Abstract
Purpose
Iron absorption in sickle cell anemia (SCA) remains unclear and studies in adults with SCA are scarce. The aim of this study was to evaluate the iron absorption SCA adults and its association with iron status and hepcidin concentration.
Methods
SCA patients (n = 13; SCAtotal) and control participants (n = 10) ingested an oral stable iron isotope (57Fe). Iron absorption was measured by inductively coupled plasma mass spectrometry (ICP-MS) 14 days after isotope administration. Patients with ≥ 1000 ng/mL serum ferritin were considered to present iron overload (IO) (SCAio+; n = 3) and others classified without IO (SCAio-; n = 10).
Results
Iron absorption in the control group ranged from 0.3 to 26.5% (median = 0.9%), while it varied from 0.3 to 5.4% in SCAio+ (median = 0.5%) and from 0.3 to 64.2% in the SCAio- (median = 6.9%). Hepcidin median values were 14.1 ng/mL (3.0–31.9 ng/mL) in SCAio-, 6.2 ng/mL (3.3–7.8 ng/mL) in SCAio + and 6.2 ng/mL (0.6–9.3 ng/mL) in control. Iron absorption was associated with ferritin level (r = − 0.641; p = 0.018) and liver iron concentration (LIC; r = − 0.786; p = 0.036) in the SCAtotal group.
Conclusion
Our data suggest that SCAio- individuals may be at risk of developing primary IO. Simultaneously, secondary IO may induce physiological adaptation, resulting in reduced iron absorption. Further studies evaluating intestinal iron absorption using larger sample sizes should be conducted to help establish a safe nutrition approach to be adopted and to ensure the security of food-fortifying public policies for these patients.
Trial registration
This trial was registered at www.ensaiosclinicos.gov.br (Identifier RBR-4b7v8pt).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sickle cell disease (SCD) is an inherited hemoglobinopathy caused by a genetic mutation that leads to the substitution of glutamic acid with valine in the beta-globin chain of hemoglobin. This mutation results in the production of hemoglobin S (Hb S) [1]. The term SCD refers to all the different Hb S genotypes, whereas sickle cell anemia (SCA) refers specifically to the homozygosity of the βS gene [2]. Approximately 275,000 children are born annually with SCD worldwide [3], and 30,000 individuals are estimated to have hemoglobinopathy in Brazil [4].
Blood transfusion is administered to treat acute conditions in SCA [5, 6] or prevent complications of the disease [6,7,8]. Despite their benefits, regular blood transfusions can lead to secondary iron overload (IO) [9, 10]. Chronically transfused patients have an excess of 0.3 to 0.5 mg/kg/day of iron [11, 12] and can store approximately 5 to 10 g of iron per year [13]. Iron becomes potentially toxic with the progression of IO, causing oxidative stress and cellular damage from free radical release [14].
Iron regulation occurs by a fine mechanism, which promotes the balance between its absorption and storage. Hepcidin – a hormone produced and secreted mainly by the liver – exerts its function by binding to ferroportin, a transmembrane cellular iron exporter expressed in various cell types [15]. The hepcidin-ferroportin complex controls the main inflows of iron into plasma: from duodenal enterocytes absorbing dietary iron, from macrophages of the reticuloendothelial system involved in the recycling of iron from senescent red blood cells (RBC), and from hepatocytes involved in iron storage. When hepcidin concentrations are high, the hepcidin-ferroportin complex is internalized and ferroportin undergoes degradation, resulting in a reduction in the absorption of intestinal iron and its bioavailability [16]. Therefore, hepcidin is induced to decrease the levels of iron circulating in blood plasma [17]. Other proteins, such as ferritin and transferrin, play essential roles in iron metabolism. Ferritin is responsible for iron storage, while transferrin facilitates iron transport. Therefore, the total iron-binding capacity (TIBC) indicates the overall amount of iron that can be bound by transferrin [18].
In SCA, the intense erythropoiesis and anemia can reduce hepcidin concentration [19]. On the other hand, the inflammatory characteristic of this disease and the iron overload due to blood transfusions can induce the transcription of hepcidin [20]. These disorders on iron metabolism can impact molecular and biochemical parameters, altering not only the expression of hepcidin, but also affecting the other iron biomarkers levels [21].
In recent studies [22, 23] that evaluated the concentration of hepcidin in SCA, the authors found that the levels of this hormone differed according to the presence or absence of IO. Hepcidin acts by reducing iron bioavailability [15]. Patients with SCA without IO had lower hepcidin concentrations compared with IO and control groups [22]. Conversely, patients with secondary IO and SCA had higher concentrations of hepcidin compared with other groups [22, 23]. These data support the hypothesis that patients with SCA may have higher iron absorption compared to individuals without the disease unless they present IO. The estimation of iron absorption is necessary to establish an adequate dietary guideline of iron intake for this population.
To our knowledge, this is the first study to evaluate iron absorption, using a stable isotope approach, in adults with SCA. In addition, it aimed to explore associations between iron absorption and markers of iron status and hepcidin concentration.
Methods
Participants
This is a pilot study and the sample size of 22 subjects (11 for each group) was calculated to test whether the mean iron absorption of the SCA group was greater than that of the control group by 22% (with an additional 10% for potential losses and refusals, making the total 26). The calculation considered a power of 80%, a significance level of 5%, and a standard deviation of 20, based on previous iron absorption data in SCA [24]. This calculation was performed using the PSS Health online version tool [25]. Male and female patients aged 19–50 years old, with SCA (Hb SS genotype), were recruited between October 2019 and March 2020 from the State Institute of Hematology Arthur Siqueira de Cavalcanti (Hemorio, Rio de Janeiro, Brazil). Individuals who met the inclusion criteria and agreed to participate in the study were included. The control group was composed of healthy volunteers without SCA with matched sex, age, and race/ethnicity. Patients with SCA who had been hospitalized for 30 days and/or had received blood transfusions 15 days before the first participation in this study were not included. Patients with SCA who had received blood transfusions during the iron absorption period were excluded. In the control group, participants with autoimmune diseases or who used medications for treatment of diabetes were not included. Women who were pregnant or experiencing menopause and/or participants who underwent bariatric surgery and/or used erythropoietin were not included. Participants were divided into two groups: the control group, consisting of individuals without SCA; and the sickle cell anemia group (SCAtotal). We additionally compared the results from two subgroups according to the presence (SCAio+) or absence (SCAio-) of IO. IO was diagnosed by using the cut-off point (serum ferritin ≥ 1,000 ng/mL) for SCD [26]. The study was conducted in accordance with the Declaration of Helsinki principles. The Ethical Committee of Hemorio (419/17; 2.788.659) and Pedro Ernesto University Hospital (2.695.418) approved the study protocol. Written informed consent was obtained from each participant.
Isotope enriched ferrous sulfate preparation
There are four naturally occurring iron stable isotopes at abundances of 5.8% (54Fe), 91.8% (56Fe), 2.1% (57Fe) and 0.3% (58Fe) [27]. The lowest abundant isotopes – 58Fe and 57Fe – are commonly used in nutritional research being usually provided as iron metal and converted to ferrous sulfate before administration [28]. Therefore, it was decided to use the iron isotope 57Fe, as it is more financially viable compared to 58Fe.
Elemental 57Fe was purchased from Cambridge Isotope Laboratories (Massachusetts, USA). At Laboratory of Analytical Development (LaDA) (Institute of Chemistry, Federal University of Rio de Janeiro, Brazil), 0.50079 g of 57Fe and 490 µL of sulfuric acid was mixed and this mixture was left in an inert atmosphere with gentle heating until the complete dissolution of metal. After complete dissolution, 50 µL of nitric acid was added to stabilize the solution and brought to a volume of 100 mL with water. The solution was parted into 1 mL aliquots and kept in an inert atmosphere until use with the participants.
Study procedure and isotope dosing
To define the dose of 57Fe to be administered, a literature review was carried out in studies with healthy adults using this stable isotope. It was observed that in different publications, 57Fe dosages varied between 5 mg and 7.5 mg [29,30,31]. In order to provide the smallest possible amount of stable iron isotope to participants with SCA, as there are no studies carried out with this approach in this hemoglobinopathy, we opted for the lowest dose (5 mg).
Participants were evaluated at day 1, with blood collection for baseline analyses and oral administration of 57Fe, and at day 14, with blood collection for measurement of the incorporation of 57Fe into erythrocytes [32,33,34]. Volunteers were instructed to fast for a period of 10 h. After blood collection at baseline, 5 mg of 57Fe, in the form of ferrous sulfate, was diluted in 100 mL of unsweetened and chemical food preservative-free orange juice (Do Bem®). The labeled juice was ingested under close supervision. Subsequently, each participant received a 40 g packet of starchy snack (cassava starch, eggs, vegetable oil and salt) without chemical food preservatives (Líder de Minas®). No other foods or liquids, except water, were allowed to be ingested for a period of at least 2 h following isotope administration.
Blood collection and isotope administration were performed at Hemorio (patients with SCA); and at Laboratory of Pathophysiology and Nutritional Biochemistry (Nutrition Institute, Rio de Janeiro State University, Brazil) (control group).
Iron isotope analysis in blood
First, 1 mL of blood collected in a heparin tube was transferred to Falcon tubes and centrifuged at 25oC for 10 min at 700 × g. Subsequently, the plasma and the white cells in the sample were removed and 2 mL of double sub-boiling distilled nitric acid (65% m/m) was added to the packed red blood cells to preserve the samples. At LaDA, packed red blood cells were transferred to microwave-sealed vessels and 1 mL of hydrogen peroxide (30%) and 1 mL of double sub-boiling distilled nitric acid were added to the RBC concentrate. The resulting mixture was placed in a microwave oven at 200 ºC for 10 min, and then cooled at 25oC. After this procedure, ultrapure water was added to this mixture up to 5 mL. Finally, the resulting extract was analyzed using inductively coupled plasma mass spectrometry (ICP-MS) (Thermo Fisher Scientific iCAP Qc, Bremen, Germany) and the 57Fe/56Fe ratio was determined.
Calculation of 57Fe incorporation into red blood cells and estimation of iron absorption
Fractional iron absorption was estimated based on the measured erythrocyte incorporation of the isotopic oral label (57Fe) and the estimated total amount of circulating iron in blood, was expressed as a percentage of the administered oral 57Fe dose [32].
The quantification of administered 57Fe incorporated into total red blood cells (57Feinc) 14 days after isotope administration was calculated as follows [32]:
R0Wnatural Fe 100.
where 57Feinc is expressed in mg, Rt is the 57Fe/56Fe ratio at a time after dosing (14 days), R0 is the 57Fe/56Fe ratio at baseline, circulating Fe is the total circulating iron (mg), Wnatural Fe is the average atomic weight of Fe (55.845 mg/mmol), NA57Fe is the natural abundance of 57Fe (2.1191%), and m57Fe is the atomic mass of 57Fe (56.935 mg/mmol).
The total circulating Fe (mg) was estimated as follows:
where Hb is blood hemoglobin concentration in g/L, BV is blood volume in liters (assumed to be 65 mL/kg body weight for adults) and 3.47 is the concentration of iron in hemoglobin (mg/g) [32].
Finally, percent iron absorption was calculated as follows:
where 57Feinc assumed incorporation of the absorbed isotope into red blood cells is 80% [32]. The limit of detection for iron absorption was 0.5%, and values < 0.5% were classified as undetectable. For statistical purposes, a value of 0.3% was assigned for values < 0.5%.
Hematological and biochemical analysis
Blood samples (days 1 and 14) were collected in EDTA-containing, heparin-containing, and clot activator gel tubes. Except for the samples collected in EDTA tubes, the others were centrifuged at 700 × g for 10 min at 25oC. Serum samples collected at day 1 were aliquoted into microtubes and stored at − 80ºC until analyses were performed. A complete blood count was performed using an automated counter Sysmex XP-300 (Sysmex, Kobe, Japan). Serum iron was analyzed using the modified Goodwin test (Bioclin, Belo Horizonte, Brazil) and total iron-binding capacity (TIBC) analysis was performed using a colorimetric method (Bioclin, Belo Horizonte, Brazil). Transferrin was measured using an immunoturbidimetry assay (Bioclin, Belo Horizonte, Brazil). The transferrin saturation index (TS) was calculated as follows: (serum iron/TIBC) × 100. Serum ferritin, bioactive hepcidin-25, and C-Reactive Protein (CRP) analyses were performed using a commercially available ELISA kit (Elabscience, Wuhan, China). In addition, Interleukin-6 (IL-6) (Merck Millipore, Darmstadt, Germany) was analyzed using ELISA.
Assessment of liver iron concentration (LIC)
Magnetic resonance imaging (MRI) procedures were conducted at the Radiology Department of Pedro Ernesto University Hospital. To evaluate liver IO, upper abdomen MRI was performed on a 1.5 Tesla Optima MR 360 (GE Healthcare, Chicago, USA), using an MRI T2* established method [35,36,37], without administration of contrast or sedation. LIC was quantified, as described, in micromoles of iron per gram of dry weight (µmol/g) of the liver with normal values being under 40 µmol/g [38].
Assessment of iron intake
For iron intake assessment, two 24-hour dietary recalls of each participant were evaluated, in which all foods and beverages consumed in the 24-hour period prior to participation in the study were reported. The software Avanutri (available at: https://www.avanutri.com.br/software_avanutri_online/) was used to estimate the daily iron intake. The median iron intake of the two 24-hour recalls was calculated, and it was analyzed whether the intake of this micronutrient reached the recommended for sex and age according to the Dietary Reference Intake recommendations [39].
Statistical analysis
The distribution of variables was analyzed for normality using the Shapiro–Wilk test. Measures of central tendency and dispersion were expressed as median and minimum and maximum values for continuous variables. For categorical variables, data were expressed as frequencies (n) and percentages (%). A Mann–Whitney U test was performed to assess the differences in continuous variables between SCAtotal and control groups. A Kruskal–Wallis test was performed on the three study groups (SCAio+, SCAio-, and control groups). Categorical variables were compared using the chi-square test (χ2) or Fisher’s exact test. Spearman´s correlation coefficient was used to measure the association between continuous variables. P values < 0.05 were considered significant. The statistical analysis was performed using the Statistical Package for Social Science software, version 22.0 (IBM SPSS, New York, USA) and graphics were performed using GraphPad Prism version 5.0 (GraphPad Software, San Diego, USA).
Results
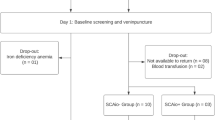
A total of 34 individuals were recruited in this study (Figs. 1), 11 for the control group, and 23 for the SCAtotal group. After blood collection and isotope administration, one participant in the control group was excluded owing to iron deficiency anemia. In the SCAtotal group, eight participants did not return for the next steps and two participants were excluded for undergoing blood transfusion. Thus, the final study population consisted of 23 participants, of whom 10 were included as a control group (60.0% females) while 13 were included in the SCAtotal group (53.8% females). The percentage of women, self-reported race, and median age of the participants did not differ between the groups, indicating homogeneity (Table 1). Among participants with SCA, six (46.2%) were using hydroxyurea.
Serum ferritin and IL-6 concentrations were higher in SCAtotal group than in control participants (p = 0.008 and p = 0.042, respectively – data not shown). Considering the participants with SCA divided into SCAio- and the SCAio + groups, as expected, both groups had lower red blood cell count and hematocrit and higher leukocytes when compared with the control group. Hepcidin did not present statistical differences among the groups, while TIBC and transferrin levels were lower in SCAio + than in the control group. Regarding inflammation markers, no significant differences were found in CRP and IL-6 levels among the three groups (Table 2).
Iron absorption was not different between control, SCAio- and SCAio + groups (Fig. 2). The SCAio + group had low iron absorption values (median: 0.5%), close to median values found in the control group (median: 0.9%). On the other hand, the SCAio- group presented an iron absorption median of 6.9%, but with a large scatter around this value.
Comparison of iron absorption (a) and hepcidin: ferritin ratio (b) among controls, SCAio− and SCAio+ groups. Differences among the three groups were tested using Kruskal−Wallis. Post hoc analysis was performed using Dunn’s Test for multiple pairwise comparisons. The asterisk indicates statistical significance (significant when p < 0.05). Iron absorption was not different among control, SCAio− and SCAio+ (p = 0.372). Abbreviations: SCAio−: sickle cell anemia without iron overload; SCAio+: sickle cell anemia with iron overload
The hepcidin: ferritin ratio of the SCAio + group is also shown in Fig. 2. The hepcidin: ferritin ratio was lower in the SCAio + group compared to SCAio- and presented a trend if compared to the control group.
Table 3 shows the iron absorption, LIC, and ferritin values for all participants. Of the 23 participants, only 7 performed MRI to assess the LIC, 5 SCAio-, and 2 SCAio + individuals. The two participants with IO had LIC above the reference range, with the diagnosis of liver IO (mild grade). The SCAio- participants presented LIC below the cut-off point.
Figure 3 shows the results of Spearman’s correlation analysis between serum ferritin, LIC, hepcidin, and iron absorption in the control individuals and patients with SCA. Iron absorption was inversely associated with serum ferritin level and LIC in the SCAtotal group. However, there was no significant correlation between ferritin level and iron absorption when adjusted for inflammation markers (IL-6: r = − 0.509; p = 0.198; PCR: r = − 0.378; p = 0.225; data not shown). Serum hepcidin level was not significantly correlated with iron absorption.
Spearman correlations of iron absorption with hepcidin (a), ferritin (b) and LIC (c) and ferritin with LIC (d). All correlations were performed using Spearman´s correlation coefficient (significant when p < 0.05). The asterisk indicates statistical significance. Spearman´s correlation (r) and p values for SCAio+ group were not presented owing to the small sample size. Abbreviations: SCAio−: sickle cell anemia without iron overload; SCAio+: sickle cell anemia with iron overload; SCAtotal, patients with and without iron overload
There were strong positive correlations between LIC and serum ferritin level in SCAtotal and in SCAio- patients. No significant correlations were found between serum hepcidin level and LIC. The lowest detectable absorption rate was 0.5% in a participant of the SCAio + group, whose serum ferritin level was 1339.0 ng/mL, while the highest iron absorption was found in the SCAio- group.
Discussion
The heightened production of RBC and the presence of hypoxia, both inherent in SCA, could potentially reduce hepcidin synthesis and contribute to elevated iron absorption [16, 19]. Conversely, the inflammatory nature of the disease, along with secondary iron overload, may hinder intestinal iron absorption [20]. These factors often coexist in individuals with SCA, underscoring the importance of comprehending iron metabolism and assessing iron absorption in these patients. Although critical for health monitoring purposes, iron absorption by the stable-isotope approach has never been evaluated in SCD. Furthermore, the very few studies that investigated iron absorption did not carry out the diagnosis of IO, in contrast to the methodology applied in the current study. Here, iron absorption was not significantly different between SCA and control groups, probably owing to the small number of individuals. Nevertheless, even though it was not significantly different compared to the control group, the iron absorption median value in the SCAio- group was almost 7 times higher than that of other groups and the highest iron absorption values (52.5% and 64.2%) were found in this group. Since SCA is a hemolytic disorder with intense erythropoietic activity, it was expected that individuals without IO would have greater iron absorption values. Despite the relatively low consumption of iron by individuals with SCA [40] in Brazil, the increasing iron fortification of foods, as well as iron supplementation, are worldwide conducted. These patients may therefore be at risk of progressively developing primary IO. Evidence from studies in experimental animal models shows that dietary iron restriction protects against both SCD vaso-occlusion and organ damage [41]. However, the effects of this restriction have not been tested in humans.
In our study, the median iron intake was 12.1 mg (11.8 mg in controls and 12.3 in the SCAtotal group), with 43.5% of all participants meeting Dietary Reference Intakes (DRI) recommendations. There was no correlation observed between iron absorption and the iron intake (data not shown). Regarding sources of dietary iron, this study corroborates with other study [40], which found that the primary sources of iron were culinary preparations based on beans and meat (beef, poultry, and pork), along with the consumption of bread and foods made from wheat and corn flour, which are mandatorily fortified with iron in Brazil. Therefore, even if the iron absorption in the SCAio- group could be considered higher than that observed in the other groups, the iron intake of the individuals analyzed here and in the study by Teixeira et al. (2023) [40] suggests that the low observed dietary iron intake does not pose a risk of primary overload.
The SCAio + group presented low iron absorption values: the lowest detectable absorption rate was 0.5%, while the highest absorption value was only 5.4%. These values are close to the median found in the control group (0.9%), suggesting that, in this group, the presence IO is not increasing intestinal iron absorption.
In 1970, an iron absorption test was performed by administering 59Fe to six adults with SCD (SS and SC genotypes were included), with iron absorption values ranging from 27.2 to 98%. Given the large scatter around the mean in all groups, no statistically significant differences were found between groups although the authors observed a tendency towards higher percentages of absorbed 59Fe in the SCA group (SS genotype) compared to SC genotype and controls [24]. This unique study [24] that evaluated iron absorption in SCD adults, used the fecal recovery method, which usually presents overestimated results [42] and did not determine whether the participants had IO.
Ferritin is widely used as an indicator of iron nutritional status, but its concentration is influenced by inflammation [43]. For this reason, the cut-off value for the classification of IO adopted in SCA is 1,000 ng/mL [26] and we also conducted a single-arm analysis involving a limited number of SCA individuals to assess LIC by MRI. In the present study, the percentage of iron absorption was negatively associated with ferritin level and LIC in SCA. Similar associations between serum ferritin level and iron absorption were found in earlier studies with normal subjects [44] and with idiopathic hemochromatosis patients [45]. Serum hepcidin levels could be higher in the SCAio + group and, consequently, these individuals could absorb less dietary iron. However, hepcidin levels did not differ between controls and the SCAtotal group and, when separated accordingly to the absence or presence of IO, the values remained without differences among all groups. Unlike ferritin, no relationship was found between serum hepcidin and iron absorption percentage in the study SCA groups. The correlation between hepcidin level and iron absorption has not been previously investigated in SCA. Previous studies determined whether serum ferritin and hepcidin levels can predict dietary iron absorption in healthy and iron-deficient individuals [46, 47]. Using a multiple linear regression model, a study in 2009 found that hepcidin level is only a modest predictor of dietary iron bioavailability in iron deficient humans [47]. The model that best-predicted iron absorption contained age, hemoglobin content, and serum ferritin level and explained 43% of the variance in iron absorption; when ferritin was excluded from the model, hepcidin level and age were predictors, but explained only 28% of the variance of iron absorption. Thus, serum ferritin seemed to be a better predictor of iron absorption than hepcidin.
A limitation of our study is the small sample size, particularly in the SCAio + group. However, the scarcity of larger samples is a limitation shared by other studies on sickle cell disease [24, 48]. Furthermore, iron absorption could not be detected in some participants. This may be attributed to the absence of high-resolution ICP-MS (HR-IC-MS) in our analysis, and the use of a 5 mg dose of 57Fe might have been insufficient for certain participants, resulting in reduced detection power. Nevertheless, the homogeneity of the participants’ characteristics is a strength of the study, as all were adults and exhibited homozygosity for the HbS gene. It is worth noting that we observed little variation in iron absorption values among people with iron overload.
Conclusions
High erythropoietic activity, which is characteristic of SCA, seems to contribute to increased intestinal iron absorption. SCAio- individuals may, therefore, be at risk of developing primary IO. Simultaneously, secondary IO may induce physiological adaptation, resulting in reduced iron absorption. Thus, the iron intake of SCAio- individuals must be carefully monitored by health professionals, as well as taken into account in the development of public policies that aim at iron fortification/supplementation. On the other hand, SCAio + individuals may not need to reduce their intake of iron-rich foods since they did not show higher iron absorption compared to the control group. Further studies evaluating intestinal iron absorption using larger sample sizes should be conducted to help establish a safe nutrition approach to be adopted and to ensure the security of food-fortifying public policies for these patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Conran N, Franco-Penteado CF, Costa FF (2009) Newer aspects of the pathophysiology of sickle cell disease vaso-occlusion. Hemoglobin 33(1):1–16. https://doi.org/10.1080/03630260802625709
Rees DC, Williams TN, Gladwin MT (2010) Sickle-cell disease. Lancet 376(9757):2018–2031. https://doi.org/10.1016/S0140-6736(10)61029-X
Modell B, Darlinson M (2008) Global epidemiology of haemoglobin disorders and derived service indicators. Bull World Health Organ 86(6):480–487. https://doi.org/10.2471/blt.06.036673
Kato GJ, Piel FB, Reid CD, Gaston MH, Ohene-Frempong K, Krishnamurti L et al (2018) Sickle cell disease. Nat Rev Dis Primers 4:18010. https://doi.org/10.1038/nrdp.2018.10
Harmatz P, Butensky E, Quirolo K, Williams R, Ferrell L, Moyer T et al (2000) Severity of iron overload in patients with sickle cell disease receiving chronic red blood cell transfusion therapy. Blood 96(1):76–79. https://doi.org/10.1182/blood.V96.1.76
Josephson CD, Su LL, Hillyer KL, Hillyer CD (2007) Transfusion in the patient with sickle cell disease: a critical review of the literature and transfusion guidelines. Transfus Med Rev 21(2):118–133. https://doi.org/10.1016/j.tmrv.2006.11.003
Adams RJ, McKie VC, Hsu L, Files B, Vichinsky E, Pegelow C et al (1998) Prevention of a first stroke by transfusions in children with sickle cell anemia and abnormal results on transcranial Doppler ultrasonography. N Engl J Med 339(1):5–11. https://doi.org/10.1056/NEJM199807023390102
Adams RJ, Brambilla D, Optimizing Primary Stroke Prevention in Sickle Cell Anemia (STOP 2) Trial Investigators (2005) Discontinuing prophylactic transfusions used to prevent stroke in sickle cell disease. N Engl J Med 353(26):2769–2778. https://doi.org/10.1056/NEJMoa050460
Inati A, Khoriaty E, Mussalam KM (2011) Iron sickle-cell disease: what have we learned over the years? Pediatr Blood Cancer 56(2):182–190. https://doi.org/10.1002/pbc.22721
Thuret I (2013) Post-transfusional iron overload in the haemoglobinopathies. C R Biol 336(3):164–172. https://doi.org/10.1016/j.crvi.2012.09.010
Cohen A, Masera G, Zoumbos N, Uysal Z, Boulet D, Watman N et al (2005) Effect of iron intake on control of body iron in patients with Thalassemia major treated with Deferasirox (Exjade®, ICL670). Blood 106(11):822. https://doi.org/10.1182/blood.V106.11.822.822
Porter J, Galanello R, Saglio G, Neufeld EJ, Vichinsky E, Capellini MD et al (2008) Relative response of patients with myelodysplastic syndromes and other transfusion-dependent anaemias to deferasirox (ICL670): a 1-yr prospective study. Eur J Haematol 80(2):168–176. https://doi.org/10.1111/j.1600-0609.2007.00985.x
Hoffbrand AV, Taher A, Cappellini MD (2012) How I treat transfusional iron overload. Blood 120(18):3657–3669. https://doi.org/10.1182/blood-2012-05-370098
Ward R (2010) An update on disordered iron metabolism and iron overload. Hematology 15(5):311–317. https://doi.org/10.1179/102453310X12647083621164
Ganz T, Nemeth E (2012) Hepcidin and iron homeostasis. Biochim Biophys Acta Mol Cell Res 1823(9):1434–1443. https://doi.org/10.1016/j.bbamcr.2012.01.014
Muckenthaler MU, Rivella S, Hentze MW, Galy B (2017) A Red Carpet for Iron Metabolism. Cell 168(3):344–361. https://doi.org/10.1016/j.cell.2016.12.034
Ganz T, Nemeth E (2006) Iron imports. IV. Hepcidin and regulation of body iron metabolism. Am J Physiol Gastrointest Liver Physiol 290(2):G199–203. https://doi.org/10.1152/ajpgi.00412.2005
Lynch S, Pfeiffer CM, Georgieff MK, Brittenham G, Fairweather-Tait S, Hurrell RF, McArdle HJ, Raiten DJ (2018) Biomarkers of Nutrition for Development (BOND)-Iron review. J Nutr 148(suppl1):1001S–1067S. https://doi.org/10.1093/jn/nxx036
Nemeth E (2008) Iron regulation and erythropoiesis. Curr Opin Hematol 15(3):169–175. https://doi.org/10.1097/MOH.0b013e3282f73335
Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, Ganz T (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101(7):2461–2463. https://doi.org/10.1182/blood-2002-10-3235
Camaschella C, Nai A, Silvestri L (2020) Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 105(2):260–272. https://doi.org/10.3324/haematol.2019.232124
Omena J, Cople-Rodrigues CS, Cardoso JDA, Soares AR, Fleury MK, Brito FSB et al (2018) Serum hepcidin concentration in individuals with sickle cell anemia: basis for the dietary recommendation of iron. Nutrients 10(4):498. https://doi.org/10.3390/nu10040498
Mangaonkar AA, Thawer F, Son J, Ajebo G, Xu H, Barrett NJ et al (2020) Regulation of iron homeostasis through the erythroferrone-hepcidin axis in sickle cell disease. Br J Haematol 189(6):1204–1209. https://doi.org/10.1111/bjh.16498
Ringelhann B, Konotey-Ahulu F, Dodu SR (1970) Studies on iron metabolism in sickle cell anaemia, sickle cell haemoglobin C disease, and haemoglobin C disease using a large volume liquid scintillation counter. J Clin Pathol 23(2):127–134. https://doi.org/10.1136/jcp.23.2.127
Borges R, Mancuso A, Camey S, Leotti V, Hirakata V, Azambuja G, Castro S (2021) Power and Sample Size for Health Researchers a tool for calculating sample size and statistical power designed for health researchers. Clin Biomed Res 40(4). https://doi.org/10.22491/2357-9730.109542
Porter J, Garbowski M (2013) Consequences and management of iron overload in sickle cell disease. Hematol Am Soc Hematol Educ Program 2013:447–456. https://doi.org/10.1182/asheducation-2013.1.447
Walczyk T, von Blanckenburg F (2002) Natural iron isotope variations in human blood. Science 295(5562):2065–2066. https://doi.org/10.1126/science.1069389
Abrams SA (1999) Using stable isotopes to assess mineral absorption and utilization by children. Am J Clin Nutr 70(6):955–964. https://doi.org/10.1093/ajcn/70.6.955
van den Heuvel EG, Muys T, Pellegrom H, Bruyntjes JP, van Dokkum W, Spanhaak S, Schaafsma G (1998) A new method to measure iron absorption from the enrichment of 57Fe and 58Fe in young erythroid cells. Clin Chem 44(3):649–654
Fidler MC, Davidsson L, Zeder C, Walczyk T, Hurrell RF (2003) Iron absorption from ferrous fumarate in adult women is influenced by ascorbic acid but not by Na2EDTA. Br J Nutr 90(6):1081–1085. https://doi.org/10.1079/bjn2003995
Junqueira-Franco MVM, Dutra de Oliveira JE, Nutti MR, Pereira HS, Carvalho JLV, Abrams SA et al (2018) Iron absorption from beans with different contents of iron, evaluated by stable isotopes. Clin Nutr ESPEN 25:121–125. https://doi.org/10.1016/j.clnesp.2018.03.120
International Atomic Energy Agency (IAEA) (2012) Assessment of iron bioavailability in humans using stable iron isotope techniques. Vienna: International Atomic Energy Agency 77 p. No.: 21
Kastenmayer P, Davidsson L, Galan P, Cherouvrier F, Hercberg S, Hurrell RF (1994) A double stable isotope technique for measuring iron absorption in infants. Br J Nutr 7(3):411–424. https://doi.org/10.1079/bjn19940148
Chen Z, Griffin IJ, Plumlee LM, Abrams SA (2005) High resolution inductively coupled plasma mass spectrometry allows rapid assessment of iron absorption in infants and children. J Nutr 135(7):1790–1795. https://doi.org/10.1093/jn/135.7.1790
Angelucci E, Brittenham GM, McLaren CE, Ripalti M, Baronciani D, Giardini C et al (2000) Hepatic iron concentration and total body iron stores in Thalassemia major. N Engl J Med 343(5):327–331. https://doi.org/10.1056/NEJM200008033430503
St Pierre TG, Clark PR, Chua-anusorn W, Fleming AJ, Jeffrey GP, Olynyk JK et al (2005) Noninvasive measurement and imaging of liver iron concentrations using proton magnetic resonance. Blood 105(2):855–861. https://doi.org/10.1182/blood-2004-01-0177
Hankins JS, McCarville MB, Loeffler RB, Smeltzer MP, Onciu M, Hoffer FA et al (2009) R2* magnetic resonance imaging of the liver in patients with iron overload. Blood 113(20):4853–4855. https://doi.org/10.1182/blood-2008-12-191643
Labranche R, Gilbert G, Cerny M, Vu KN, Soulières D, Olivié D et al (2018) Liver iron quantification with MR imaging: a primer for radiologists. Radiographics 38(2):392–412. https://doi.org/10.1148/rg.2018170079
Institute of Medicine (US) Panel on Micronutrients (2001) Dietary reference intakes for. In: Vitamin A, Vitamin K (eds) Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc. National Academies Press (US)
Teixeira TV, Da Silva ACF, Rodrigues CDSC, Brito FDSB, Canella DS, Citelli M (2023) Food Consumption of people with Sickle Cell Anemia in a Middle-Income Country. Nutrients15(6):1478. https://doi.org/10.3390/nu15061478
Li H, Kazmi JS, Lee S, Zhang D, Gao X, Maryanovich M et al (2023) Dietary iron restriction protects against vaso-occlusion and organ damage in murine sickle cell disease. Blood 141(2):194–199. https://doi.org/10.1182/blood.2022016218
Sarria B, Dainty JR (2010) Comparison of faecal monitoring and area under the curve techniques to determine iron absorption in humans using stable isotope labelling. J Trace Elem Med Biol 24(3):157–160. https://doi.org/10.1016/j.jtemb.2010.01.010
Sudarev VV, Dolotova SM, Bukhalovich SM, Bazhenov SV, Ryzhykau YL, Uversky VN et al (2023) Ferritin self-assembly, structure, function, and biotechnological applications. Int J Biol Macromol 224:319–343. https://doi.org/10.1016/j.ijbiomac.2022.10.126
Cook JD, Lipschitz DA, Miles LEM, Finch CA (1974) Serum ferritin as a measure of iron stores in normal subjects. Am J Clin Nutr 27(7):681–687. https://doi.org/10.1093/ajcn/27.7.681
Lynch SR, Skikne BS, Cook JD (1989) Food iron absorption in idiopathic hemochromatosis. Blood 74(6):2187–2193
Roe MA, Collings R, Dainty JR, Swinkels DW, Fairweather-Tait SJ (2009) Plasma hepcidin concentrations significantly predict interindividual variation in iron absorption in healthy men. Am J Clin Nutr 89(4):1088–1091. https://doi.org/10.3945/ajcn.2008.27297
Zimmermann MB, Troesch B, Biebinger R, Egli I, Zeder C, Hurrell RF (2009) Plasma hepcidin is a modest predictor of dietary iron bioavailability in humans, whereas oral iron loading, measured by stable-isotope appearance curves, increases plasma hepcidin. Am J Clin Nutr 90(5):1280–1287. https://doi.org/10.3945/ajcn.2009.28129
Erlandson ME, Walden B, Stern G, Hilgartner MW, Wehman J, Smith CH (1962) Studies on congenital hemolytic syndromes, IV. Gastrointestinal absorption of iron. Blood 19:359–378
Acknowledgements
The authors express their gratitude to the volunteers who participated in this study. They would also like to acknowledge the excellent technical assistance provided by Isis Rodrigues, Viviane F.Meneses, Clarice M. Carvalho, Elizabeth Pereira, Verônica Barbosa, and Valdilene L. Souza.
Funding
This study was supported by the Ministry of Health (process # 777022/2012); Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (process # 408401/2017-6); Coordenação de Aperfeiçoamento de Pessoal de Nível Superior – Brasil (CAPES) (Finance Code 001); Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (process # E-26-010.100930/2018 and E-26/200.963/2022).
Author information
Authors and Affiliations
Contributions
MC, JO, FFB, and CSCR designed the research; JO, MC, and VMV conducted the research; JO, VMV, MC, BFB and RES conducted the laboratory analysis; FFB and CMD helped to interpret the data and provided critical suggestions and comments; GFJ and ASR conducted the MRI procedures; JO, FFB and MC performed the statistical analysis, wrote the manuscript, and had primary responsibility for the final content. All authors read, contributed and approved the final manuscript. None of the authors declared any personal or financial conflict of interest.
Corresponding authors
Ethics declarations
Ethical approval
The study was conducted in accordance with the Declaration of Helsinki principles. The Ethical Committee of Hemorio (419/17; 2.788.659) and Pedro Ernesto University Hospital (2.695.418) approved the study protocol. Written informed consent was obtained from each participant.
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Omena¹, J., Bezerra¹, F.F., Voll¹, V.M. et al. Iron absorption in adults with sickle cell anemia: a stable-isotope approach. Eur J Nutr 63, 2163–2172 (2024). https://doi.org/10.1007/s00394-024-03417-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-024-03417-8