: the CAPRICORN Echo Substudy. Circulation 109:201–206. doi:10.1161/01.CIR.0000108928.25690.94
Felker GM, Hasselblad V, Hernandez AF, O’Connor CM (2009) Biomarker-guided therapy in chronic heart failure: a meta-analysis of randomized controlled trials. Am Heart J 158:422–430. doi:10.1016/j.ahj.2009.06.018
Gaborit F, Bosselmann H, Tonder N, Iversen K, Kumler T, Kistorp C, Soletormos G, Goetze JP, Schou M (2015) Association between left ventricular global longitudinal strain and natriuretic peptides in outpatients with chronic systolic heart failure. BMC Cardiovasc Disord 15:92. doi:10.1186/s12872-015-0063-8
Groenning BA, Nilsson JC, Sondergaard L, Fritz-Hansen T, Larsson HB, Hildebrandt PR (2000) Antiremodeling effects on the left ventricle during beta-blockade with metoprolol in the treatment of chronic heart failure. J Am Coll Cardiol 36:2072–2080. doi:10.1016/S0735-1097(00)01006-8
Grothues F, Smith GC, Moon JC, Bellenger NG, Collins P, Klein HU, Pennell DJ (2002) Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional echocardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am J Cardiol 90:29–34. doi:10.1016/S0002-9149(02)02381-0
Hama N, Itoh H, Shirakami G, Nakagawa O, Suga S, Ogawa Y, Masuda I, Nakanishi K, Yoshimasa T, Hashimoto Y et al (1995) Rapid ventricular induction of brain natriuretic peptide gene expression in experimental acute myocardial infarction. Circulation 92:1558–1564. doi:10.1161/01.CIR.92.6.1558
Hansson NH, Sorensen J, Harms HJ, Kim WY, Nielsen R, Tolbod LP, Frokiaer J, Bouchelouche K, Dodt KK, Sihm I, Poulsen SH, Wiggers H (2017) Myocardial oxygen consumption and efficiency in aortic valve stenosis patients with and without heart failure. J Am Heart Assoc. doi:10.1161/JAHA.116.004810
Heiberg E, Sjogren J, Ugander M, Carlsson M, Engblom H, Arheden H (2010) Design and validation of Segment-freely available software for cardiovascular image analysis. BMC Med Imaging 10:1. doi:10.1186/1471-2342-10-1
Heusch G, Botker HE, Przyklenk K, Redington A, Yellon D (2015) Remote ischemic conditioning. J Am Coll Cardiol 65:177–195. doi:10.1016/j.jacc.2014.10.031
Heusch G, Libby P, Gersh B, Yellon D, Bohm M, Lopaschuk G, Opie L (2014) Cardiovascular remodelling in coronary artery disease and heart failure. Lancet 383:1933–1943. doi:10.1016/S0140-6736(14)60107-0
Hofmann U, Frantz S (2013) How can we cure a heart “in flame”? A translational view on inflammation in heart failure. Basic Res Cardiol 108:356. doi:10.1007/s00395-013-0356-y
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG, Clarke SC, Shapiro LM, Schofield PM, O’Sullivan M, Dutka DP (2009) Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation 119:820–827. doi:10.1161/CIRCULATIONAHA.108.809723
Horiuchi M (2017) Ischemic preconditioning: potential impact on exercise performance and underlying mechanisms. J Phys Fitness Sports Med 6:15–23. doi:10.7600/jpfsm.6.15
Izawa KP, Watanabe S, Osada N, Kasahara Y, Yokoyama H, Hiraki K, Morio Y, Yoshioka S, Oka K, Omiya K (2009) Handgrip strength as a predictor of prognosis in Japanese patients with congestive heart failure. Eur J Cardiovasc Prev Rehabil 16:21–27. doi:10.1097/HJR.0b013e32831269a3
Jones H, Hopkins N, Bailey TG, Green DJ, Cable NT, Thijssen DH (2014) Seven-day remote ischemic preconditioning improves local and systemic endothelial function and microcirculation in healthy humans. Am J Hypertens. doi:10.1093/ajh/hpu004
Jones H, Nyakayiru J, Bailey TG, Green DJ, Cable NT, Sprung VS, Hopkins ND, Thijssen DH (2015) Impact of eight weeks of repeated ischaemic preconditioning on brachial artery and cutaneous microcirculatory function in healthy males. Eur J Prev Cardiol 22:1083–1087. doi:10.1177/2047487314547657
Kono Y, Fukuda S, Hanatani A, Nakanishi K, Otsuka K, Taguchi H, Shimada K (2014) Remote ischemic conditioning improves coronary microcirculation in healthy subjects and patients with heart failure. Drug Des Dev Ther 8:1175–1181. doi:10.2147/DDDT.S68715
Konstantinov IE, Arab S, Li J, Coles JG, Boscarino C, Mori A, Cukerman E, Dawood F, Cheung MM, Shimizu M, Liu PP, Redington AN (2005) The remote ischemic preconditioning stimulus modifies gene expression in mouse myocardium. J Thorac Cardiovasc Surg 130:1326–1332. doi:10.1016/j.jtcvs.2005.03.050
Logeart D, Thabut G, Jourdain P, Chavelas C, Beyne P, Beauvais F, Bouvier E, Solal AC (2004) Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol 43:635–641. doi:10.1016/j.jacc.2003.09.044
McDonald MA, Braga JR, Li J, Manlhiot C, Ross HJ, Redington AN (2014) A randomized pilot trial of remote ischemic preconditioning in heart failure with reduced ejection fraction. PLoS One 9:e105361. doi:10.1371/journal.pone.0105361
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, Gonzalez-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Authors/Task Force M (2016) 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37:2129–2200. doi:10.1093/eurheartj/ehw128
Pryds K, Kristiansen J, Neergaard-Petersen S, Nielsen RR, Schmidt MR, Refsgaard J, Kristensen SD, Botker HE, Hvas AM, Grove EL (2017) Effect of long-term remote ischaemic conditioning on platelet function and fibrinolysis in patients with chronic ischaemic heart failure. Thromb Res 153:40–46. doi:10.1016/j.thromres.2017.03.008
Rohailla S, Clarizia N, Sourour M, Sourour W, Gelber N, Wei C, Li J, Redington AN (2014) Acute, delayed and chronic remote ischemic conditioning is associated with downregulation of mTOR and enhanced autophagy signaling. PLoS One 9:e111291. doi:10.1371/journal.pone.0111291
Schmidt M, Jacobsen JB, Lash TL, Botker HE, Sorensen HT (2012) 25 year trends in first time hospitalisation for acute myocardial infarction, subsequent short and long term mortality, and the prognostic impact of sex and comorbidity: a Danish nationwide cohort study. BMJ 344:e356. doi:10.1136/bmj.e356
Schmidt MR, Pryds K, Botker HE (2014) Novel adjunctive treatments of myocardial infarction. World J Cardiol 6:434–443. doi:10.4330/wjc.v6.i6.434
Shimizu M, Saxena P, Konstantinov IE, Cherepanov V, Cheung MM, Wearden P, Zhangdong H, Schmidt M, Downey GP, Redington AN (2010) Remote ischemic preconditioning decreases adhesion and selectively modifies functional responses of human neutrophils. J Surg Res 158:155–161. doi:10.1016/j.jss.2008.08.010
Sloth AD, Schmidt MR, Munk K, Kharbanda RK, Redington AN, Schmidt M, Pedersen L, Sorensen HT, Botker HE, Investigators C (2014) Improved long-term clinical outcomes in patients with ST-elevation myocardial infarction undergoing remote ischaemic conditioning as an adjunct to primary percutaneous coronary intervention. Eur Heart J 35:168–175. doi:10.1093/eurheartj/eht369
Sneed NV, Paul S, Michel Y, VanBakel A, Hendrix G (2001) Evaluation of 3 quality of life measurement tools in patients with chronic heart failure. Heart Lung 30:332–340. doi:10.1067/Mhl.2001.118303
Stanton T, Leano R, Marwick TH (2009) Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2:356–364. doi:10.1161/CIRCIMAGING.109.862334
Tanaka D, Suga T, Tanaka T, Kido K, Honjo T, Fujita S, Hamaoka T, Isaka T (2016) Ischemic preconditioning enhances muscle endurance during sustained isometric exercise. Int J Sports Med 37:614–618. doi:10.1055/s-0035-1565141
Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54:1747–1762. doi:10.1016/j.jacc.2009.05.015
Wei M, Xin P, Li S, Tao J, Li Y, Li J, Liu M, Li J, Zhu W, Redington AN (2011) Repeated remote ischemic postconditioning protects against adverse left ventricular remodeling and improves survival in a rat model of myocardial infarction. Circ Res 108:1220–1225. doi:10.1161/CIRCRESAHA.110.236190
Yamaguchi T, Izumi Y, Nakamura Y, Yamazaki T, Shiota M, Sano S, Tanaka M, Osada-Oka M, Shimada K, Miura K, Yoshiyama M, Iwao H (2014) Repeated remote ischemic conditioning attenuates left ventricular remodeling via exosome-mediated intercellular communication on chronic heart failure after myocardial infarction. Int J Cardiol 178C:239–246. doi:10.1016/j.ijcard.2014.10.144
Acknowledgements
We thank Casper Carlson Elkjær and Anja Helveg Larsen from Department of Cardiology, Aarhus University Hospital, Aarhus, Denmark, and Maria Krejberg Skou and Dagmar Margrethe Lybæk Sieg from Department of Physiotherapy and Occupational Therapy, Aarhus University Hospital, Aarhus, Denmark for technical assistance.
Author information
Authors and Affiliations
Contributions
KP, RRN, HEB, and MRS designed the experiments. KP did the data analysis and drafting of the manuscript. All authors participated in data acquisition and critical revision of the manuscript.
Corresponding author
Ethics declarations
Funding
This study was funded by The Danish Council for Strategic Research (11-115818), Kirsten Anthonius’ Mindelegat, The Danish Heart Foundation, Direktør Kurt Bønnelycke og hustru fru Grethe Bønnelyckes Fond and The Novo Nordisk Foundation Interdisciplinary Synergi Programme. The study was designed, conducted, analyzed, interpreted, and reported independently of all funding sources.
Conflict of interest
HEB and MRS are shareholders in CellAegis Devices Inc. KP, RRN, AJ, MSH, SR, JR, WYK, and AKP declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Pryds, K., Nielsen, R.R., Jorsal, A. et al. Effect of long-term remote ischemic conditioning in patients with chronic ischemic heart failure. Basic Res Cardiol 112, 67 (2017). https://doi.org/10.1007/s00395-017-0658-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-017-0658-6