Abstract
Purpose
To clarify the association between dietary diversity and inflammatory status in Japanese workers.
Methods
Of 1,460 men and women aged 20–64 years in 2010 (baseline), those who were followed-up at least once between 2011 and 2018 were included in this study; 1,433 participants and 745 participants were included in the cross-sectional and longitudinal analyses, respectively. Dietary intake was assessed using a food frequency questionnaire at baseline, and the dietary diversity score was determined using the Quantitative Index for Dietary Diversity (QUANTIDD). High-sensitivity C-reactive protein (hs-CRP) was taken to indicate inflammatory status at the baseline and follow-up surveys. In the cross-sectional analysis using baseline data, a generalized linear model was used to calculate adjusted means and 95% confidence intervals (CIs) for hs-CRP according to the QUANTIDD score. In the longitudinal analysis, generalized estimating equations were used to calculate the adjusted mean (95% CI) for hs-CRP in follow-up according to the QUANTIDD score at baseline.
Results
In the cross-sectional analysis, the hs-CRP concentration in male participants was significantly lower in those who had a high QUANTIDD score (adjusted mean [95% CI]: 0.074 [0.009—0.140] mg/dL in the lower group vs. 0.038 [-0.029—0.105] mg/dL in the higher group, p-value = 0.034). In the longitudinal analysis, the hs-CRP concentration of male participants also tended to be lower in those with higher QUANTIDD scores (p-value = 0.103). In both the cross-sectional and longitudinal analyses in women, there was no significant difference between the lower and higher QUANTIDD score groups.
Conclusion
These findings suggest that, in male Japanese workers, higher dietary diversity might be important for maintaining a low inflammatory status.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An unbalanced diet, characterized as containing an insufficient intake of healthy food, has become a public health problem in Japan. According to the National Health and Nutrition Survey, the intake of vegetables, fruits, and beans has been decreasing, and their intake is lowest in the 20–30-year age group in the Japanese population [1]. The Japanese dietary guidelines recommend eating a variety of food groups [2]. Previous studies conducted in countries other than Japan found that adherence to dietary diversity was associated with a low risk of non-communicable diseases [3,4,5].
High-sensitivity C-reactive protein (hs-CRP) is a known marker of inflammation that is associated with increased risk of chronic diseases such as type 2 diabetes mellitus [6, 7], cardiovascular disease [8,9,10], and many types of cancer [11,12,13,14].
Among the potential risk factors for chronic diseases, which include genetic and lifestyle factors, diet is one of the most modifiable [15]. Dietary factors modulate inflammatory status by suppressing immune responses [16]. Some previous studies have found that intake of some food groups, including cereals [17], fish and/or fish oil [16], and nuts [18], has the effect of significantly reducing inflammatory marker levels. In addition, several previous studies have demonstrated an association between dietary pattern type and inflammatory markers such as hs-CRP [19,20,21,22,23,24,25].
Many countries have developed nutritional policies for preventing chronic disease [26]. One such policy is dietary diversity, which has been shown to be critical in a high-quality diet [2, 27]. Dietary diversity varies among countries due to differences in dietary composition, food culture, and habitual dietary intake, and may influence the development of chronic diseases. However, the associations between dietary diversity and inflammatory markers such as hs-CRP in the Japanese population have yet to be elucidated. Thus, we analyzed both the cross-sectional and longitudinal associations between dietary diversity and hs-CRP concentration in Japanese workers.
Methods and materials
Study design and participants
The present cross-sectional analysis was based on data from the third wave (from June 2010 to February 2011) of an occupation-based dynamic cohort established in Tokushima Prefecture in Japan, the details of which have been reported elsewhere [28]. In brief, the participants underwent a voluntary health examination on an annual basis. The first wave of the annual examination was conducted from June 2008 to February 2009 and involved 821 participants (550 men, 271 women; age range 20–60 years). The participants were followed up every year. New male and female workers aged ≥ 20 years were also recruited annually. After the fifth wave, participants’ dietary intakes and physical activity were followed up by assessments performed every 5 years.
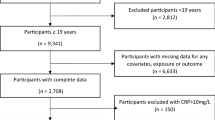
The present study participants were 1,460 men and women aged 20–64 years living in Tokushima Prefecture, Japan, in the third wave. Among these, those who had no or incomplete data for any inflammatory marker (n = 5), incomplete data for smoking habit, drinking habit, or physical activity, or a medical history of allergic diseases at baseline (n = 13) were excluded. Also excluded were participants who had a medical history of cancer, heart disease, or stroke at baseline (n = 9). The data of a final total of 1,433 participants (1,058 men and 375 women; age range, 20–64 years) were included in the cross-sectional analysis.
For the longitudinal analysis, we selected subjects who participated in the third-wave survey (baseline) and the fourth- to eleventh-wave surveys (follow-up surveys) at least once: the fourth study survey (June 2011 to February 2012, n = 1,349), fifth study survey (June 2012 to February 2013, n = 1,399), sixth study survey (June 2013 to February 2014, n = 1,432), seventh study survey (June 2014 to February 2015, n = 1,414), eighth study survey (June 2015 to February 2016, n = 1,431), ninth study survey (June 2016 to February 2017, n = 1,455), tenth study survey (June 2017 to February 2018, n = 1,394), and eleventh study survey (June 2018 to February 2019, n = 1,373). Although we also conducted surveys after 2019, we thought that the COVID-19 pandemic would affect the assessment of hs-CRP, so a longitudinal analysis using data from the third- to eleventh-wave surveys was conducted. Of the 1,433 participants included in the cross-sectional analysis, those who did not participate in the follow-up survey at least once were excluded (n = 230). Almost all of the reasons for missing data in the present study were random events, such as moving out of the prefecture, changing jobs to another company, retiring, or cancelling the measurement visit due to work schedule changes. A final total of 1,203 subjects (886 men and 317 women) were included in the longitudinal analysis (Fig. 1).
The study was conducted according to the ethical standards of the Declaration of Helsinki, and the protocol was approved by the institutional review board of Tokushima University Hospital (ethical approval No. 2868). Written, informed consent was obtained from all participants.
Dietary assessment
The participants were asked about meals taken in the past one month and used a food frequency questionnaire (FFQ) to determine the frequency of food intake (FFQg ver. 2.0; Kenpakusha Inc., Tokyo, Japan). Participants were asked about the frequency and amount of all foods consumed at each meal, and they were asked how many times and how much of each of the food groups was consumed per week. The amount of food consumed (per week) was calculated as the product of frequency of eating and the amount of each food at each meal; in the FFQg, such food intake is calculated for the following 17 food groups: cereals; potatoes and starches; deep yellow vegetables; other vegetables and mushrooms; algae; pulses; fish, mollusks and crustaceans; meat; eggs; milk and milk products; fruits; confectionaries; beverages; sugar and sweeteners; nuts and seeds; fats and oils; seasonings and spices [29]. The validity of the FFQg was verified by comparing food intake amounts for seven consecutive days using the weighting method [30].
The dietary diversity score was calculated using the Quantitative Index for Dietary Diversity (QUANTIDD) developed by Katanoda et al. [31]. The QUANTIDD score is calculated as the proportion of foods that contribute to total energy, for the amount of food and the number of food groups, following the formula:
,
where prop(j) is the proportion of each food group(s) j contributing to total energy or nutrient intake, and n is the number of food groups. The possible score ranges from 0 to 1. In the present study, we estimated scores based on the intake of 12 main food groups, excluding confectionaries, beverages, sugar and sweeteners, fats and oils, and seasonings and spices. Beverages were excluded as components of dietary diversity in this study. Beverages are liquid foods that have a different density than solid foods. In addition, beverages in the Japanese food composition table include many kinds of liquid foods that might affect chronic outcomes differently, such as alcohol, tea, coffee, and soft drinks (fruit-flavored and colored drinks), but not including fresh fruit juice or milk. The reason for the exclusion of beverages as a component of the dietary diversity score was to avoid difficulties in measuring beverages that contain a mix of alcohol, tea, and soft drinks. In addition, confectionaries, sugar and sweeteners, fats and oils, and seasonings and spices were also excluded in this study. The dietary guidelines do not recommend the consumption of sugary food (including added sugar), sodium, and saturated fat. These food groups, such as confectionaries, sugar and sweeteners, fats and oils, and seasonings and spices, include non-recommended nutrient contents (added sugar, sodium, and saturated fat). The reason for the exclusion of confectionaries, sugar and sweeteners, fats and oils, and seasonings and spices was to avoid difficulty in interpreting dietary diversity including non-recommended food groups. In addition, because the range of QUANTIDD scores is narrow, we divided the participants into two groups by QUANTIDD score using a sex-specific median (higher group: above median value, lower group: below median) to more clearly assess the effect of QUANTIDD score differences. In this study, the lower group was defined as the control group.
Dietary patterns as a correlation matrix for the 17 food groups were assessed using principal component factor analysis. The principal components were selected on the basis of eigenvalues > 1.3 and interpretability (Supplemental Table 1). Principal component scores were saved for each individual and were used as continuous variables. The first principal component was termed the “healthy pattern” and contained higher factor loadings for the ten food groups: cereals; potatoes and starches; deep yellow vegetables; other vegetables and mushrooms; algae; pulses; fish, mollusks and crustaceans; fruits; sugars and sweeteners; and seasonings and spices. This pattern explained 21.6% of the variance. The second principal component, termed the “Western pattern”, contained higher factor loadings for the four food groups of meat, eggs, beverages, and fats and oils, and it explained 9.5% of the variance. The third component, termed the “sweetener pattern”, contained higher factor loadings for the three food groups of milk and milk products, confectionaries, and nuts and seeds, and it explained 7.7% of the variance. The scores of the three principal components were saved for each participant and used in the analysis.
Blood inflammatory markers
For serum biochemical measurements in both the baseline survey and all follow-up surveys, venous blood was taken via an antecubital vein in the morning of the survey, and all hs-CRP values were measured. The participants were requested not to eat from after dinner to the time of examination the next day as much as possible and underwent the medical health check-up the following day at their worksite. Hs-CRP concentration was determined using a commercially available latex turbidimetric immunoassay (LT CRP-HS II, Wako Pure Chemical Ltd., Osaka, Japan).
Other measurements
The following measurements were also collected at baseline in this study. Height was measured to the nearest 0.1 cm, with participants standing without shoes or sandals. Body weight was measured to the nearest 100 g, with participants wearing light clothing. The body mass index (BMI) was calculated using the formula: BMI = weight (kg)/[height (m)]2. Daily values of physical activity (MET-hours/week) were calculated using the International Physical Activity Questionnaire [32]. The following information was obtained by a self-administered questionnaire: medical history of hypertension and allergy (binary: yes or no), education level (categorical: elementary, junior high and high school, tertiary college, career college and junior college, college, and graduate school or other), drinking habit (categorical: current, former, never), and smoking habit (categorical: current, former, never). Subjects completed the questionnaire before attending the physical examination day.
Statistical analysis
The basic characteristics of participants by the QUANTIDD score were dichotomized by sex. Continuous variables are presented as mean ± standard deviation (SD) or median (25 percentile, 75 percentile) values, and simple mean comparisons were calculated using Student’s t-test or the Mann–Whitney test. Categorical variables are expressed as numbers (percentages, %), and proportions were compared using the chi-squared test.
The confounding variables defined in this study included baseline age, energy intake [33], BMI [34], physical activity [35], smoking habit [36], drinking habit [37], education level [38], medical history of hypertension [8,9,10, 39], allergy [40], and dietary patterns in both cross-sectional and longitudinal analyses.
In the cross-sectional analysis, a generalized linear model was used to calculate the adjusted mean and 95% confidence interval (CI) for hs-CRP by the QUANTIDD score after controlling for the following variables; when the hs-CRP level was compared between two groups (lower group and higher group) of the QUANTIDD score, the lower group of the QUANTIDD score was used as the reference group. The confounding variables were (1) age-adjusted model, age (continuous: years); (2) Model 1, age-adjusted model + energy intake (continuous: kcal/day), BMI (continuous: kg/m2), physical activity (continuous: MET-hours/week), smoking habit (categorical: current, former, never), drinking habit (categorical: current, former, never), education level (categorical: elementary, junior high and high school, tertiary college, career college and junior college, college, and graduate school or other), and medical history of hypertension and allergy (binary: yes. no); (3) Model 2, Model 1 + healthy dietary pattern (continuous: score); (4) Model 3, Model 1 + Western dietary pattern (continuous: score); and (5) Model 4, Model 1 + sweetener dietary pattern (continuous: score).
In the longitudinal analysis, cumulative data during follow-up surveys were analyzed using generalized estimating equations (GEEs). GEEs take into account the dependency of repeated observations within participants. An additional advantage of GEEs is that missing values can also be used during the analysis. Thus, subjects who were lost to follow-up surveys after the baseline survey were also included in the analyses. GEE models were fitted by the GENLIN syntax in SPSS. This procedure corresponds to generalized linear models. In the present analyses, compound symmetry was specified for the correlation structure. GEE analyses were used to estimate the adjusted mean and 95% CI for hs-CRP in the follow-up surveys by the QUANTIDD score at baseline after controlling for the following variables; when the cumulative hs-CRP level was compared between two groups (lower group and higher group) of the QUANTIDD score, the lower group of the QUANTIDD score was used as the reference group. The confounding variables were: (1) age-adjusted model, age and follow-up time (continuous: years); (2) Model 1, Age-adjusted model + energy intake, BMI, physical activity, smoking habit, drinking habit, education level, and medical history of hypertension and allergy; (3) Model 2, Model 1 + healthy dietary pattern; (4) Model 3, Model 1 + Western dietary pattern; and (5) Model 4, Model 1 + sweetener dietary pattern.
As one of the sensitivity analyses (total number = 1,203, cumulative number = 8,003), the longitudinal association between the QUANTIDD score and hs-CRP was assessed using both baseline (2010) and follow-up dietary data (2011, 2012 and 2017). In addition, as another sensitivity analysis (total number = 1,203, cumulative number = 10,827), the longitudinal association between the QUANTIDD score and hs-CRP after imputing missing outcome data with estimated mean values from previously measured outcome data, when information about hs-CRP level during follow-up was lost, was confirmed.
All statistical analyses were performed separately for each sex using SPSS (IBM Corporation, Tokyo, Japan) version 28.0 for Windows. All statistical tests were based on two-sided probabilities. All p-values < 0.05 were considered significant.
Results
Participants’ characteristics
Table 1 lists the participants’ characteristics by QUANTIDD score and sex in the cross-sectional analysis. In men and women, the higher QUANTIDD score groups tended to have higher dietary diversity scores (p-value < 0.001). In both sexes, there was also a greater number of participants with a current drinking habit in the higher QUANTIDD score group than in the lower group. In women, participants who had higher dietary diversity tended to be older, and there was a greater number of participants with higher education in men according to QUANTIDD score.
Table 2 lists the cumulative number of participants and follow-up ratio in the longitudinal analysis. The cumulative number of participants was 5,990 for men and 2,013 for women. During the 8-year follow-up period, the mean cumulative follow-up years were 4.08 ± 2.29 years for men and 4.01 ± 2.33 years for women, and mean cumulative participation was 6.78 ± 2.46 times for men and 6.35 ± 2.56 times for women.
Table 3 shows the participant characteristics by QUANTIDD score and sex in the longitudinal analysis. The participants’ basic characteristics were similar in both the longitudinal and cross-sectional analyses.
Association of the dietary diversity score with hs-CRP
In the cross-sectional analysis (Table 4), the age-adjusted mean and 95% CI of hs-CRP in male participants were lower in the higher QUANTIDD score group (0.084 [0.061—0.107] mg/dL) than in the lower QUANTIDD score group (0.116 [0.093—0.139] mg/dL) (p-value = 0.057). Table 4 shows the multivariate-adjusted mean and 95% CI of hs-CRP according to the QUANTIDD score in the cross-sectional analysis (Model 1). In male participants, the adjusted mean and 95% CI of hs-CRP were significantly lower in the higher QUANTIDD score group (0.038 [-0.029—0.105] mg/dL) than in the lower QUANTIDD score group (0.074 [0.009—0.140] mg/dL) (p-value = 0.034). To determine whether the associations between dietary diversity and hs-CRP were confounded by dietary quality, such as dietary pattern, we further adjusted the scores for healthy dietary pattern, Western dietary pattern, and sweetener dietary pattern. After controlling for each dietary pattern score, a significant inverse association between the QUANTIDD score and hs-CRP was maintained in men. The adjusted mean and 95% CI for hs-CRP were 0.078 (0.011—0.145) mg/dL in the lower QUANTIDD score group and 0.035 (-0.033—0.102) mg/dL in the higher QUANTIDD score group (p-value = 0.030) after controlling for healthy dietary pattern score. After controlling for Western dietary pattern score, the adjusted mean and 95% CI for hs-CRP were 0.074 (0.009—0.140) mg/dL in the lower QUANTIDD score group and 0.037 (-0.030—0.104) mg/dL in the higher QUANTIDD score group (p-value = 0.028). After controlling for sweetener dietary pattern score, the adjusted mean and 95% CI for hs-CRP were 0.074 (0.009—0.140) mg/dL in the lower QUANTIDD score group and 0.038 (-0.028—0.105) mg/dL in the higher QUANTIDD score group (p-value = 0.034).
Table 5 shows the multivariate-adjusted mean and 95% CI of hs-CRP according to QUANTIDD score in the longitudinal analysis. In this analysis, men in the higher QUANTIDD score group tended to have a lower concentration of hs-CRP (adjusted mean and 95% CI: 0.099 [0.076—0.122] mg/dL in the lower QUANTIDD score group vs. 0.084 [0.062—0.105] mg/dL in the higher QUANTIDD score group, p-value = 0.103), though the difference was not significant (Model 1). In addition, after controlling for each dietary pattern score, a slight inverse association was maintained in men between QUANTIDD score and hs-CRP (p-value = 0.136 in Model 2, 0.098 in Model 3, and 0.103 in Model 4).
In women, there was no significant association between dietary diversity and hs-CRP in either the cross-sectional or longitudinal analysis.
To assess the longitudinal association between the QUANTIDD score and hs-CRP levels using both baseline and follow-up dietary data, a sensitivity analysis (total number = 1,203, cumulative number = 8,003) was performed and showed similar associations using both baseline and follow-up dietary data (Supplemental Table 4). In addition, a sensitivity analysis was performed using an imputed dataset (total number = 1,203, cumulative number = 10,827), and it showed similar associations using the imputed dataset (Supplemental Table 5). Thus, the main associations between the QUANTIDD score and hs-CRP levels were robust.
Discussion
The present study showed that, in the cross-sectional analysis, hs-CRP concentration in men was significantly lower in those who had a higher QUANTIDD score (Table 4). The longitudinal analysis also showed that hs-CRP concentration tended to be lower in men who had a higher QUANTIDD score, and was close to significance (Table 5). In women, however, no significant difference in hs-CRP concentration was observed between those with lower and higher QUANTIDD scores. In two sensitivity analyses, similar longitudinal associations were found in both sexes (Supplemental Tables 4 and 5).
An inverse association between dietary diversity and concentration of hs-CRP was shown in the present male Japanese workers (Tables 4 and 5, and Supplemental Tables 4 and 5). These results are consistent with those of several previous studies that investigated the association between dietary pattern and serum CRP concentration [20, 22, 23, 25]. Although some previous studies have reported that a Western dietary pattern (i.e., high intakes of red meat, refined grains, processed meat, and high-fat dairy products) is associated with increased concentration of serum CRP [19,20,21,22], an association between the healthy and/or prudent dietary pattern (e.g., high intakes of vegetables, fruit, and fish) and decreased concentration of serum CRP has also been reported [20, 22, 23]. In particular, two cross-sectional studies conducted in the Japanese population [23, 25] showed that a healthy or prudent dietary pattern decreased the serum CRP concentration. The present participants consumed higher amounts of vegetables, fruit, soy, and fish and lower amounts of cereals as the QUANTIDD score increased (Supplemental Tables 2 and 3). Therefore, the dietary diversity score used in the present study might reflect the dietary characteristics of each of the healthy, prudent, and non-Western dietary patterns. We further adjusted the healthy dietary pattern score to determine whether the associations between dietary diversity and hs-CRP were confounded by dietary quality, and we found that a significant inverse association between the QUANTIDD score and hs-CRP was maintained in men (Table 4). Furthermore, after controlling for Western dietary pattern score, a significant inverse association was clear in men (Table 4). The present results and those of previous studies suggest that a higher dietary diversity score, which indicates healthier dietary characteristics, might reduce the concentration of hs-CRP in Japanese adults. Accordingly, eating a variety of food groups might have direct protective effects against inflammatory markers in addition to the effects mediated by diet quality.
The possible reason for the present results is that a higher dietary diversity score indicates healthier nutritional habits. It has been shown that dietary diversity is related to food quality [27]. Furthermore, diets with greater variety are associated with increased intakes of essential micronutrients including vitamin C, calcium, and dietary fiber, while having low carbohydrate intake [41]. Indeed, the present study participants who had high QUANTIDD scores consumed higher amounts of several healthy food groups: deep yellow vegetables; other vegetables and mushrooms; algae; pulses; fish, mollusks and crustaceans; milk and milk products; and fruits, nuts, and seeds. Previous studies have reported that the beneficial combinations of antioxidant vitamins and fiber contained in some food groups, including deep yellow vegetables, other vegetables and mushrooms, algae, pulses and fruit, may partly explain this inverse association between healthy dietary pattern and CRP [42,43,44]. Thus, consuming various types of foods that suggest higher dietary quality might be associated with decreased hs-CRP, through protective actions such as antioxidant activity by nutrients such as antioxidants.
In the present study, the association between dietary diversity and concentration of hs-CRP differed between men and women (Tables 4 and 5, and Supplemental Tables 4 and 5). This might mean that the effect of dietary diversity on inflammatory markers such as hs-CRP is stronger in men than in women. It is known that factors such as age [45], body mass [34], and sex hormones [46, 47] are related to inflammatory status. In the present study, female participants had healthier demographic factors (e.g. higher dietary diversity, younger age, higher proportion of normal body type) than those in men. These characteristics might reduce the variation of hs-CRP more in women than in men: the median concentration of hs-CRP (interquartile range) at baseline was 0.040 (0.020–0.083) mg/dL in men and 0.020 (0.010–0.050) mg/dL in women (p-value < 0.001) (data not shown). Therefore, a dietary diversity effect might have been seen in men with more variable hs-CRP. Another possible reason for the sex difference is a difference in variation of the QUANTIDD score. The present female participants had higher dietary diversity than did the male participants: the median QUANTIDD score (interquartile range) at baseline was 0.82 (0.76–0.87) mg/dL in men and 0.86 (0.81–0.90) mg/dL in women (p-value < 0.001) (data not shown).
The present study has some limitations. First, the present study included both cross-sectional and longitudinal analyses. Although the follow-up time was probably not too short (the median cumulative follow-up was 4 years) to assess changes in outcome, the follow-up rate decreased to about half in subsequent follow-up years (Table 2). To minimize this effect, we used GEE models in which missing values can also be used during analysis. Almost all previous studies that have assessed the association between dietary pattern and CRP level were cross-sectional in design [19,20,21,22,23,24,25]. Therefore, longitudinal study findings are important for this research area. In addition, there were no significant differences in blood pressure, blood glucose, or BMI between the two QUANTIDD score groups for about 8 years in the present population (data not shown), whereas in contrast, the trend for hs-CRP approached significance. Since high concentrations of hs-CRP, indicating a chronic inflammatory state, occur in the pre-stages of various lifestyle-related diseases (e.g. hypertension, diabetes mellitus, and obesity), inflammatory markers such as hs-CRP might be useful predictors of lifestyle-related disease. Second, since the study participants lived in a limited area and included only Japanese workers, the present findings might not be generalizable to other populations. We acknowledge that more detailed information regarding occupation type is important, but these data could not be obtained. Third, the sample size of this study, especially in women, was small. Fourth, food consumption was assessed only at baseline. When the secular change of the QUANTIDD score during the 8 follow-up years was confirmed, there were no significant change in women, but there was a U-shaped secular change in men during follow-up (Supplemental Table 6). Therefore, the longitudinal association between the QUANTIDD score and hs-CRP was assessed using both baseline and follow-up dietary data as a sensitivity analysis, and a similar longitudinal association was confirmed (Supplemental Table 4). Thus, the main associations between the QUANTIDD score and hs-CRP appear to be robust. Finally, since the distribution of hs-CRP concentration was skewed, and the range of concentration of hs-CRP was narrow, in the present study, we compared hs-CRP concentrations between two QUANTIDD score groups. Therefore, we could not assess the trend between dietary diversity and hs-CRP concentration.
In conclusion, the results of this study suggest that a higher dietary diversity score might reduce the hs-CRP concentrations in Japanese male workers. Further larger longitudinal studies are needed to confirm this finding.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Ministry of Health, Labour and Welfare (2017) The National Health and Nutrition Survey in Japan 2017. https://www.mhlw.go.jp/content/001066645.pdf. Accessed 18th September 2023 (In Japanese)
Ministry of Agriculture, Forestry and Fisheries (2016) The Dietary Guidelines for Japanese. https://www.maff.go.jp/j/syokuiku/attach/pdf/shishinn-9.pdf. Accessed 18th September 2023 (In Japanese)
Wang SS, Lay S, Yu HN, Shen SR (2016) Dietary Guidelines for Chinese Residents (2016): comments and comparisons. J Zhejiang Univ Sci B 17:649–656. https://doi.org/10.1631/jzus.B1600341
Kant AK, Block G, Schatzkin A, Ziegler RG, Nestle M (1991) Dietary diversity in the US population, NHANES II, 1976–1980. J Am Diet Assoc 91:1526–1531. https://doi.org/10.1016/S0002-8223(21)01428-0
Lee BL (2011) Dietary guidelines in Singapore. Asia Pac J Clin Nutr 20:472–476. https://apjcn.nhri.org.tw/server/APJCN/20/3/472.pdf
Hu FB, Meigs JB, Li TY, Rifai N, Manson JE (2004) Inflammatory markers and risk of developing type 2 diabetes in women. Diabetes 53:693–700. https://doi.org/10.2337/diabetes.53.3.693
Fischer CP, Perstrup LB, Berntsen A, Eskildsen P, Pedersen BK (2005) Elevated plasma interleukin-18 is a marker of insulin-resistance in type 2 diabetic and non-diabetic humans. Clin Immunol 117:152–160. https://doi.org/10.1016/j.clim.2005.07.008
Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GDO, Pepys MB, Gudnason V (2004) C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med 350:1387–1397. https://doi.org/10.1056/NEJMoa032804
Ridker PM, Silvertown JD (2008) Inflammation, C-reactive protein, and atherothrombosis. J Periodontol 79:1544–1551. https://doi.org/10.3389/fimmu.2018.00430
Verma S, Devaraj S, Jialal I (2006) Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation 113:2135–2150.
Coussens LM, Werb Z (2002) Inflammation and cancer. Nature 420:860–867. https://doi.org/10.1038/nature01322
Aleksandrova K, Jenab M, Boeing H, Jansen E, Bueno-de-Mesquita HB, Rinaldi S, Riboli E, Overvad K, Dahm CC, Olsen A, Tjønneland A, Boutron-Ruault M, Clavel-Chapelon F, Morois S, Palli D, Krogh V, Tumino R, Vineis P, Panico S, Kaaks R, Rohrmann S, Trichopoulou A, Lagiou P, Trichopoulos D, van Duijnhoven FJB, Leufkens AM, Peeters PH, Rodríguez L, Bonet C, Sánchez M, Dorronsoro M, Navarro C, Barricarte A, Palmqvist R, Hallmans G, Khaw K-T, Wareham N, Allen NE, Spencer E, Romaguera D, Norat T, Pischon T (2010) Circulating C-reactive protein concentrations and risks of colon and rectal cancer: a nested case-control study within the European prospective investigation into Cancer and Nutrition. Am J Epidemiol 172:407–418. https://doi.org/10.1093/aje/kwq135
Erlinger TP, Platz EA, Rifai N, Helzlsouer KJ (2004) C-reactive protein and the risk of incident colorectal cancer. JAMA 291:585–590. https://doi.org/10.1001/jama.291.5.585
Balkwill F, Mantovani A (2001) Inflammation and cancer: back to Virchow? Lancet 357:539–545. https://doi.org/10.1016/S0140-6736(00)04046-0
Ahmed SM, Clasen ME, Donnelly JE (1998) Management of dyslipidemia in adults. Am Fam Physician 57:2192–2204.
Giugliano D, Ceriello A, Esposito K (2006) The effects of diet on inflammation: emphasis on the metabolic syndrome. J Am Coll Cardiol 48:677–685. https://doi.org/10.1016/j.jacc.2006.03.052
Lefevre M, Jonnalagadda S (2012) Effect of whole grains on markers of subclinical inflammation. Nutr Rev 70:387–396. https://doi.org/10.1111/j.1753-4887.2012.00487.x
Barbaresko J, Koch M, Schulze MB, Nöthlings U (2013) Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev 71:511–527. https://doi.org/10.1111/nure.12035
Fung TT, Rimm EB, Spiegelman D, Rifai N, Tofler GH, Willett WC, Hu FB (2001) Association between dietary patterns and plasma biomarkers of obesity and cardiovascular disease risk. Am J Clin Nutr 73:61–67. https://doi.org/10.1093/ajcn/73.1.61
Lopez-Garcia E, Schulze MB, Fung TT, Meigs JB, Rifai N, Manson JE, Hu FB (2004) Major dietary patterns are related to plasma concentrations of markers of inflammation and endothelial dysfunction. Am J Clin Nutr 80:1029–1035. https://doi.org/10.1093/ajcn/80.4.1029
Nettleton JA, Steffen LM, Mayer-Davis EJ (2006) Dietary patterns are associated with biochemical markers of inflammation and endothelial activation in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Clin Nutr 83:1369–1379. https://doi.org/10.1093/ajcn/83.6.1369
Esmaillzadeh A, Kimiagar M, Mehrabi Y, Azadbakht L, Hu FB, Willett WC (2007) Dietary patterns and markers of systemic inflammation among Iranian women. J Nutr 137:992–998. https://doi.org/10.1093/jn/137.4.992
Nanri A, Yoshida D, Yamaji T, Mizoue T, Takayanagi R, Kono S (2008) Dietary patterns and C-reactive protein in Japanese men and women. Am J Clin Nutr 87:1488–1496. https://doi.org/10.1093/ajcn/87.5.1488
Eilat-Adar S, Mete M, Nobmann ED, Xu J, Fabsitz RR, Ebbesson SOE, Howard BV (2009) Dietary patterns are linked to cardiovascular risk factors but not to inflammatory markers in Alaska Eskimos. J Nutr 139:2322–2328. https://doi.org/10.3945/jn.109.110387
Nanri H, Nakamura K, Hara M, Higaki Y, Imaizumi T, Taguchi N, Sakamoto T, Horita M, Shinchi K, Tanaka K (2011) Association between dietary pattern and serum C-reactive protein in Japanese men and women. J Epidemiol 21:122–131. https://doi.org/10.2188/jea.je20100110
Lachat C, Van Camp J, De Henauw S, Matthys C, Larondelle Y, Winter A, Kolsteren P (2005) A concise overview of national nutrition action plans in the European Union Member States. Public Health Nutr 8:266–274. https://doi.org/10.1079/PHN2004691
Nakamoto M, Yun Y, Omine M, Mori S, Shuto E, Nakamoto A, Hata A, Aki N, Shikama Y, Bando Y, Ichihara T, Minagawa T, Tamura A, Kuwamura Y, Funaki M, Saka T (2020) Dietary diversity and characteristics of lifestyle and awareness of health in Japanese workers: a cross-sectional study. J Med Invest 67:255–264. https://doi.org/10.2152/jmi.67.255
Nakamoto M, Omine M, Yun Y, Shuto E, Nakamoto A, Hata A, Aki N, Shikama Y, Bando Y, Ichihara T, Minamigawa T, Tamura A, Kuwamura Y, Funaki M, Sakai T (2019) Associations of dietary diversity with allergic diseases in Japanese workers: a cross-sectional study. Asia Pac J Clin Nutr 28:857–869. https://doi.org/10.6133/apjcn.201912_28(4).0023
Ministry of Health, Labour and Welfare (2015) Standard Tables of Food Composition in Japan (Seventh Revised Edition) https://www.mext.go.jp/component/english/__icsFiles/afieldfile/2017/10/02/1385123_Standard-Table_Chap.1_Prov_1.pdf. Accessed 20th March 2019
Takahashi K, Yoshimura Y, Kaimoto T, Kunii D, Komatsu T, Yamamoto S (2001) Validation of a food frequency questionnaire based on food groups for estimating individual nutrient intake. J Nutr (Tokyo) 59:221–232 (In Japanese). https://doi.org/10.5264/eiyogakuzashi.59.221
Katanoda K, Kim H-S, Matsumura Y (2006) New Quantitative Index for Dietary Diversity (QUANTIDD) and its annual changes in the Japanese. Nutrition 22:283–287. https://doi.org/10.1016/j.nut.2005.06.014
Craig CL, Marshall AL, Sjöström M, Bauman AE, Booth ML, Ainsworth BE, Pratt M, Ekelund U, Yngve A, Sallis JF, Oja P (2003) International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc 35:1381–1395. https://doi.org/10.1249/01.mss.0000078924.61453.fb
Meng Y, Kautz A (2022) An evidence review of the association of immune and inflammatory markers with obesity-related eating behaviors. Front Immunol 15(13):902114. https://doi.org/10.3389/fimmu.2022.902114
Choi J, Joseph L, Pilote L (2013) Obesity and C-reactive protein in various populations: a systematic review and meta-analysis. Obes Rev 14:232–244. https://doi.org/10.1111/obr.12003
Silva FM, Duarte-Mendes P, Teixeira AM, Soares CM, Ferreira JP (2024) The effects of combined exercise training on glucose metabolism and inflammatory markers in sedentary adults: a systematic review and meta-analysis. Sci Rep. 14:1936. https://doi.org/10.1038/s41598-024-51832-y
Xu Z, Wang W, Liu Q, Li Z, Lei L, Ren L, Deng F, Guo X, Wu S (2022) Association between gaseous air pollutants and biomarkers of systemic inflammation: a systematic review and meta-analysis. Environ Pollut 292(Pt A) 118336. https://doi.org/10.1016/j.envpol.2021.118336
Imhof A, Froehlich M, Brenner H, Boeing H, Pepys MB, Koenig W (2001) Effect of alcohol consumption on systemic markers of inflammation. Lancet 357:763–767. https://doi.org/10.1016/S0140-6736(00)04170-2
Liu RS, Aiello AE, Mensah FK, Gasser CE, Rueb K, Cordell B, Juonala M, Wake M, Burgner DP (2017) Socioeconomic status in childhood and C reactive protein in adulthood: a systematic review and meta-analysis. J Epidemiol Community Health 71:817–826. https://doi.org/10.1136/jech-2016-208646
Chrysohoou C, Pitsavos C, Panagiotakos DB, Skoumas J, Stefanadis C (2004) Association between prehypertension status and inflammatory markers related to atherosclerotic disease: the ATTICA Study. Am J Hypertens 17:568–573. https://doi.org/10.1016/j.amjhyper.2004.03.675
Shimoda T, Obase Y, Kishikawa R, Iwanaga T (2015) Serum high-sensitivity C-reactive protein can be an airway inflammation predictor in bronchial asthma. Allergy Asthma Proc 36:e23–e28. https://doi.org/10.2500/aap.2015.36.3816
Bernstein MA, Tucker KL, Ryan ND, O’Neill EF, Clements KM, Nelson ME, Evans WJ, Fiatarone Singh MA (2002) Higher dietary variety is associated with better nutritional status in frail elderly people. J Am Diet Assoc 102:1096–1104. https://doi.org/10.1016/S0002-8223(02)90246-4
Ma Y, Griffith JA, Chasan-Taber L, Olendzki BC, Jackson E, Stanek EJ 3rd, Li W, Pagoto SL, Hafner AR, Ockene IS (2006) Association between dietary fiber and serum C-reactive protein. Am J Clin Nutr 83:760–766. https://doi.org/10.1093/ajcn/83.4.760
Watzl B, Kulling SE, Möseneder J, Barth SW, Bub A (2005) A 4-wk intervention with high intake of carotenoid-rich vegetables and fruit reduces plasma C-reactive protein in healthy, nonsmoking men. Am J Clin Nutr 82:1052–1058. https://doi.org/10.1093/ajcn/82.5.1052
Wannamethee SG, Lowe GDO, Rumley A, Bruckdorfer KR, Whincup PH (2006) Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr 83:567–574, quiz 726–727. https://doi.org/10.1093/ajcn.83.3.567
Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL (2005) The origins of age-related proinflammatory state. Blood 105:2294–2299. https://doi.org/10.1182/blood-2004-07-2599
Kupelian V, Chiu GR, Araujo AB, Williams RE, Clark RV, McKinlay JB (2010) Association of sex hormones and C-reactive protein levels in men. Clin Endocrinol (Oxf) 72:527–533. https://doi.org/10.1111/j.1365-2265.2009.03713.x
Störk S, Bots ML, Grobbee DE, van der Schouw YT (2008) Endogenous sex hormones and C-reactive protein in healthy postmenopausal women. J Intern Med 264:245–253. https://doi.org/10.1111/j.1365-2796.2008.01946.x
Acknowledgements
The authors wish to thank the study participants and our colleagues for their assistance with the survey.
Funding
This work was supported in part by The Knowledge Cluster Initiative (Tokushima Health and Medicine Cluster) (http://www.mext.go.jp/a_menu/kagaku/chiiki/cluster/index.htm) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MF), by Grants-in-Aid for research from Tokushima Prefecture (MF) (http://www.pref.tokushima.jp/), by Grants-in-Aid for Young Scientists (B) (25860439) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (AH) (http://www.jsps.go.jp/english/index.html), by Grants-in JSPS KAKENHI (Grant Number the Grant-in-Aid for Scientific Research (C) 20K05925) from the Ministry of Education, Culture, Sports, Science and Technology of Japan (MN), and by 3rd -time Grants from the Tanuma Green House Foundation (2021) (MN). The funders/sponsors had no role in the design, conduct, or reporting of the study or in the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
All authors (M. N., K. T., B. T. T., A. T., K. Y., A. N., A. H., N. A., Y. S., Y. B., T. I., T. M., A. T., Y. K., M. F., and T. S.) conceptualized the study and collected the data. Measurements and data analysis were performed by M. N., K. T., B. T. T., A. T., K. Y., and T. S. A. N. provided nutritional advice regarding the interpretation of the data. M. N. drafted the manuscript with the assistance of S. T. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The study was conducted according to the ethical standards of the Declaration of Helsinki, and the protocol was approved by the institutional review board of Tokushima University Hospital (ethical approval No. 2868). Written consent was obtained from all participants.
Conflict of interest
All authors state that they have no conflicts of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nakamoto, M., Torami, K., Bui, T.T. et al. Associations between dietary diversity and high sensitive C-reactive protein among Japanese workers: findings of a cross-sectional and longitudinal study. Eur J Nutr 63, 1915–1927 (2024). https://doi.org/10.1007/s00394-024-03389-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-024-03389-9