Abstract
Purpose
Sub-optimal food choices contribute to the risk of multiple non-communicable diseases (NCDs) which can be mitigated by improving diet quality. Food consumption patterns may partly account for variation of NCD risks in population subgroups in China. This study aimed to evaluate the risk of diet-related NCDs of observed Chinese diets, and to assess the potential reduction in NCD risks by adhering to certain diet recommendations.
Methods
Dose-response meta-analyses were used to derive relative risks between three diet-related NCDs and consumption of 15 food groups. 24-h dietary recall data of 12,809 adults from the 2011 China Health and Nutrition Survey were used to estimate the diet-related summed risks (SRs) of NCDs. Twelve Chinese provinces were aggregated into five regions, and stratified by age, gender, overweight status, education, income, and urbanicity. The Chinese Dietary Guideline-2016 (CDG-2016) and the EAT-Lancet diet were used as recommended diets.
Results
Associations between SRs and gender, age, educational level, income level, and urbanicity were observed. No association was found between SRs and overweight status. Both diet recommendations have lower SRs compared to observed diets among all regions. The food groups that contributed most to the variation of the SRs of diet-related NCDs in China were high consumption of red meat and refined grains, and low consumption of whole grains, fruits, and legumes.
Conclusion
To address the heterogeneity in diet-related NCD risks, focusing on region-specific dietary practical is imperative for Chinese population, in order to propose tailored guidance to adhere to diet recommendations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sub-optimal diets and food choices pose obstacles to achieving the upcoming 2030 targets of Sustainable Development Goals (SDGs), particularly those related to improved nutrition (Goal 2) and good health and well-being (Goal 3) [1, 2]. Globally, an estimated eleven million deaths and 255 million disability-adjusted life years (DALYs) were attributed to dietary risk factors in recent years [3]. Yet, many low- and middle-income countries, including China, are unlikely to achieve the health-related SDGs [4]. In China, dietary risk factors are responsible for more than 2 million deaths and nearly 47 million DALYs per year through non-communicable chronic diseases (NCDs), and these numbers are rising [3, 5]. Furthermore, unhealthy diets that include an excessive amount of sodium and red meat and inadequate amounts of whole grains and fruits were the leading dietary risk factors for death (17.4% of total risk factors) and DALYs (11.6% of total risk factors) in China [6].
Heterogeneity in diet quality and subsequent health implications are typically associated with the geographic, demographic, and socio-economic characteristics of consumers [7]. As a highly-populated, large country with diverse food cultures between regions, China is inevitably faced with large heterogeneity in food consumption patterns across regions [3]. Demographic and socio-economic variables have been found to be related to specific dietary patterns, for example, households with higher incomes have higher diet quality [8]. Women have higher dietary quality scores than men [9], and older Chinese people have been shown to more frequently consume plant-based foods such as grains and vegetables [10].
Previous studies have developed methodologies to quantify life expectancy, and healthy life years gained or lost according to population-level dietary patterns [3, 11, 12]. However, a single health problem would not be embodied by overall health risks. Therefore, specific diet-related NCDs are of great interest to population groups with higher risk dietary patterns. In China, as of 2020, the prevalence of type 2 diabetes is as high as 138 million (11%) in the adult population, with that of coronary heart disease being 118 million (9.4%) [6], and colorectal cancer 0.44 million (the third-highest incidence rate among all types of cancers) [13]. Data from recent studies has shown that nearly 47% of deaths caused by NCDs were attributed to these three NCDs [3]. Hence, because of their prevalence and public health consequences, this study investigated coronary heart disease, type 2 diabetes, and colorectal cancer, in relation to diets in different socio-economic and demographic population subgroups and regions in China.
To support consumers in making healthier food choices, food based dietary recommendations have been developed for public health targets [14]. The Chinese dietary guideline 2016 (CDG-2016) is food-based and aims to provide advice to Chinese citizens on a healthy diet, taking into account both the risk of diet-related diseases and nutrient adequacy [15]. Additionally, the EAT-Lancet reference diet (hereafter referred to as EAT-Lancet diet) is a recommended diet that not only takes health into account, but also considers diet-related environmental impacts [16]. Both of these recommended diets are food group-based and designed to reduce diet-related health risk and improve diet quality.
The objectives of our study were (1) to gain insight into the heterogeneity of diet-related NCD risks (coronary heart disease, type 2 diabetes, and colorectal cancer) across socio-economic and demographic variables among five regions in China, and (2) to investigate the potential effects of adherence to recommended diets to reduce or prevent diet-related NCD risks.
Methods
Identification of diet-related NCD risks for each food group
The diet-related NCD risks associated with specific food groups were derived from dose-response meta-analyses. These analyses examined the relationships between the consumption (expressed in serving size of a food group) of certain food groups and the corresponding NCDs. To investigate potential NCD risks, relative risks (RRs) obtained from prospective cohort studies in meta-analyses were used as indicators of the diet-related NCD risks. These RRs were assumed to reflect causal relationships between food consumption (exposure) and disease risks. In order to collect reliable data, the same literature search strategy, inclusion criteria, and data extraction methods from previous studies were followed [12, 17]. In particular, we explore the pooled relative risks of 15 food groups: whole grain, refined grain (The definition is based on whether the bran was removed at the time of processing), fruits, vegetables, legume, nut, tuber, aquatic products, dairy, eggs, poultry, red meat (fresh beef, donkey, horse, lamb, or pork), sugar-sweetened beverages (SSBs), The health effects of tubers were collected from potatoes only. Olive oil was used as a proxy for plant oils because of the high unsaturated fatty acids content and is considered to contain similar nutrients as other vegetable oils. To estimate the association between sodium intake and the risk of health outcomes, we calculted the estimated value of 24-hour urine collection sodium according to 24-hour diet recall data on sodium with a conversion factor of 0.86, since the RR derived from meta-analysis studies was only collected by 24-hour urinary sodium [18].
We performed a literature search with PubMed (2000-March, 2023) and Web of Science (2000-March, 2023) databases. The letters, comments, narrative reviews, and animal studies were excluded. Studies were included if they were meta-analyses involving cohort studies, studied the impacts of certain food consumption, and reported the associations with the three NCDs we selected. To minimize the potential influence of uncontrolled confounders on the risks of NCDs associated with food consumption, meta-analyses that effectively controlled for various confounding variables, including age, sex, energy intake, smoking status, alcohol intake, etc., were selected as eligible evidence [17]. In addition, the most recent meta-analyses were chosen as they encompassed a larger number of cohort studies and included a greater number of participants.
Study population
The China Health and Nutrition Survey (CHNS) is an ongoing longitudinal household survey with the goal to investigate the association between the economic, sociological, and demographic transformation in China. The survey procedures are described in more detail elsewhere [19, 20].
In our study, data from the 2011 round of the CHNS was used. The survey was conducted across 12 provinces and municipalities, which were grouped into 5 regions, i.e., the East (Shandong and Jiangsu provinces), the Northeast (Heilongjiang and Liaoning provinces), the Central areas (Hunan, Hubei, and Henan provinces), the Southwest (Guangxi, Guizhou, and Chongqing provinces), and Metropolitan areas (Beijing and Shanghai municipalities). All adult participants (age ≥ 18) were included, except pregnant or lactating women (n = 89) and participants with implausible energy intakes (Z-score < -5 or > 5, n = 12) [21]. The final study population included 12,809 eligible participants.
Dietary assessment
The dietary consumption data were collected with 24-hour dietary recalls over three consecutive days and the consumption of salt and edible oils were assessed by examinating the food inventory at household level for the same three consecutive days [22]. The three consecutive days during which detailed household food consumption data have been collected were randomly allocated from Monday to Sunday and are almost equally balanced across the seven days of the week for each sampling unit. Household food consumption has determined by examining changes in inventory from the beginning to the end of each day, in combination with a weighing and measuring technique. All processed foods (including edible oils and salt) remaining after the last meal before initiation of the survey have been weighed and recorded. All purchases, home production, and processed snack foods have been recorded. Whenever foods have been brought into the household unit, they have been weighed, and preparation waste has been estimated when weighing was not possible. At the end of the survey, all remaining foods have been again weighed and recorded. Additionally, all field workers have been trained by nutritionists who are otherwise professionally engaged in nutrition work in their own counties and who have participated in other national surveys. Almost all interviewers have been graduates of post-secondary schools; many have had four-year degrees. In addition, three days of specific training in the collection of dietary data have been provided for this survey.
By linking dietary consumption data to the China Food Composition Table 1 [23], quantities of food consumption were calculated for each participant using the average values from three-day dietary intake data and were standardized per 2,000 kcal. Additionally, for the purpose of comparison, the reference food consumption values in CDG-2016 and EAT-Lancet diet were also standardized to 2,000 kcal (energy-standardized reference values are shown in Supplementary Table 1). The Chinese Healthy Eating Index (CHEI), a validated dietary index to evaluate diet quality according to CDG-2016, was calculated for each participant. The scoring formula and validation of CHEI have been described elsewhere (Supplementary Table 2) [24]. This CHEI index served as a validated indicator to prove the reliability of the estimated SR of diet-related NCDs in the present study.
Aggregation of relative risks for diet-related NCDs
To assess the overall health risks associated with each food group within the context of complete diets, the summed risk (SR) was calculated. The SR represents the combined risk-increasing or risk-reducing potential of a complete diet on the corresponding diet-related non-communicable diseases (NCDs), following the methods outlined by Schwingshackl et al. in 2019 [25]. Food components that were not included in the current dose-response meta-analyses were assumed to have neutral health effects in either direction, including the dose-response relationship between sodium and type 2 diabetes, as well as oils, refined grains, sodium and colorectal cancer [11]. Since the dose-response association of RR between food groups and NCDs are not linear [25], piecewise linear associations fittings were subsequently applied to compute RR values of food groups in a given consumption interval for coronary heart disease, type 2 diabetes, and colorectal cancer. The linear regressions on each interval were carried out in three steps: (1) The piecewise intervals for the association splines were determined based on the observed food consumption values. The specific scales of these intervals for each food group can be found in Supplementary Tables 3 to 5; (2) Within each interval, a linear fit was applied, assuming that each short interval on the curve could be approximated as a linear segment; and (3) The corresponding RRs of diet-related NCDs were calculated for each food group within these specific intervals, taking into account their associations with the respective NCDs.
To calculate the final SR, risk-reducing and risk-increasing food groups were calculated separately by multiplying the RR of their specific consumption, then subtracting the summed RR of risk-increasing food groups from (1 - summed RR of risk-reducing food groups) [25]. A SR > 1 indicates that the complete diet increased the total risk for a given disease, whereas a SR < 1 indicates that the diet reduced risk for a given disease. The formula (1) is as follows:
where RRreduction is the aggregate of relative risks of risk-reducing food groups i and is obtained by multiplying RRi for a specific disease by xi, the quantity of consumption of i. RRincrease is the aggregate of relative risks of risk-increasing food groups j, and is obtained by multiplying RRj for a specific disease by xj, he quantity of consumption of j.
Demographic and socio-economic and covariates
Demographic and socio-economic variables were recorded during the survey period, which included age, gender, overweight status, education level, income level, and urbanicity. Age groups were allocated into three categories: elderly people (aged ≥ 65), middle-aged adults (aged 45–64), and young adults (aged 18–44). Overweight status was divided into 2 levels based on BMI: overweight (BMI ≥ 25 kg/m2) and normal (BMI < 25 kg/m2) [7]. Education level was divided into three categories according to the original classification in questionnaires: Higher education (technical or vocational degree or higher), Middle education (including lower and upper middle school), and Lower education (primary school and lower). Tertiles of income at the total population level were used to define high, middle and low income levels in our study. Two categories of urbanicity were defined by municipalities and administrative divisions in China: urban areas and rural areas [26]. Based on these covariates, population subgroups were formed for each of the five regions.
Statistical analysis
To investigate whether food groups that reduced or increased risk of one NCD also exhibited similar effects on the risks of other NCDs, Spearman’s rank correlation was used to test the correlations of RRs between the three NCDs [17]. Daily food consumption was summarized using the mean, median, and the 95% confidence interval (CI) for 15 food groups per 2,000 kcal in the observed diets.
For the characteristic of participants, the Chi-square test was used to compare unordered categorical variables, and the Kruskal-Wallis rank test was used to compare ordered categorical variables. Differences in food consumption between observed diets and recommended diets were tested using one way ANOVA. And p values < 0.05 were considered statistically significant for all analyses [7]. To evaluate the consistency of the estimated SRs of the three diet-related NCDs (dependent variable) and their association with the CHEI (independent variable), Pearson correlation coefficients (r) were calculated between each set of two of the three NCD risks [24]. Multivariable linear regression models were employed to explore the association between the estimated SRs and gender, age groups, overweight, educational level, income level, and urbanicity. The statistical analyses were conducted using STATA 16.1 (StataCorp, USA) for Windows, and R 4.1.2 was used for data visualization.
Results
Relative risks across 15 food groups
After identifying eligible studies, eleven dose-response meta-analyses were used to extract RR data, which contain a total of 134 cohort studies on coronary heart diseases, 166 cohort studies on type 2 diabetes, and 130 cohort studies on colorectal cancer (Supplementary Table 6). Fourteen food groups (whole grains, refined grains, fruits, vegetables, legumes, nuts, tubers, aquatic products, dairy, eggs, poultry, red meat, sugar-sweetened beverages (SSBs), plant oils (hereafter referred to as oils)) and one nutrient (sodium) with reliable evidence of RRs were included in our study [25, 27,28,29,30,31,32,33,34,35,36,37,38,39].
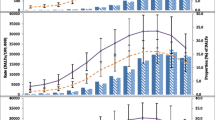
The findings revealed that food groups with risk-reducing properties (RR < 1) were whole grains, fruits, vegetables, and nuts, whereas risk-increasing food groups (RR > 1) were red meat, refined grains, sodium, and SSBs (Fig. 1, the serving sizes of the food groups are listed in Supplementary Table 7). Spearman’s rank correlations indicated that food groups positively or negatively associated with one diet-related NCD risk tended to have similar influences on the risk of other NCDs (Supplementary Table 8).
Relative risks of coronary heart disease, type 2 diabetes, and colorectal cancer for different food groups per additional serving size. Relative risks were derived from meta-analyses. The serving sizes of the food groups are listed in Supplementary Table 2. Points with RR < 1 and RR > 1 are labelled in green and red dots, respectively. Grey dots indicate no association. Error bars: 95% confidence intervals. RRs: relative risks. SSBs: sugar-sweetened beverages
Baseline characteristics and food consumption
Characteristics of the 12,809 adult participants (47% men and 53% women, respectively) in the CNHS 2011 are shown in Table 1, and the flow chat of participants is in Supplementary Fig. 1. Among the regions, Metropolitan areas had the highest proportion (42%) of young adults, while the East and Southwest regions had highest percentage of elderly people (20%). Approximately 40% of participants were overweight (BMI ≥ 25 kg/m2) in East, Northeast, and Metropolitan areas, whereas overweight was relatively lower (30%) in Southwest. Education levels showed regional variations, with the proportion of participants with lower education ranging from 12% in metropolitan areas to 47% in the Southwest. Moreover, the largest proportion of participants with high income was observed in Metropolitan areas (66%), while this ranged from 16 to 37% across the other four regions. Urban residents accounted for over 60% of the total population in Metropolitan areas, whereas the proportion was less than half in the other four regions.
The energy-standardized food consumption in grams of observed diets in five Chinese regions are shown in Supplementary Table 9. Consumption of whole grains, nuts, and dairy in observed diets among all regions were lower than the recommendations in both recommended diets. For example, the consumption of whole grains was 13.8 g per day (14% and 12% of the reference values in the CDG-16 and the EAT-Lancet diet, respectively). In contrast, consumption above the recommended levels was observed for red meat, sodium, and refined grains. Vegetable consumption in observed diets was the closet to the reference value of 300 g and 240 g per day as recommended in the CDG-16 and the EAT-Lancet diet, respectively.
Reliability of the estimated summed risks
Inverse associations were observed between CHEI and the estimated SRs for coronary heart disease, type 2 diabetes, and colorectal cancer (Fig. 2). While no association was observed between CHEI and the estimated SRs of type 2 diabetes, there was a strong positive correlation (Pearson correlation coefficient, r = 0.80) between estimated SRs of type 2 diabetes and the estimated SRs of colorectal cancer (Supplementary Table 10).
Correlations between the Chinese Healthy Eating Index (CHEI) and the estimated summed risks (SRs) of coronary heart disease (panel A; r = -0.48), type 2 diabetes (panel B; r = -0.01), and colorectal cancer (panel C; r = -0.25). The red points in each panel represented the CDG-2016, with a 100 out of 100 CHEI score and 0.42 (95% CI: 0.20, 0.85), 0.48 (95% CI: 0.26, 0.91), and 0.55 (95% CI: 0.40, 0.78) of estimated SRs for coronary heart disease, type 2 diabetes, and colorectal cancer, respectively. CDG-2016: Chinese dietary guideline-2016
Diet-related NCD risks of observed and recommended diets
Figure 3 illustrates the SRs of coronary heart disease, type 2 diabetes, and colorectal cancer for the observed diets, the CDG-2016, and the EAT-Lancet diet. Compared to the observed diets in five regions, substantial reductions in SRs were observed in both the CDG-2016 and the EAT-Lancet diet for coronary heart disease (CDG-2016: 0.42, 95% CI 0.20–0.85; EAT-Lancet diet: 0.37, 95% CI 0.17–0.80), type 2 diabetes (CDG-2016: 0.48, 95% CI 0.26 to 0.91; EAT-Lancet diet: 0.46, 95% CI 0.22 to 1.06), and colorectal cancer (CDG-2016: 0.55, 95% CI 0.40 to 0.78; EAT-Lancet diet: 0.59, 95% CI 0.39 to 0.97).
Contribution of summed risks by relative risk (RR) of each food group for coronary heart disease, type 2 diabetes, and colorectal cancer in observed and recommended diets. Stacked bar plots represent the distribution of summed risk for each food group within these diets. The bars below zero are risk-reducing food groups that decrease the risk of NCDs (1-RRreduction), while the bars beyond zero are risk-increasing food groups that increase the risk of NCDs (RRincrease-1). SSBs: sugar-sweetened beverages. Total: total average observed diet
When comparing the observed diets between the five regions in China, the diets in Southwest region showed the highest risk of coronary heart disease (SR: 1.23; 0.79 to 1.91), followed by diets in Central (SR: 1.18, 0.74 to 1.83), Northeast (SR: 1.18, 0.70 to 1.78), East (SR: 1.17, 0.73 to 1.80), and Metropolitan regions (SR: 1.15, 0.70 to 1.85). Similarly, the diets in Southwest had higher SRs for both type 2 diabetes and colorectal cancer compared to the average diet across all regions (1.19, 95% CI 0.87 to 1.52 and 0.99, 95% CI 0.82 to 1.17 respectively).
The RRs, presented in Fig. 4, were further analyzed to determine the contributions of different food groups to the estimated SRs of three diet-related NCDs. Comparing the CDG-2016 and the EAT-Lancet diets with the other regions, lower consumption of nuts (4.8 g per day) was found to increase the risk of coronary heart disease and type 2 diabetes. Additionally, high consumption of refined grains (423.0 g per day) and sodium (3.3 g per day), in particular, increased considerably risk of coronary heart disease. Furthermore, red meat consumption substantially increased the risk of type 2 diabetes and colorectal cancer. In the Southwest region, the RRs of red meat for type 2 diabetes and colorectal cancer were 1.20 (95% CI 1.00 to 1.37) and 1.15 (95% CI 1.00 to 1.27). Similarly, in Metropolitan areas, the RRs of red meat were 1.19 (95% CI 1.00 to 1.27) and 1.14 (95% CI 1.00 to 1.27) for type 2 diabetes and colorectal cancer. Low dairy consumption (32.2 g per day) and whole grains consumption (13.9 g per day) increased the risk of colorectal cancer in all regions. Finally, the vegetables reduced SRs across the three NCDs between observed and recommended diets similarly.
The NCD risks in socio-economic and demographic subgroups
Associations between participant characteristics and the diet-related NCD risks are presented in Table 2, more detailed information can be found in Supplementary Tables 11 to 13, with the estimated SRs of three NCDs ranging from 0.87 (95% CI 0.70 to 1.03) to 1.29 (95% CI 0.79 to 1.92). Differences in SRs were observed in multivariable linear regression models according to age groups (elderly people had higher risks), and urbanicity (higher risks in rural areas) for coronary heart disease. For type 2 diabetes and colorectal cancer, significant differences were observed according to gender (females having lower SRs than males), age groups (young adults had higher risks), educational level (highly educated people had lower estimated SRs), and income level (higher risks associated with higher income). No differences were observed in estimated SRs according to overweight status. The largest variations in SRs have been found according to age groups. Specifically, elderly people had the highest risk of coronary heart disease, which was 6.6% higher compared to the risk in young adults.
Discussion
Based on the meta-analyses of relative risks between NCDs and food groups, we found that the food groups whole grains, fruits, vegetables, and nuts likely reduce risks for coronary heart disease, type 2 diabetes, and colorectal cancer, whereas red meat, refined grains, sodium and SSBs increase these risks. The observed diets in all regions had increased SRs for coronary heart disease and type 2 diabetes, but a reduced SR for colorectal cancer. Both the CDG-2016 and the EAT-Lancet diet showed an inverse association with the diet-related NCD risks. The hypothesis was confirmed by the associations between diet-related NCD risks and socio-economic and demographic factors. The largest disparities in SRs were found between age categories, where the participants aged over 65 had around 10% lower risks compared to those young adults (age ≤ 44). Moreover, the SRs were lower for females vs. males, low-income level versus other income levels, high educational level versus lower educational levels, and urban residents versus rural residents, although for overweight status there was no difference observed.
Validity and reliability of the RR data for specific food groups is crucial to our study. The RR data we derived align with a previous study [17], the food groups whole grains, fruits, vegetables, legumes, nuts, dairy and plant oils were associated with reduced risks, and additional servings of salt, red meat, SSBs, and refined grains were associated with increased risks for all these three NCDs. Although a high sodium intake is an important contributing factor for increasing risks of various NCDs [40, 41], we only found a dose-response relationship for RR between sodium and coronary heart disease. The reliability of our estimated SRs is supported by their consistent associations with the CHEI scores, which served as an independent method to assess the healthiness of the diet. Inverse associations were shown between the CHEI and the estimated SRs of all three diet-related NCDs.
Allthough the observed variation in diet-related health outcomes across the regions is small, it corresponds to findings from other studies in China. In areas in close proximity to the east coast, the diet quality is reported to be higher than in northern regions [9], which is in line with lower estimated SRs in the eastern region compared to diets in the northeast region and can be partially attributed to the higher consumption of seafood in these areas. In coastal regions such as the East and Metropolitan Areas, the consumption of aquatic products almost meets the recommended level in the CDG-2016 and exceeded the EAT-Lancet recommendations [42]. Moreover, the southwest ares had the highest risks for all three diet-related NCDs according to our estimates. This might be led by the coinstantaneous higher red meat and lower whole grains consumption among these areas. Batis and colleagues [43] reported that people living in south areas tend to consume more high-fat meat and refined grains rather than whole grains, and other two studies identified that southwest areas’ consumers had higher red meat and refined rice intake with the spicy-eating habits [44, 45].
Although geographical regions play a role in variations of diet-related NCD risks, it is important to emphasize that socio-economic and demographic factors, like age, gender, educational level, income level, and urbanicity, also considerably contribute to these differences. Among these factors, the most notable associations were observed between estimated SRs and age groups. In our data, young adults had higher risks of colorectal cancer and type 2 diabetes based on their current food consumption patterns. By contrast, they had the lowest risks of coronary heart disease among all age groups. This was mostly due to the excessive sodium intake in diets of elderly people (more than double the reference value) [46, 47]. The lower risks of type 2 diabetes and colorectal cancer among elderly individuals can be attributed to their higher consumption of plant-based foods and lower intake of red meat, which aligns with previous research findings [48]. However, in the case of coronary heart disease, the increased risk associated with high sodium intake in elderly people’s diets outweighed the reduction of risk from the other food groups. With respect to gender, a study that investigated the association of diet quality with major cardiometabolic risk factors showed a 33% lower risk for women compared to men after adjusting for potential confounders [49], which is consistent with the differences in the estimated SR for coronary heart disease between females and males observed in our study. When measuring the healthiness of observed diets from CHNS 2011, large differences were found based for education level and urbanicity. People with high educational level had a 19% higher CHEI score versus those with lower educational level (58.4 vs. 49.3). Yuan and colleagues [24] also observed that high education level positively associated with higher CHEI score with a coefficient of 9.06 (95%CI: 8.47, 9.65). This finding aligns with our estimated SR of coronary heart disease, as diets of participants who had higher education backgrounds in Northeast, East, and Southwest were associated with lower risks compared to those with lower education levels. The results for urbanicity in the aforementioned study also match with our results: diets in urban areas had a 12% higher CHEI score than those in rural areas. Another study also supports our findings regarding the effect of income inequality on diet-related NCD risks [24]. This study indicated that improving affordability of diets could potentially alleviate diet-related health problems among lower-income populations.
We did not observe an association between overweight and the estimated SRs. Using BMI as the measure of overweight status is not without controversy [50]. It is an inexpensive and easy measure of body composition and that reflects time-integrated energy balance, but it is only weakly associated to energy intake and physical activity at the time of dietary assessment [51]. Moreover, as BMI is known as a determinant of over/underreporting of food and energy intake, we avoided a potential bias in the diet-BMI association by standardizing for energy intake when calculating SRs based on food groups [52].
Some limitations of our study should also be noted. Firstly, we assumed that the associations between food groups and diseases were as linearity on each given intervals. Under this assumption, the results we have obtained may be different in smaller intervals compared to analyzing with splines. However, using linear models in our study did not yield a large difference in estimated NCD risks compared to non-linear regressions, which is consistent with the results of Schwingshackl et al. [53]. Secondly, given the rapid changes occurring in China over the past years, food consumption data from 2011 is a relatively long time ago and might behind the times. However, the CHNS data beyond 2011 are not accessible through open access channels. In addition, the temporal changes in dietary patterns are relatively small over the past two decades. Therefore, it is unlikely that using data from CHNS 2011 would seriously affect the generalizability of our conclusions. Thirdly, based on the existing dose-response meta-analysis data, we specifically addressed three NCDs mentioned in our study, and thus omitted other NCDs. However, analyzing all NCDs is infeasible, and in the Chinese context these three NCDs represent the most prevalent NCDs with significant susceptibility to dietary factors [6, 54, 55]. Finally, the food consumption data we used were from 2011 because newer national data were not available, although it is likely that food consumption patterns have changed over time [56, 57]. Thus, there is a possibility that certain deviations would have been overlooked. For example, meat consumption has been persistently growing in recent years, which would result in increasing risks on meat-related NCDs [6]. Our study also has several strengths. Firstly, the meta-analyses used in our study were deemed high-quality via the NutriGrade scoring system and the AMSTAR-2 tools [12]. Secondly, our approach enables the estimation of disease outcomes pertaining to participants with diverse socio-economic and demographic attributes. This allows a more nuanced understanding of the specific impact of dietary habits in various demographic population subgroups and regions. Thirdly, we employed a risk-based assessment approach to evaluate the NCD risks. By deriving the RR data from dose-response meta-analyses, we obtained more detailed information about three individual NCDs rather than a generalized measure of overall health status at a lower resolution. Finally, the RR data were based on the most comprehensive and up-to-date dose-response meta-analyses, which mitigated the uncertainties associated with relying solely on findings from individual studies.
Conclusion
Substantial disparities in diet-related risks of coronary heart disease, type 2 diabetes, and colorectal cancer were found to be associated with socio-economic and demographic factors, such as region and age, in China. To address this heterogeneity, specific data on food consumption patterns is needed for every region in China, to identify potential innovative policies as well as tailored promotions to adhere to diet recommendations.
Data availability
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval from the corresponding author, data link: https://www.cpc.unc.edu/projects/china/data/datasets
References
Ladha JK, Jat ML, Stirling CM et al (2020) Chapter Two - Achieving the sustainable development goals in agriculture: The crucial role of nitrogen in cereal-based systems. In: Sparks DL (ed) Advances in Agronomy. Academic Press, pp 39–116
Rabbi MF, Hasan M, Kovács S (2021) Food Security and Transition towards sustainability. Sustainability 13:12433. https://doi.org/10.3390/su132212433
Afshin A, Sur PJ, Fay KA et al (2019) Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the global burden of Disease Study 2017. Lancet 393:1958–1972. https://doi.org/10.1016/S0140-6736(19)30041-8
Sustainable Development Goals In: United Nations Development Programme. https://www.undp.org/sustainable-development-goals. Accessed 6 Jul 2022
Rath S, Yu P, Srinivas S (2018) Challenges of non-communicable diseases and sustainable development of China and India. Acta Ecol Sin 38:117–125. https://doi.org/10.1016/j.chnaes.2017.06.012
Chinese Nutrition Society (2020) 2020 Report on Chinese Nutrition and Chronic Disease. The Sata Council Information Office, Beijing, China
Mertens E, Kuijsten A, Dofková M et al (2019) Geographic and socioeconomic diversity of food and nutrient intakes: a comparison of four European countries. Eur J Nutr 58:1475–1493. https://doi.org/10.1007/s00394-018-1673-6
He Y, Fang Y, Yang X, Ding G (2017) Establishment and application of China Healthy Diet Index. CAJ 39:436–441. https://doi.org/10.13325/j.cnki.acta.nutr.sin.2017.05.011
Xu X, Hall J, Byles J, Shi Z (2015) Assessing Dietary Quality of Older Chinese people using the Chinese Diet Balance Index (DBI). PLoS ONE 10:e0121618. https://doi.org/10.1371/journal.pone.0121618
Shen X-M, Huang Y-Q, Zhang X-Y et al (2020) Association between dietary patterns and prediabetes risk in a middle-aged Chinese population. Nutr J 19:77. https://doi.org/10.1186/s12937-020-00593-1
Stylianou KS, Fulgoni VL, Jolliet O (2021) Small targeted dietary changes can yield substantial gains for human health and the environment. Nat Food 2:616–627. https://doi.org/10.1038/s43016-021-00343-4
Fadnes LT, Økland J-M, Haaland ØA, Johansson KA (2022) Estimating impact of food choices on life expectancy: a modeling study. PLOS Med 19:e1003889. https://doi.org/10.1371/journal.pmed.1003889
Sung H, Ferlay J, Siegel RL et al (2021) Global Cancer statistics 2020: GLOBOCAN estimates of incidence and Mortality Worldwide for 36 cancers in 185 countries. CA Cancer J Clin 71:209–249. https://doi.org/10.3322/caac.21660
He P, Baiocchi G, Hubacek K et al (2018) The environmental impacts of rapidly changing diets and their nutritional quality in China. Nat Sustain 1:122–127. https://doi.org/10.1038/s41893-018-0035-y
Chinese Nutrition Society (2022) 2022 Chinese Dietary Guideline, 1st edn. People’s Medical Publishing House, Beijing, China
Willett W, Rockström J, Loken B et al (2019) Food in the Anthropocene: the EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 393:447–492. https://doi.org/10.1016/S0140-6736(18)31788-4
Clark MA, Springmann M, Hill J, Tilman D (2019) Multiple health and environmental impacts of foods. Proc Natl Acad Sci USA 116:23357–23362. https://doi.org/10.1073/pnas.1906908116
McLean R, Cameron C, Butcher E et al (2019) Comparison of 24-hour urine and 24-hour diet recall for estimating dietary sodium intake in populations: a systematic review and meta-analysis. J Clin Hypertens 21:1753–1762. https://doi.org/10.1111/jch.13729
Zhang B, Zhai FY, Du SF, Popkin BM (2014) The China Health and Nutrition Survey, 1989–2011. Obes Rev 15:2–7. https://doi.org/10.1111/obr.12119
Popkin BM, Du S, Zhai F, Zhang B (2010) Cohort Profile: the China Health and Nutrition Survey—monitoring and understanding socio-economic and health change in China, 1989–2011. Int J Epidemiol 39:1435–1440. https://doi.org/10.1093/ije/dyp322
Aggarwal V, Gupta V, Singh P et al (2019) Detection of Spatial Outlier by Using Improved Z-Score Test. In: 2019 3rd International Conference on Trends in Electronics and Informatics (ICOEI). IEEE, Tirunelveli, India, pp 788–790
He K, Du S, Xun P et al (2011) Consumption of monosodium glutamate in relation to incidence of overweight in Chinese adults: China Health and Nutrition Survey (CHNS). AJCN 93:1328–1336. https://doi.org/10.3945/ajcn.110.008870
Yang Y, Wang G, Pan X (2009) China Food Composition table. China Institute of Nutrition and Food Safety, China CDC, Peking
Yuan Y-Q, Li F, Dong R-H et al (2017) The development of a Chinese healthy eating Index and its application in the General Population. Nutrients 9:977. https://doi.org/10.3390/nu9090977
Bechthold A, Boeing H, Schwedhelm C et al (2019) Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 59:1071–1090. https://doi.org/10.1080/10408398.2017.1392288
Mao L, Zhang Y, Wang W et al (2020) Plant-sourced and animal-sourced monounsaturated fatty acid intakes in relation to mortality: a prospective nationwide cohort study. Eur J Nutr 59:1989–1998. https://doi.org/10.1007/s00394-019-02048-8
Schwingshackl L, Hoffmann G, Lampousi A-M et al (2017) Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol 32:363–375. https://doi.org/10.1007/s10654-017-0246-y
Schwingshackl L, Schwedhelm C, Hoffmann G et al (2018) Food groups and risk of colorectal cancer. Int J Cancer 142:1748–1758. https://doi.org/10.1002/ijc.31198
Halvorsen RE, Elvestad M, Molin M, Aune D (2021) Fruit and vegetable consumption and the risk of type 2 diabetes: a systematic review and dose–response meta-analysis of prospective studies. BMJ Nutr Prev Health 4:519–531. https://doi.org/10.1136/bmjnph-2020-000218
Aune D, Keum N, Giovannucci E et al (2016) Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med 14:207. https://doi.org/10.1186/s12916-016-0730-3
Schwingshackl L, Schwedhelm C, Hoffmann G, Boeing H (2019) Potatoes and risk of chronic disease: a systematic review and dose–response meta-analysis. Eur J Nutr 58:2243–2251. https://doi.org/10.1007/s00394-018-1774-2
Abete I, Romaguera D, Vieira AR et al (2014) Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Brit J Nutr 112:762–775. https://doi.org/10.1017/S000711451400124X
Yang X, Li Y, Wang C et al (2020) Meat and fish intake and type 2 diabetes: dose–response meta-analysis of prospective cohort studies. Diabetes Metab 46:345–352. https://doi.org/10.1016/j.diabet.2020.03.004
Shi Y, Yu P-W, Zeng D-Z (2015) Dose–response meta-analysis of poultry intake and colorectal cancer incidence and mortality. Eur J Nutr 54:243–250. https://doi.org/10.1007/s00394-014-0705-0
Martínez-González MA, Dominguez LJ, Delgado-Rodríguez M (2014) Olive oil consumption and risk of CHD and/or stroke: a meta-analysis of case–control, cohort and intervention studies. Brit J Nutr 112:248–259. https://doi.org/10.1017/S0007114514000713
Ma Y, He FJ, Sun Q et al (2022) 24-Hour urinary sodium and Potassium Excretion and Cardiovascular Risk. New Engl J Med 386:252–263. https://doi.org/10.1056/NEJMoa2109794
Jayedi A, Shab-Bidar S (2020) Fish Consumption and the risk of Chronic Disease: an Umbrella Review of Meta-analyses of prospective cohort studies. Adv Nutr 11:1123–1133. https://doi.org/10.1093/advances/nmaa029
Tieri M, Ghelfi F, Vitale M et al (2020) Whole grain consumption and human health: an umbrella review of observational studies. Int J Food Sci Nutr 71:668–677. https://doi.org/10.1080/09637486.2020.1715354
Chen G-C, Tong X, Xu J-Y et al (2016) Whole-grain intake and total, cardiovascular, and cancer mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr 104:164–172. https://doi.org/10.3945/ajcn.115.122432
Jayedi A, Ghomashi F, Zargar MS, Shab-Bidar S (2019) Dietary sodium, sodium-to-potassium ratio, and risk of stroke: a systematic review and nonlinear dose-response meta-analysis. Clin Nutr 38:1092–1100. https://doi.org/10.1016/j.clnu.2018.05.017
Kolahdouz-Mohammadi R, Soltani S, Clayton ZS, Salehi-Abargouei A (2021) Sodium status is associated with type 2 diabetes mellitus: a systematic review and meta-analysis of observational studies. Eur J Nutr 60:3543–3565. https://doi.org/10.1007/s00394-021-02595-z
Huang L, Wang H, Wang Z et al (2019) Regional disparities in the association between cereal consumption and metabolic syndrome: results from the China Health and Nutrition Survey. Nutrients 11:764. https://doi.org/10.3390/nu11040764
Batis C, Sotres-Alvarez D, Gordon-Larsen P et al (2014) Longitudinal analysis of dietary patterns in Chinese adults from 1991 to 2009. Br J Nutr 111:1441–1451. https://doi.org/10.1017/S0007114513003917
Huang W Analysis of dietary pattern and regional dietary structure in Sichuan
McDougall JI (2021) Globalization of Sichuan hot pot in the new era. Asian Anthropol 20:77–92. https://doi.org/10.1080/1683478X.2020.1779970
Aburto NJ, Ziolkovska A, Hooper L et al (2013) Effect of lower sodium intake on health: systematic review and meta-analyses. BMJ 346:f1326–f1326. https://doi.org/10.1136/bmj.f1326
Alderman MH (2006) Evidence relating Dietary Sodium to Cardiovascular Disease. J AM COLL NUTR 25. https://doi.org/10.1080/07315724.2006.10719575:256S-261S
Li L, Ouyang Y, Wang H et al (2022) Disparities in fresh fruit and vegetable intake by sociodemographic and behavioural factors among adults in China. Public Health Nutr 25:649–656. https://doi.org/10.1017/S1368980020003274
Wang Z, Siega-Riz AM, Gordon-Larsen P et al (2018) Diet quality and its association with type 2 diabetes and major cardiometabolic risk factors among adults in China. Nutr Metab Cardiovasc Dis 28:987–1001. https://doi.org/10.1016/j.numecd.2018.06.012
Wyatt SB, Winters KP, Dubbert PM (2006) Overweight and obesity: prevalence, consequences, and causes of a growing Public Health Problem. AM J MED SCI 331:166–174. https://doi.org/10.1097/00000441-200604000-00002
Stubbs CO, Lee AJ (2004) The obesity epidemic: both energy intake and physical activity contribute. MED J AUSTRALIA 181:489–491. https://doi.org/10.5694/j.1326-5377.2004.tb06406.x
Huang TT-K, Roberts SB, Howarth NC, McCrory MA (2005) Effect of Screening out Implausible Energy Intake Reports on relationships between Diet and BMI. Obes Res 13:1205–1217. https://doi.org/10.1038/oby.2005.143
Schwingshackl L, Knüppel S, Michels N et al (2019) Intake of 12 food groups and disability-adjusted life years from coronary heart disease, stroke, type 2 diabetes, and colorectal cancer in 16 European countries. Eur J Epidemiol 34:765–775. https://doi.org/10.1007/s10654-019-00523-4
GBD 2019 Results Tool In: Institute for Health Metrics and Evaluation. https://vizhub.healthdata.org/gbd-results. Accessed 2 Jun 2022
Shen X, Vaidya A, Wu S, Gao X (2016) The diabetes epidemic in China: an Integrated Review of National Surveys. Endocr Pract 22:1119–1129. https://doi.org/10.4158/EP161199.RA
Gaubatz P (1999) China’s Urban Transformation: patterns and processes of morphological change in Beijing, Shanghai and Guangzhou. Urban Stud 36:1495–1521. https://doi.org/10.1080/0042098992890
Huang L, Wang Z, Wang H et al (2021) Nutrition transition and related health challenges over decades in China. Eur J Clin Nutr 75:247–252. https://doi.org/10.1038/s41430-020-0674-8
Acknowledgements
The authors appreciate Karin Borgonjen and João Paulo from the Global Nutrition chair group, Wageningen University, and Mo Li and Xinyue Ding from China Agricultural University, for their help with statistical suggestions and data cleaning. We are grateful to all the participants in our study and all the staff working for the China Health and Nutrition Survey 2011.
Funding
This work was supported by the Agricultural Green Development Ph. D Program (No. 201913043) of China Agricultural University and Wageningen University and Research.
Author information
Authors and Affiliations
Contributions
Zhiyao Chang: Methodology, Software, Formal analysis, Writing – Original Draft, Visualization. Elise F. Talsma: Writing – Review & Editing, Supervision. Hongyi Cai: Validation, Formal analysis. Shenggen Fan: Writing - Review & Editing. Yuanying Ni: Funding acquisition. Xin Wen: Supervision, Funding acquisition. Pieter van’t Veer: Conceptualization, Writing – Review & Editing. Sander Biesbroek: Methodology, Writing – Review & Editing, Supervision.
Corresponding author
Ethics declarations
Ethical approval
The ethics approval was obtained by the review board from the University of North Carolina at Chapel Hill, National Institute for Nutrition and Food Safety, China Center for Disease Control. The data were anonymized for the analysis, and the manuscript does not contain clinical studies or patient data.
Conflict of interests
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chang, Z., Biesbroek, S., Cai, H. et al. Heterogeneity in diet-related non-communicable disease risks in a Chinese population. Eur J Nutr (2024). https://doi.org/10.1007/s00394-024-03481-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00394-024-03481-0