Abstract
Background
We aim to report the latest pooled analyses to evaluate the additive efficacy and safety of probiotics in the treatment of ulcerative colitis (UC).
Methods
We systematically searched the relevant literature investigating the efficacy and/or safety of probiotics in patients with UC from PubMed, Embase and Web of Science up to January 2023. Two researchers independently screened the literature, extracted data, and evaluated the quality of the included studies according to the inclusion and exclusion criteria. Any discrepancies throughout these processes were solved by consensus. All statistical analyses were performed by Review Manager version 5.4 and Stata version 15.0.
Results
A total of 13 articles were included in the pooled analyses, and the studies were all randomized controlled trials with a total of 930 patients. There were no significant differences between the probiotics and placebo groups concerning demographic and baseline characteristics. For patients with active UC, the probiotic group boosted the remission rate by 87% compared to the placebo group, but failed to reach a statistical difference (OR: 1.87; 95% CI 0.98, 3.57; P = 0.06, I2 = 67%); furthermore, there were no statistical differences in maintenance of clinical remission, clinical response, change in UCDAI scores, or mucosal healing outcomes in the probiotic group compared to the placebo group. For patients in clinical remission, the clinical relapse rates were significantly lower in the probiotic group than in the placebo group (OR: 0.34; 95% CI 0.14, 0.79; P = 0.01). Moreover, this study did not observe a significant difference between the two groups for general adverse events rate (OR: 1.98; 95% CI 0.69, 5.68; P = 0.20).
Conclusion
Probiotic-assisted therapy may be effective in inhibiting UC recurrence in patients in clinical remission without increasing the risk of treatment-related adverse events; furthermore, probiotics may increase the rate of clinical remission in patients with active UC. However, caution is needed when interpreting the clinical efficacy of probiotics in improving the clinical outcome of patients with active UC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC) is a common disease of the digestive system. As an idiopathic, chronic disease characterized by diffuse mucosal inflammation and mainly affecting the rectum and sigmoid colon, its typical clinical symptoms are chronic or subacute bloody diarrhea and abdominal pain [1]. UC is a subtype of inflammatory bowel disease (IBD). The extensively accepted hypothesis for the pathogenesis of IBD is complicated interactions between genetic and environmental factors and the host immune system, resulting in excessive immune responses and chronic intestinal mucosal inflammation. Progress in next-generation sequencing technology has identified changes in the composition and function of the gut microbiome in IBD patients [2, 3]. For instance, microbiome samples from IBD patients are detected with less overall diversity and abundance of anti-inflammatory groups than those from healthy subjects. Intestinal flora is strongly associated with IBD and is significantly altered and less diverse in patients with IBD compared to healthy individuals. Analyses of IBD cohort data from France, the United States, Israel, and Germany suggest that patients with CD and UC have a lower diversity of gut flora and significantly different gut flora than healthy individuals. In addition, there were differences in the gut flora of patients in the outbreak and remission phases [4].
Treatment with certain probiotic strains, such as VSL#3 and E. coli Nissle 1917, is an effective form of therapy that can induce remission in patients with mild to moderate UC. To date, the effectiveness of probiotics, prebiotics and synbiotics in inducing or maintaining remission in patients with CD has not been proven. Fecal microbiota transplantation (FMT) has also been reported to be potentially beneficial in the course of IBD, especially UC [5]. Some studies have shown that probiotics can alter the mucosal immune system, improve intestinal barrier function, increase bacterial diversity, and inhibit the growth of potential pathogenic bacteria. However, these results lack consistency in patients, and the outcomes of studies on the effectiveness of probiotics for ulcerative colitis are different [6, 7]. Therefore, we conducted a meta-analysis of those studies on the use of probiotics in ulcerative colitis to provide more persuasive evidence for evidence-based medicine and to explore more effective and safe therapies for patients with UC.
Materials and methods
Literature search
The present evidence-based analysis was carried out following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA 2020 statement) [8] and was registered in the PROSPERO (CRD42023393294). The PRISMA 2020 checklist is shown in Supplementary Table 1. We performed a systematic literature search using PubMed, Embase, and Web of Science up to January 2023 for studies investigating the efficacy and/or safety of probiotics in patients with ulcerative colitis and published in English. We searched the databases using the following terms: “probiotic”, “probiotics”, “ulcerative colitis”, “colitis, ulcerative”, “colitis”, “ulcerative”, and “colitis gravis”. The specific search strategies are presented in Table 1. In addition, the reference lists of all eligible studies were manually reviewed. Two investigators completed this process independently. Any divergences in the literature search were solved by consensus.
Identification of eligible studies
Studies were eligible if they met the following criteria: (1) the study design was randomized controlled trial; (2) studies compared probiotics with placebo in terms of efficacy and/or safety; (3) patients could be active, ranging from mild to severe extent or in clinical remission; (4) patients received or did not receive conventional UC therapy; (5) studies evaluated at least one of the following outcomes: clinical remission, clinical remission maintenance, clinical response, clinical relapse, change in various scoring scales of UC, such as Mayo scores and UCDAI scores, mucosal healing, and laboratory indicators related to UC closely, major and general adverse events; and (6) sufficient data to calculate odds ratio (OR) or weighted mean difference (WMD). The exclusion criteria were as follows: (1) reviews, letters, editorial comments, case reports, conference abstracts, pediatric articles and non-English articles were excluded. (2) Interventions were not probiotics but pre-probiotics, synbiotics, symbiotic therapy or probiotic food. (3) The control groups were not administered placebo. Two investigators conducted this process independently. Any divergences in opinions were resolved by discussion until a consensus was reached.
Quality assessment
Literature quality was independently assessed by two investigators using the Cochrane Handbook 5.1.0 risk scale for bias. The evaluation included the following terms: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Three evaluation outcomes including low risk, high risk and unclear risk were assigned to every study aspect. Studies with more “low risk” bias evaluations were regarded as superior. Two authors severally assessed the quality of all included studies. Any disagreement during this process was discussed.
Data extraction
The data we extracted from studies were as followed, classified into four: (1) basic information of each study: first author, study period, country, follow-up, methods characteristics; (2) demographic and clinical characteristics: sample size, age, gender, body mass index(BMI), smoking, disease duration, ulcerative colitis disease activity index (UCDAI) scores at entry, clinical activity index (CAI) scores at entry, number of previous relapse; (3) intervention measures: type and dose of probiotic, concomitant treatment, medication time; (4) outcomes: clinical remission, clinical remission maintenance, clinical response, clinical relapse, change of various scoring scale of UC such as Mayo scores and UCDAI scores, mucosal healing, laboratory indices related to UC closely, major or any adverse events. We calculated the mean ± standard deviation via the validated mathematical method provided continuous variables in studies were reported as median with range or interquartile [9, 10]. When data were missing or not reported in the study, we contacted the corresponding authors to obtain completed data if available. This process was completed by two investigators independently. In case of disagreement on opinions, a third one would join and consult together to reach a consensus.
Statistical analysis
Review Manager version 5.4 (Cochrane Collaboration, Oxford, UK) was applied for statistical analysis. The odds ratio (OR) and weight mean difference (WMD) were used as effect indices for dichotomous and continuous data, respectively. All metrics were reported with 95% confidential intervals (CIs). The heterogeneity in studies was evaluated by the chi-squared (χ2) test (Cochran’s Q) and inconsistency index (I2) (38). I2 > 50% or Chi2 p-value < 0.1 was considered significant heterogeneity. A random-effects model was adopted; otherwise, a fixed-effects model was used. In addition, we performed one-way sensitivity analyses to evaluate the stability of outcomes with significant heterogeneity. Publication bias was rated by funnel plots using Review Manager 5.4 version (Cochrane Collaboration, Oxford, UK) and Egger’s regression tests using Stata 15.0 version (Stata Corp, College Station, TX, USA) for outcomes with 10 or more included studies. A p-value < 0.05 was considered statistically significant publication bias.
Results
Literature search
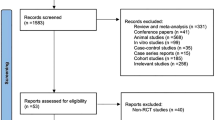
The specific flowchart of the search and selection process is presented in Fig. 1. A total of 6497 relevant articles in PubMed (n = 1032), Embase (n = 2722), and Web of Science (n = 2733) were systematically obtained through a literature search. After removing duplicate articles, 4341 titles and abstracts were reviewed. Finally, 13 full-text articles involving 930 patients (496 probiotic versus 434 placebo) were included in the pooled analysis, of which studies were all random controlled trials [11,12,13,14,15,16,17,18,19,20,21,22,23]. Table 2 shows the basic information and characteristics of each study. The bias risk assessment of each study is shown in Figs. 2 and 3.
Demographic and baseline characteristics
There were no significant differences among the two groups concerning age (WMD: −0.06; 95% CI −1.15, 1.39; P = 0.94), gender (male/total, OR: 0.93; 95% CI 0.69, 1.25; P = 0.62), BMI (WMD: 0.99; 95% CI 0.01,1.96; P = 0.05), smoking (OR: 1.02; 95% CI0 .36,2.87; P = 0.98), disease duration (WMD: 0.23; 95% CI −0.45,0.91; P = 0.67), number of previous relapse (WMD: −0.09; 95% CI −0.33, 0.14; P = 0.44), UCADI score at entry (WMD: −0.00; 95% CI −0.27,0.27; P = 0.98), CAI score at entry (WMD: −0.86; 95% CI −3.42, 1.52; P = 0.48), disease location: pancolitis (OR: 0.91; 95% CI 0.63,1.30; P = 0.59); left-sided colitis (OR:0.99; 95% CI 0.71,1.37; P = 0.94); proctosigmoiditis (OR:1.16; 95% CI 0.81, 1.66; P = 0.43) (Table 3).
Clinical remission
In the outcome of achieving clinical remission, a total of 10 studies enrolled 695 patients (376 patients in the probiotic group and 319 in the placebo group). Meta-analysis found that the probiotic group boosted the remission rate by 87% compared to the placebo group, but failed to reach a statistical difference (OR: 1.87; 95% CI 0.98, 3.57; P = 0.06, I2 = 67%) (Fig. 4).
Clinical remission maintenance
Two studies analyzed the maintenance of clinical remission, comprising 78 patients (43 in the probiotic group versus 35 in the placebo group). Meta-analysis showed that the probiotic group was no better than the placebo group in the maintenance of clinical remission (OR: 2.42; 95% CI 0.84, 6.96; P = 0.10; I2 = 0%) (Fig. 5).
Clinical relapse
The clinical relapse outcome included 3 studies comprising 104 patients (51 in the probiotic group versus 53 in the placebo group). Meta-analysis showed a lower rate of clinical recurrence in the probiotic group than in the placebo group (OR: 0.34; 95% CI 0.14, 0.79; P = 0.01, I2 = 28%) (Fig. 6).
Clinical response
Six studies containing patients (248 in the probiotic group versus 247 in the placebo group) were included in the analysis. Pooled results revealed a similar clinical response between the two groups (OR: 2.25; 95% CI 0.79, 6.42; P = 0.13), but heterogeneity existed (I2 = 79%, P = 0.0003) (Fig. 7).
UCDAI score changes
Two articles reported data on UCDAI score changes, including 194 patients (101 probiotic versus 93 placebo). No significant difference was detected between the two groups (WMD: −0.95; 95% CI − 3.04, 1.13; P = 0.37), but statistically significant heterogeneity was observed (I2 = 96%, p < 0.00001) (Fig. 8).
Mucosal healing
There were three studies reporting data on mucosal healing, including 224 patients (113 probiotic versus 111 placebo). No significant difference was detected between the two groups in terms of mucosal healing (OR: 1.14; 95% CI 0.26, 4.96; P = 0.86), but statistically significant heterogeneity was observed (I2 = 77%, p < 0.01) (Fig. 9).
Mean change in hemoglobin
Two studies were available for hemoglobin data with 105 patients (52 probiotic versus 53 placebo). No significant difference was observed between the two groups in the mean change in hemoglobin (WMD: 0.32; 95% CI −0.49, 1.14; P = 0.43), but significant heterogeneity was detected (I2 = 66%, P = 0.08) (Fig. 10).
Mean change in hematocrit
A total of 2 studies with 105 patients (52 probiotic versus 53 placebo) were included in the analysis for mean change of hematocrit. No significant difference was observed between the two groups for the mean change in hematocrit (WMD: 0.92; 95% CI −1.33, 3.17; P = 0.42), but significant heterogeneity was detected (I2 = 75%, P = 0.05) (Fig. 11).
Mean change in WBC count
Two articles were included in the analysis of WBC changes, containing 105 patients (52 probiotic versus 53 placebo). There was no significant difference in the change in WBCs between the two groups (WMD: 0.01; 95% CI −0.77, 0.79; P = 0.97), and the pooled results were not heterogeneous (I2 = 0%, P = 0.96) (Fig. 12).
Mean change in CRP
The data on the mean change in CRP were extracted from two studies involving 105 patients (52 probiotic versus 53 placebo). The combined results showed no significant difference in the change in CRP between the two groups (WMD: −0.26; 95% CI −0.65, 0.12; P = 0.18) as well as heterogeneity (I2 = 0%, P = 0.49) (Fig. 13).
Major adverse events
Twelve studies reported major adverse events involving 869 patients (462 probiotic versus 407 placebo). Only 1 patient in the probiotic group of one study had a major adverse event (Fig. 14).
General adverse events
Data on general adverse events were attained from twelve studies consisting of 869 patients (462 probiotic versus 407 placebo). There was no statistically significant difference between the two groups (OR: 1.98; 95% CI 0.69, 5.68; P = 0.20). However, heterogeneity existed (I2 = 72%, P = 0.002) (Fig. 15).
Publication bias
We conducted a publication bias assessment for clinical remission and general adverse events through funnel plots and Egger’s test. Funnel plots for the two outcomes all indicated that there was slight publication bias (Figs. 16, 17). Egger’s tests were not statistically significant, and the P-values for clinical remission and general adverse events were 0.469 and 0.125, respectively.
Sensitivity analysis
We conducted sensitivity analysis for clinical remission, general adverse events, clinical response, and mucosal healing to evaluate the stability of these outcomes with high heterogeneity. Clinical remission results included a total of 10 studies, and the combined results and heterogeneity did not change when the data of the other 9 studies were excluded one by one except Petersen 2014 (Fig. 18). When the data of Petersen 2014 were removed, the pooled result changed (OR: 2.23; 95% CI 1.37, 3.65; P = 0.001), and the heterogeneity became inapparent (I2 = 38%, P = 0.12), indicating that Petersen 2014 was sensitive to the combined results (OR) and brought heterogeneity to the results (Fig. 19). Twelve studies were included in general adverse events, and the OR did not change when the data of each study were excluded one by one (Fig. 20), demonstrating that the result was stable. When the data of Petersen 2014 were removed, heterogeneity became unobvious (I2 = 47%, P = 0.09), indicating that Petersen 2014 brought heterogeneity to the results (Fig. 21). In the same way as above, we conducted sensitivity analyses for clinical response (Fig. 22) and mucosal healing (Fig. 23), which showed that the ORs of the two outcomes both changed to be statistically significant after removal of Miele 2009 and Petersen 2014, respectively (Figs. 24, 25).
Discussion
Ulcerative colitis, a chronic disease with a variable course of remission and recurrence, belongs to inflammatory bowel disease (IBD) together with Crohn's disease. Current treatment strategies for IBD involve first induction of remission, followed by maintenance of remission. In terms of patients with UC, the conventional therapies are topical or systemic 5-aminosalicylic acids (5-ASA), immunomodulators and corticosteroids. However, it has been reported that only approximately 40% of patients achieve clinical remission at the end of a year on the basis of current treatment. In addition, there are problems such as the high cost of biotherapy, high risk of complications and eventual surgical intervention, prompting the exploration of new treatment modalities [24, 25]. Since the role of the gut microbiota has been confirmed in the etiology of UC, the application of probiotics to alleviate inflammation and induce intestinal homeostasis seems a promising approach with huge potential in the treatment of UC [26].
Several important findings we obtained from the merged results are as follows: For patients with mild to moderate clinical activity, first, although the combined results showed that compared with conventional UC therapy, probiotics as add-on treatment did not induce clinical remission better, there was significant heterogeneity in the combined results. We evaluated the publication bias by funnel plot and Egger’s test, suggesting no publication bias (P = 0.469). Then, we carried out sensitivity analysis. After the data in Petersen 2014 were excluded, the OR changed, which indicated that clinical remission improved after the addition of probiotics, and the original results lacked stability. Moreover, the heterogeneity changed from significant to insignificant, indicating that the data of this study brought heterogeneity to the results. Notably, the study conducted by Petersen et al. is the only study to find that adjunctive probiotic therapy significantly reduces the rate of clinical remission for patients with UC. The main reason for this contradictory finding may be that patients in the probiotic group received fewer azathioprine and/or steroid enemas than those receiving placebo [17]. Therefore, caution should be exercised when interpreting the ineffectiveness of probiotics for clinical remission rates in UC, given the significant heterogeneity and instability of the results.
Second, the merged results did not show that the addition of probiotics improved the clinical response, but the heterogeneity of the results was obvious. Sensitivity analysis revealed that the results were not stable, and Miele 2009 brought heterogeneity to the results. After the removal of this study, the OR changed to significant, and the heterogeneity decreased into unobvious. The contradictory results reported by Miele et al. may be due to the fact that the vast majority of patients in the probiotic group achieved clinical remission, whereas the rate of clinical remission was lower in the placebo group. Therefore, relatively, the clinical response rate of the probiotic group was significantly lower than that of the placebo group [22]. So, caution should be exercised when interpreting the ineffectiveness of probiotics for clinical response rates in UC. Third, for mucosal healing, the combined results showed no statistical significance but apparent heterogeneity. We excluded studies one by one, and after the removal of Petersen 2014, the OR changes showed statistical significance, and the heterogeneity disappeared, so the original results were unstable. However, due to the small number of studies and insufficient sample size, more clinical studies are needed to confirm the effect of probiotics as additional therapy on mucosal healing. For patients in remission, probiotics as additional treatment did not have a better effect of maintaining clinical remission. However, as there were only two included studies with insufficient sample sizes, it was impossible to conduct subgroup analysis or explore the source of heterogeneity by other means, and this result could not fully explain the maintenance effect of probiotics on clinical remission. However, probiotics showed a better effect in inhibiting UC relapse. We did not find that probiotics had an effect on laboratory indicators, but only two studies reported these indicators, among which the heterogeneity of hemoglobin and erythrocyte volume was obvious, and we did not explore the source of the heterogeneity. In terms of safety, only one major adverse event was reported in 1 study and could not be pooled for analysis. For general adverse events, there was no publication bias in this result (Egger’s test, P = 0.125), and no change in OR occurred after each individual study was excluded in sensitivity analysis, so the result was relatively stable.
Previous meta-analyses have scrutinized the effectiveness of probiotics in individuals with IBD. Derwa et al. [27] conducted a meta-analysis involving both adult patients with UC and CD, comparing probiotics with 5-ASA or a placebo. The findings indicated no discernible advantage in inducing remission of active ulcerative colitis compared to a placebo. Notably, the study included 31 children with a mean age of 12 years, broadening the scope of investigation. The conclusions drawn aligned with previous research. In contrast to the present meta-analysis, Derwa et al. [27] encompassed a study comparing probiotics with 5-ASA, revealing that probiotics were comparable to 5-ASA in preventing the recurrence of ulcerative colitis. Additionally, a separate study focusing on the VSL#3 strain demonstrated its efficacy in the remission of active ulcers. Mahboube et al. [28] conducted a meta-analysis inclusive of both children and adults, featured not only a probiotic intervention but also a probiotic and Biotime intervention study. This study delved into the roles of different probiotics and Biotime in achieving remission of active UC. Our meta-analysis differs from it, encompassing a more extensive array of studies and focusing primarily on adult patients with UC. In addition, in terms of interventions, we included only studies that compared probiotics with placebo and excluded studies that used synthetic probiotics as interventions. This approach helps to better assess the effectiveness of probiotics as adjunctive therapies for UC and reduces clinical heterogeneity.
Presently, an expanding body of research underscores the significance of gut microbiota dysbiosis in the onset and progression of IBD. Consequently, approaches like probiotics, prebiotics, and fecal microbiota transplantation have garnered increased attention for treatment. Studies have revealed that probiotics offer therapeutic benefits in IBD through diverse mechanisms. Probiotics play a pivotal role in modulating immunity and inflammation, improving gut microbiota by curtailing lipopolysaccharide levels, boosting mucus secretion, safeguarding tight junction proteins, fortifying intestinal barrier function, and fostering T cell differentiation towards Th2 cells. This differentiation enhances the production of Th2 cell cytokines such as IL-4 and IL-10 [29]. Additionally, probiotics suppress the excessive activation of the NF-κB pathway, diminish the production and release of pro-inflammatory cytokines, and stimulate the generation of anti-inflammatory cytokines [30]. Furthermore, by augmenting the production of short-chain fatty acids, they lower the pH in the intestinal environment, inhibiting the growth of potential pathogenic microorganisms [31].
However, we must acknowledge several limitations of this meta-analysis. Firstly, the sample size of the included RCT was small, and there may be potential publication bias. Secondly, significant heterogeneity and instability were present in some outcomes, which reduced the credibility of the results. Thirdly, due to the variety of probiotics used in the included RCTs, we were unable to conduct subgroup analysis according to the type of probiotics. Fourthly, most of the included RCTs were followed up for less than one year, and the long-term efficacy of probiotics in adjuvant treatment of UC needs further research to be confirmed.
Conclusion
Meta-analysis found that adjuvant probiotic therapy for active UC does not seem to significantly improve clinical remission and clinical response. But for patients in clinical remission, probiotics can effectively inhibit the recurrence of UC. In addition, probiotics did not increase the risk of treatment-related adverse events compared with placebo. Given the significant heterogeneity and instability, caution should be exercised in interpreting the ineffectiveness of probiotics in improving clinical remission and clinical response for patients with active UC. More large-scale, multi-center, double-blind RCTs are needed to further evaluate the additive efficacy and safety of probiotics in the treatment of UC.
Data availability
The data that support the findings of this study are available from the corresponding anthor upon reasonable request.
References
da Silva BC, Lyra AC, Rocha R, Santana GO (2014) Epidemiology, demographic characteristics and prognostic predictors of ulcerative colitis. World J Gastroenterol 20:9458–9467. https://doi.org/10.3748/wjg.v20.i28.9458
Nishida A, Inoue R, Inatomi O, Bamba S, Naito Y, Andoh A (2018) Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin J Gastroenterol 11:1–10. https://doi.org/10.1007/s12328-017-0813-5
Shen ZH, Zhu CX, Quan YS, Yang ZY, Wu S, Luo WW, Tan B, Wang XY (2018) Relationship between intestinal microbiota and ulcerative colitis: mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J Gastroenterol 24:5–14. https://doi.org/10.3748/wjg.v24.i1.5
Federici S, Kredo-Russo S, Valdés-Mas R, Kviatcovsky D, Weinstock E, Matiuhin Y, Silberberg Y, Atarashi K, Furuichi M, Oka A, Liu B, Fibelman M, Weiner IN, Khabra E, Cullin N, Ben-Yishai N, Inbar D, Ben-David H, Nicenboim J, Kowalsman N, Elinav E (2022) Targeted suppression of human IBD-associated gut microbiota commensals by phage consortia for treatment of intestinal inflammation. Cell 185(16):2879-2898.e24. https://doi.org/10.1016/j.cell.2022.07.003
Akutko K, Stawarski A (2021) Probiotics, prebiotics and synbiotics in inflammatory bowel diseases. J Clin Med 10(11):2466. https://doi.org/10.3390/jcm10112466
Glassner KL, Abraham BP, Quigley EMM (2020) The microbiome and inflammatory bowel disease. J Allergy Clin Immunol 145:16–27. https://doi.org/10.1016/j.jaci.2019.11.003
Shiba T, Aiba Y, Ishikawa H, Ushiyama A, Takagi A, Mine T, Koga Y (2003) The suppressive effect of bifidobacteria on Bacteroides vulgatus, a putative pathogenic microbe in inflammatory bowel disease. Microbiol Immunol 47:371–378. https://doi.org/10.1111/j.1348-0421.2003.tb03368.x
Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D (2021) The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 372:n71. https://doi.org/10.1136/bmj.n71
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27:1785–1805. https://doi.org/10.1177/0962280216669183
Park SK, Kang SB, Kim S, Kim TO, Cha JM, Im JP, Choi CH, Kim ES, Seo GS, Eun CS (2022) Additive effect of probiotics (Mutaflor) on 5-aminosalicylic acid therapy in patients with ulcerative colitis. Korean J Intern Med 37:949–957. https://doi.org/10.3904/kjim.2021.458
Agraib LM, Yamani MI, Tayyem R, Abu-Sneineh AT, Rayyan YM (2022) Probiotic supplementation induces remission and changes in the immunoglobulins and inflammatory response in active ulcerative colitis patients: a pilot, randomized, double-blind, placebo-controlled study. Clin Nutr ESPEN 51:83–91. https://doi.org/10.1016/j.clnesp.2022.08.020
Bjarnason I, Sission G, Hayee BH (2019) A randomised, double-blind, placebo-controlled trial of a multi-strain probiotic in patients with asymptomatic ulcerative colitis and Crohn’s disease. Inflammopharmacology 27:465–473. https://doi.org/10.1007/s10787-019-00595-4
Vejdani R, Bahari A, Zadeh AM, Azmi M, Ebrahimi-Daryani N, Hashtroudi AA, Mehr AJ, Abdollahi M, Sayyah A, Zali MR, Bari Z, Bakhshipour A (2017) Effects of Lactobacillus casei probiotic on mild to moderate ulcerative colitis: a placebo controlled study. Indian J Med Sci 69:24–28
Tamaki H, Nakase H, Inoue S, Kawanami C, Itani T, Ohana M, Kusaka T, Uose S, Hisatsune H, Tojo M, Noda T, Arasawa S, Izuta M, Kubo A, Ogawa C, Matsunaka T, Shibatouge M (2016) Efficacy of probiotic treatment with Bifidobacterium longum 536 for induction of remission in active ulcerative colitis: a randomized, double-blinded, placebo-controlled multicenter trial. Dig Endosc 28:67–74. https://doi.org/10.1111/den.12553
Yoshimatsu Y, Yamada A, Furukawa R, Sono K, Osamura A, Nakamura K, Aoki H, Tsuda Y, Hosoe N, Takada N, Suzuki Y (2015) Effectiveness of probiotic therapy for the prevention of relapse in patients with inactive ulcerative colitis. World J Gastroenterol 21:5985–5994. https://doi.org/10.3748/wjg.v21.i19.5985
Petersen AM, Mirsepasi H, Halkjær SI, Mortensen EM, Nordgaard-Lassen I, Krogfelt KA (2014) Ciprofloxacin and probiotic Escherichia coli Nissle add-on treatment in active ulcerative colitis: a double-blind randomized placebo controlled clinical trial. J Crohns Colitis 8:1498–1505. https://doi.org/10.1016/j.crohns.2014.06.001
Oliva S, Di Nardo G, Ferrari F, Mallardo S, Rossi P, Patrizi G, Cucchiara S, Stronati L (2012) Randomised clinical trial: the effectiveness of Lactobacillus reuteri ATCC 55730 rectal enema in children with active distal ulcerative colitis. Aliment Pharmacol Ther 35:327–334. https://doi.org/10.1111/j.1365-2036.2011.04939.x
Tursi A, Brandimarte G, Papa A, Giglio A, Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, Modeo ME, Rodino’ S, D’Amico T, Sebkova L, Sacca’ N, Di Giulio E, Luzza F, Imeneo M, Larussa T, Di Rosa S, Annese V, Danese S, Gasbarrini A (2010) Treatment of relapsing mild-to-moderate ulcerative colitis with the probiotic VSL#3 as adjunctive to a standard pharmaceutical treatment: a double-blind, randomized, placebo-controlled study. Am J Gastroenterol 105:2218–2227. https://doi.org/10.1038/ajg.2010.218
Matthes H, Krummenerl T, Giensch M, Wolff C, Schulze J (2010) Clinical trial: probiotic treatment of acute distal ulcerative colitis with rectally administered Escherichia coli Nissle 1917 (EcN). BMC Complem Altern Med 10:13. https://doi.org/10.1186/1472-6882-10-13
Sood A, Midha V, Makharia GK, Ahuja V, Singal D, Goswami P, Tandon RK (2009) The probiotic preparation, VSL#3 induces remission in patients with mild-to-moderately active ulcerative colitis. Clin Gastroenterol Hepatol 7:1202-1209.e1201. https://doi.org/10.1016/j.cgh.2009.07.016
Miele E, Pascarella F, Giannetti E, Quaglietta L, Baldassano RN, Staiano A (2009) Effect of a probiotic preparation (VSL#3) on induction and maintenance of remission in children with ulcerative colitis. Am J Gastroenterol 104:437–443. https://doi.org/10.1038/ajg.2008.118
Wildt S, Nordgaard I, Hansen U, Brockmann E, Rumessen JJ (2011) A randomised double-blind placebo-controlled trial with Lactobacillus acidophilus La-5 and Bifidobacterium animalis subsp. lactis BB-12 for maintenance of remission in ulcerative colitis. J Crohns Colitis 5:115–121. https://doi.org/10.1016/j.crohns.2010.11.004
Hirten RP, Sands BE (2021) New therapeutics for ulcerative colitis. Annu Rev Med 72:199–213. https://doi.org/10.1146/annurev-med-052919-120048
Derikx LAAP, Dieleman LA, Hoentjen F (2016) Probiotics and prebiotics in ulcerative colitis. Best Pract Res Clin Gastroenterol 30:55–71. https://doi.org/10.1016/j.bpg.2016.02.005
Štofilová J, Kvaková M, Kamlárová A, Hijová E, Bertková I, Guľašová Z (2022) Probiotic-based intervention in the treatment of ulcerative colitis: conventional and new approaches. Biomedicines 10:2236. https://doi.org/10.3390/biomedicines10092236
Derwa Y, Gracie DJ, Hamlin PJ, Ford AC (2017) Systematic review with meta-analysis: the efficacy of probiotics in inflammatory bowel disease. Aliment Pharmacol Ther 46(4):389–400. https://doi.org/10.1111/apt.14203
Ganji-Arjenaki M, Rafieian-Kopaei M (2018) Probiotics are a good choice in remission of inflammatory bowel diseases: a meta analysis and systematic review. J Cell Physiol 233(3):2091–2103. https://doi.org/10.1002/jcp.25911
Cristofori F, Dargenio VN, Dargenio C, Miniello VL, Barone M, Francavilla R (2021) Anti-inflammatory and immunomodulatory effects of probiotics in gut inflammation: a door to the body. Front Immunol 12:578386. https://doi.org/10.3389/fimmu.2021.578386
Celiberto LS, Bedani R, Rossi EA, Cavallini DC (2017) Probiotics: The scientific evidence in the context of inflammatory bowel disease. Crit Rev Food Sci Nutr 57(9):1759–1768. https://doi.org/10.1080/10408398.2014.941457
Roy S, Dhaneshwar S (2023) Role of prebiotics, probiotics, and synbiotics in management of inflammatory bowel disease: Current perspectives. World J Gastroenterol 29(14):2078–2100. https://doi.org/10.3748/wjg.v29.i14.2078
Funding
The authors received no financial support for the research, authorship, and/or publication of this article. This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
(1) Conception and design: Xinyue Wang; (2) Administrative support: Chunyu Zhou, Shaohui Zhang; (3) Literature search: Xinyue Wang, Wenqin Xiao; (4) Literature inclusion and exclusion: Xinyue Wang, Yanmei Guo;(5)Literature quality assessment: Xinyue Wang, Yixiang Ma; (6) Data extraction and analysis: Xinyue Wang, Yixiang Ma; (7) Manuscript writing: Xinyue Wang. All authors contributed to the article and agreed with the submitted version.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, X., Zhou, C., Zhang, S. et al. Additive efficacy and safety of probiotics in the treatment of ulcerative colitis: a systematic review and meta-analysis. Eur J Nutr 63, 1395–1411 (2024). https://doi.org/10.1007/s00394-023-03307-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-023-03307-5