Abstract
Purpose
The objective of this study was to investigate the association between intake of seafood and plant-derived n-3 polyunsaturated fatty acids (PUFA) and development of total atherosclerotic cardiovascular disease (ASCVD) and acute major ischemic events.
Methods
A total of 53,909 men and women were enrolled between 1993 and 1997 into the Danish Diet, Cancer and Health cohort and followed through nationwide Danish registries for development of total ASCVD defined as a first registration of myocardial infarction, peripheral artery disease, or ischemic stroke due to large artery atherosclerosis or small-vessel occlusion. At recruitment, the intake of the major marine n-3 PUFA, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) and the plant-derived n-3 PUFA, alpha-linolenic acid (ALA), was assessed using a validated food frequency questionnaire. Statistical analyses were conducted using sex-stratified multivariable Cox proportional hazard regression models.
Results
During a median of 13.5 years of follow-up, 3958 participants developed ASCVD including 3270 patients with an acute major ischemic event. In multivariable analyses including adjustment for established risk factors, we found no associations for intake of ALA, but indications of inverse associations between intake of EPA, DHA and EPA + DHA and the rate of total ASCVD and acute major ischemic events.
Conclusions
A high intake of marine n-3 PUFA was associated with a lower risk of total ASCVD and acute major ischemic events, whereas no association could be demonstrated for the plant-derived ALA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
N-3 polyunsaturated fatty acids (PUFA) can be divided into marine- and plant-based n-3 PUFA. The major biologically active marine n-3 PUFA are derived from seafood and include eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA), while the major plant-derived n-3 PUFA, alpha-linolenic acid (ALA), is found in green plants, grain products, walnuts, some seeds, and vegetable oils based on canola, soybean, and flaxseed [1].
Marine n-3 PUFA have been subject to extensive research since the 1970s where Bang, Dyerberg, and colleagues based on findings in Greenland Inuit hypothesized that EPA might protect against atherosclerosis and thrombosis [2]. Beneficial effects of EPA and DHA may among others include anti-inflammatory, anti-thrombotic, and anti-arrhythmic effects, and a lowering of heart rate, blood pressure, plasma triglycerides and platelet reactivity, and possibly also stabilisation of atherosclerotic plaques [3,4,5,6,7]. ALA is the most abundant n-3 PUFA in Western countries—especially in the Mediterranean area—and has been hypothesized to be an important mediator of the protective effect on vascular disease provided by a Mediterranean diet [8, 9]. Also, ALA has attracted public health interest due to its role as a precursor for marine n-3 PUFA and has been suggested to be a sustainable plant-based alternative to EPA [1]. However, only 8–12% ALA consumed may be converted to EPA (and less than 1% to DHA) in most humans [1], and therefore, a high intake of ALA would be needed to achieve biologically relevant amounts of EPA through intake of ALA. Limited knowledge exists on the biological effects of ALA and findings have been inconsistent, but a few studies have suggested that ALA might exert anti-inflammatory, anti-thrombotic, and anti-arrhythmic effects [10]. Such effects could be exerted by ALA per se and/or through conversion of ALA into EPA [1, 10].
Several prospective observational studies have reported that marine n-3 PUFA were associated with a lower risk of coronary heart disease (CHD) and in particular fatalities from CHD, which might be mediated by a reduction in fatal ventricular arrhythmias [11,12,13]. The effect of marine n-3 PUFA on the other major atherosclerotic vascular diseases ischemic stroke and peripheral artery disease (PAD) has been less studied. However, a few studies have indicated that a high intake of marine n-3 PUFA might be associated with a lower risk of ischemic stroke, but most studies have reported neutral findings regarding intake of marine n-3 PUFA and the risk of ischemic stroke [14, 15]. On the other hand, a few studies have suggested that intake of ALA might be associated with a lower risk of incident CHD [16,17,18], fatal CHD [18,19,20,21], and sudden cardiac death [22], but most studies have reported neutral findings between ALA intake and the risk of CHD, ischemic stroke, and PAD [15]. Previous investigations of individual types of ASCVD outcomes have provided insight into the underlying biology of exposure to marine and plant-derived n-3 PUFA, and the different associations observed might be due to the effect of these fatty acids not being uniform across subtypes of ASCVD. However, the public health relevance of recommendation of consumption of marine and plant-derived n-3 PUFA may merely depend on the relation with a composite of total ASCVD rather than one of its components.
The objectives of this study were to investigate the associations between intake of marine n-3 PUFA (EPA, DHA, and EPA + DHA) and of ALA and risk of the incident composite endpoints of total ASCVD and acute major ischemic events.
Methods
Study population
The current study was based on data from the Diet, Cancer and Health cohort. This cohort study has been described in detail previously [23]. Briefly, citizens born in Denmark aged 50–64 years who were living in the greater areas of Copenhagen and Aarhus without a previous diagnosis of cancer were invited to participate. Eligible cohort members were identified through the Danish Civil Registration System. The enrollment began in December 1993 and ended in May 1997 [23].
The current study was conducted using a traditional follow-up design. We excluded participants with a cancer diagnosis before baseline that due to processing delay had not been registered in the Danish Cancer Registry at the time of invitation. Also, participants registered with a diagnosis of myocardial infarction (MI), stroke, and PAD before baseline were excluded. Finally, participants with missing information on exposures and/or established ASCVD risk factors were excluded assuming that these were missing at random.
The study was conducted according to the Declaration of Helsinki. All participants gave written informed consent, including prospective data collection from national registers, and the study was approved by the relevant scientific Ethical Committees and the Danish Data Protection Agency [23]. The current study was approved by the Danish Data Protection Agency (2008-58-0028-2017-225).
Assessment of dietary intake of n-3 PUFA
All participants received a 192-item food frequency questionnaire (FFQ) and were asked to report their average intake the past year. The consumption of foods and beverages was reported within 12 categories. All FFQs were optically scanned and checked for missing values, and uncertainties were clarified by technicians during enrollment [23]. The daily intake of EPA, DHA, and ALA was estimated for each participant using the software program FoodCalc based on Danish composition tables and standardized recipes and portion sizes. N-3 PUFA from supplements were not included in estimated intakes of n-3 PUFA. The intake of n-3 PUFA was energy-adjusted using the residual method [24]. The FFQ filled in at baseline had previously been validated against 7-day weighed diet records and found useful for categorizing individuals according to their intake of energy and PUFA [25].
Covariates
All participants were asked to fill in a questionnaire on social and lifestyle factors, such as length of schooling, smoking habits, and physical activity during enrollment [23]. Also, self-reported information on hypercholesterolemia, hypertension or diabetes mellitus, and relevant medications used to treat these conditions were collected. The questionnaires were checked for reading errors and missing information by laboratory technicians who also undertook anthropometric measurements including height, weight, and waist circumference of the participants [23]. Information of alcohol consumption was calculated from the FFQ. We retrieved information on registered International Classification of Disease (ICD) discharge diagnoses of kidney insufficiency at baseline by record linkage with the nationwide Danish National Patient Register (DNPR) (ICD-8: 79299 & ICD-10: DN18, DN181, DN182, DN183, DN184; DN185, DN188 and DN189).
Outcome assessment
The outcome measures were incident total ASCVD and acute major ischemic events. ASCVD was defined as incident MI, ischemic stroke due to large artery atherosclerosis or small-vessel occlusion and PAD, while acute major ischemic events were defined as MI and ischemic stroke due to presumed large artery atherosclerosis or small-vessel occlusion of cerebral arteries. Information on incident ASCVD cases was obtained through record linkage with the cohort participants and registered discharge diagnoses registered in the nationwide DNPR [26]. Participants who were registered with either a primary or secondary discharge diagnosis of MI (ICD-8: 410-410.99 and ICD-10: I21), stroke (ICD-8: 430, 431, 433, 434, 436.01, or 436.90 and ICD-10: I60, I61, I63 or I64), or PAD (ICD-8: 44,390, 44,500, 44,509, 44,590, 44,599, 44,020, 44,030 and ICD-10: I702, I702A, I739A-C) were considered as potential cases. Furthermore, information from the Danish Cause of Death Registry [27] was used to identify potential fatal cases of MI. Also, all cases of cardiac arrest (ICD-8: 427.27 and ICD-10: I46) were included as cases of MI if the arrest after case validation was considered to be of cardiac origin. All potential cases of MI from baseline through 2003 had previously been validated based on a complete review of medical records with a positive predictive value of MI above 92% when the diagnosis was obtained from a hospital ward [28]. Therefore, from January 2004 and onwards, all participants who were registered with a diagnosis of MI from a hospital ward were accepted as cases for the current study without further validation. All other diagnoses of MI were validated by examining all relevant diagnoses and procedure codes in the DNPR. All potential ischemic stroke cases were validated by a physician with neurological experience and classified into ischemic stroke subtypes according to the Trial of Org 10,172 in Acute Stroke Treatment (TOAST) classification based on clinical findings, brain imaging, imaging of extra cranial arteries, laboratory tests, electrocardiograms, and other relevant findings registered within their medical records [29, 30]. Ischemic stroke cases were restricted to ischemic strokes of presumed atherosclerotic origin, including ischemic stroke due to large artery atherosclerosis and ischemic stroke due to small-vessel occlusions [29, 31]. All potential PAD cases were validated by a trained vascular surgeon based on registered clinical data, such as relevant symptoms, imaging, ankle–brachial index and/or toe brachial index, and other relevant findings in their medical records [32].
Participants were followed from baseline until the first registration of incident ASCVD, death, emigration, or end of follow-up in November 2009.
Statistical analyses
Hazard rate ratios (HRs) with 95% confidence intervals, calculated using Cox proportional hazard regression, were used as measures of association. We used age as underlying time scale and the analyses were adjusted for sex by allowing for separate baseline hazards among men and women. Exposures of interest were modelled as continuous variables using restricted cubic splines with four knots to describe the shape of the observed associations using the median intake as reference. The knots were placed at fixed percentiles at the 5th, 35th, 65th, and 95th percentile as recommended by Harrell [33]. The spline functions were tested against a horizontal line using Wald’s test and plotted for the 98% central range. Categorical analyses were conducted by categorizing the exposures of interest into quintiles using the lowest quintile as reference.
We included covariates into three different models specified prior to data analysis. In model 1A, we adjusted for baseline age, while in model 1B (main model for interpretation), we additionally adjusted for established ASCVD risk factors including length of schooling ( ≤ 7, 8–10, or > 10 years), smoking (never, former, current 1–14, 15–24 or > 24 g/d), physical activity (inactive, moderately inactive, moderately active, or active), waist circumference (continuous, cm), body mass index (continuous, kg/m2), and alcohol intake (continuous, g/d). In model 2, we additionally adjusted for self-reported history of hypercholesterolemia and/or use of lipid-lowering medication (yes, no or unknown), hypertension and/or use of anti-hypertensive medication (yes, no or unknown), and diabetes mellitus and/or use of insulin. All continuous covariates were entered into the models using restricted cubic splines with five knots to ensure a detailed adjustment for these covariates.
In supplemental analyses, we adjusted for potential dietary risk factors, including total energy intake, intake of fiber (g/day), glycemic load, saturated fatty acids (g/day), monounsaturated fatty acids (g/day), linoleic acid (g/day), and EPA + DHA or ALA depending on the exposure of interest using restricted cubic splines with three knots.
The proportional hazard assumption was evaluated for each model by plotting the scaled Schoenfeld residuals against age at event.
A radar plot was used to describe intake of EPA+DHA and ALA as indicators of the underlying dietary pattern related to consumption of these fatty acids. We calculated an energy-adjusted intake of selected foods and beverages among participants in the highest and lowest quintile of EPA+DHA and ALA intake and divided by the overall median intake in the cohort.
Data were analysed using Stata statistical software (version 16; StataCorp LP, US). A P value below 0.05 was considered statistically significant and measures of association that did not reach this value of conventional statistically significance were described as indications of associations when considered appropriate [34].
Results
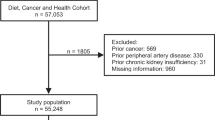
A total of 160,725 eligible men and women were invited to participate in the Diet, Cancer and Health cohort of whom 57,053 were enrolled. We excluded a total of 3144 participants, because they had a diagnosis of cancer, ASCVD or chronic kidney insufficiency before enrollment, or if information on exposures or covariates was missing (Supplemental Fig. 1). During a median of 13.5 years of follow-up, we identified 3958 participants that developed ASCVD including 3270 with an acute major ischemic event. Baseline characteristics of the cohort and participants that developed ASCVD and acute major ischemic events are shown in Table 1. The underlying dietary pattern to intake of EPA + DHA and ALA is shown in Fig. 1. This radar plot showed several differences in the underlying dietary pattern related to intake of EPA + DHA and ALA, respectively. Thus, participants in the highest quintile of EPA + DHA had as expected a higher intake of fish, but also a higher intake of alcohol, soft drinks and juices, vegetable oils and mayonnaises, meat, vegetables, eggs and poultry, and a lower intake of butter and other animal fat and snacks, sugar and confectionary compared to participants in the lowest quintile of EPA + DHA intake. When compared to participants in the lowest quintile of ALA intake, participants with the highest intake of ALA had a higher intake of vegetable oils and mayonnaises, butter and other animal fat, margarines, meat and alcohol as well as a higher intake of eggs, meat, fish, potatoes, refined cereals, snacks, sugar and confectionary and a lower intake of fruit, vegetables and dairy products.
Marine n-3 PUFA and ASCVD and acute major ischemic events
The results of minimally adjusted analyses (model 1A) are shown in Supplemental Figs. 2+3. In multivariable analyses including adjustment for established risk factors (model 1B), we found statistically non-significant indications of lower rates of ASCVD (Fig. 2) and acute major ischemic events (Fig. 3) in subjects with the highest intake of EPA, DHA, and EPA + DHA. In analyses including additional adjustment for hypertension, hypercholesterolemia, and diabetes mellitus (model 2), we observed statistically significant inverse associations between intake of EPA and the rate of ASCVD (Fig. 4) and between EPA, DHA, and EPA + DHA and the rate of acute major ischemic events (Fig. 5), while inverse, but statistically non-significant, indications of associations were found between DHA and EPA + DHA and the rate of ASCVD (Fig. 4). A similar pattern of association was observed in analyses including adjustment for established risk factors and dietary factors (model 3) compared to analyses including adjustments according to model 1B and model 2 (Supplemental Figs. 4+5). Our findings indicate that at least 1 g/day of EPA + DHA may be needed to achieve cardiovascular benefits.
Associations between n-3 PUFA and the rate of incident ASCVD in multivariable analyses included adjustment for established risk factors (model 1B). The gray area represents the 95% confidence bonds. The median intake of the exposures of interest was used as reference and the dashed lines represent the 20th, 40th, 60th, and 80th percentile of intake. The spline curve is plotted on logscale and the exposure ranges were restricted to the 1st to the 99th percentile of intake
Associations between n-3 PUFA and the rate of acute major ischemic events in multivariable analyses included adjustment for established risk factors (model 1B). The gray area represents the 95% confidence bonds. The median intake of the exposures of interest was used as reference and the dashed lines represent the 20th, 40th, 60th, and 80th percentile of intake. The spline curve is plotted on logscale and the exposure ranges were restricted to the 1st to the 99th percentile of intake
Associations between n-3 PUFA and the rate of incident ASCVD in multivariable analyses included adjustment for established risk factors and potential intermediates (model 2). The gray area represented the 95% confidence bonds. The median intake of the exposures of interest was used as reference and the dashed lines represent the 20th, 40th, 60th, and 80th percentile of intake. The spline curve is plotted on logscale and the exposure ranges were restricted to the 1st to the 99th percentile of intake
Associations between n-3 PUFA and the rate of acute major ischemic events in multivariable analyses included adjustment for established risk factors and potential intermediates (model 2). The shaded area represents the 95% confidence bonds. The median intake of the exposures of interest was used as reference and the dashed lines represent the 20th, 40th, 60th, and 80th percentile of intake. The spline curve is plotted on logscale and the exposure ranges were restricted to the 1st to the 99th percentile of intake
Categorical analyses of intake of marine n-3 PUFA and the rate of ASCVD and acute major ischemic events are given in Table 2, while supplemental analyses including adjustment for established risk factors and dietary factors (model 3) are shown in Supplemental Table 1. Similar patterns of association were observed in analyses conducted among men and women separately (Supplemental Table 2+3).
ALA and ASCVD and acute major ischemic events
In multivariable analyses including adjustment for established risk factors (model 1B), no association was found between intake of ALA and ASCVD (Fig. 2) and acute major ischemic events (Fig. 3). Also, no association was found in analyses including additional adjustment for hypertension, hypercholesterolemia, and diabetes mellitus (Figs. 4, 5) and analyses including adjustment for established ASCVD risk factors and dietary factors (Supplemental Figs. 4+5)
Categorical analyses of intake of ALA and the rate of ASCVD and acute major ischemic events for model 1A, 1B and 2 are shown in Table 2, while supplemental analyses including adjustment for established risk factors and dietary factors (model 3) are given in Supplemental Table 1. Also, no association was found in analyses conducted among men and women separately (Supplemental Tables 2+3).
Discussion
In this large follow-up study, we found statistically non-significant indications of lower rates of ASCVD and acute major ischemic events with intake of EPA and/or DHA, while no association was found for ALA. In analyses including additional adjustment for hypercholesterolemia, hypertension, and diabetes mellitus, we observed statistically significant inverse associations between intake of EPA and the rate of ASCVD and between intake of EPA and/or DHA and the rate of acute major ischemic events. Similar patterns of association were observed in analyses conducted among men and women separately. Our findings suggest that the associations between EPA and DHA and the risk of total ASCVD and acute major ischemic events were modest and that an intake of at least 1 g/day might be needed to achieve cardiovascular benefits. An intake of 1 g/day of EPA + DHA can be obtained through intake of 50–100 g of oily fish daily, while 300–400 g of lean fish daily would be needed to obtain this amount of EPA + DHA.
We used a composite endpoint of incident MI, ischemic stroke of atherosclerotic origin, and PAD to investigate the associations between marine and plant-derived n-3 PUFA and development of total ASCVD. Investigation of a composite of total ASCVD may be of greater public health interest than examining one of its components, but only a few prospective observational studies have investigated this. However, in the Women’s Health study, no associations were found between intake of EPA+DHA or ALA and the rate of major cardiovascular events using a composite of MI, total stroke, and cardiovascular death [35].
Composite outcomes have frequently been reported in randomized clinical supplementation trials, and supplementation with marine n-3 PUFA has shown conflicting results, and therefore, their role in lowering the risk of CVD has been debated [36, 37]. Two recent trials did not support a beneficial effect of marine n-3 supplementation on CVD risk [38, 39], but in a previous meta-analysis including 13 trials, it was reported that supplementation with marine n-3 PUFA may be associated with a modest lower risk of a composite of total CVD including non-fatal MI, non-fatal stroke, death from CVD, or hospitalization due to a cardiovascular cause [40]. Most previous trials have investigated marine n-3 supplementation in subjects with or at high risk of CVD using doses of 0.4 to 1.8 g/day of marine n-3 PUFA [40]. Interestingly, the REDUCE-IT trial investigated supplementation of a daily intake of 4 g purified EPA ethyl ester against placebo on the risk of CVD in subjects with or at high risk of CVD and mildly elevated triglycerides [41]. After nearly 5 years of follow-up, participants randomized to EPA (705 events) had a markedly lower risk of a composite endpoint of cardiovascular death, non-fatal MI, non-fatal stroke, coronary revascularization, and unstable angina pectoris compared to placebo (901 events) [41]. In contrast, the recently published STRENGTH trial showed no beneficial effect of a daily supplementation with 4 g carboxylic acid formulation of EPA and DHA among subjects with or at high risk of ASCVD on a composite endpoint of cardiovascular death, non-fatal MI, non-fatal stroke, coronary revascularization, or unstable angina pectoris requiring hospitalization (785 events) compared with controls (795 events) [39]. The VITAL study is the only large trial that have investigated an effect of primary prevention of marine n-3 PUFA supplementation [42]. Here, a total of 25,871 participants were randomized to a daily intake of 840 mg EPA+DHA and/or vitamin D or placebo and followed for a median of 5.3 years. A modest statistically non-significant lower risk of a composite of major cardiovascular events including MI, stroke, or death from CVD causes was found. An analysis of the individual components of the composite showed a statistically significant lower risk of MI, while no associations were found for cardiovascular death or stroke. An advantage of our study was the use of nationwide registries to follow the participants for a median of 13.5 years as, to our knowledge, no trials have investigated supplementation with marine n-3 PUFA beyond a median of 7.4 years of follow-up, which is a relatively short time as atherosclerosis develops over decades [40]. Furthermore, the relatively high intake of fish in the Diet, Cancer and Health cohort allowed us to investigate the associations between intake of marine n-3 PUFA and development of total ASCVD that corresponds to therapeutically doses of marine n-3 PUFA that has been applied in previous trials. Our data suggest that an intake of more than 1g/day of EPA+DHA may be needed to achieve cardiovascular benefit. However, it should be emphasized that intakes of n-3 PUFA were indicators of complex underlying dietary patterns with beneficial effects on ASCVD. Therefore, estimated intakes of n-3 PUFA cannot be directly compared to fish oil supplements, partly because fish oil supplements are taken on top of the habitual diet, while fish and other seafood are eaten instead of other food items (e.g., red meat) but also because fish may be part of a different dietary pattern than the food it replaces. Human and animal studies have suggested that marine n-3 PUFA may act to stabilize atherosclerotic plaques making them less vulnerable to rupture by decreasing infiltration of inflammatory and immune cells into the plaques and strengthen the fibrous cap around plaques [7, 43], which could contribute to a lower risk of acute major ischemic events as observed in our study.
Limitations
Selection bias cannot be ruled out, but was not considered a major concern, because information on exposures was collected independently of the outcomes and all cases were identified through nationwide registries with very limited loss to follow-up (< 0.6%). Information on intake of n-3 PUFA was likely subject to measurement error as we only had information on diets obtained through a single FFQ designed to assess the average intake of selected foods and beverages during the previous year. Measurement error of dietary exposures is inevitable, although it might be reduced through energy adjustment [24]. Measurement error of our exposures of interest probably occurred at random as information on diet was collected independently of the outcomes of interest, which may have resulted in an attenuation of the observed associations and loss of statistical power.
We followed the participants for a median of 13.5 years and repeated dietary measurements would indeed have been preferable to reduce random measurement error and to capture potential changes in dietary habits over time. Another limitation was that we did not have information on the amount of n-3 PUFA obtained through supplements. We limited our case definition to diagnoses of ischemic stroke and PAD and cases of MI with a high validity, and therefore, information bias related to misclassification of cases was not considered a concern.
We included detailed adjustment for potential risk factors defined prior to data analysis, but despite this, residual confounding cannot be ruled out.
Our study population consisted of Danish, middle-aged men and women, who had survived until enrollment without a diagnosis of cancer, chronic kidney disease or ASCVD. Also, our study population was recruited from selected areas in Denmark and included almost exclusively Caucasians and those enrolled had a higher socioeconomic position compared with invited non-participants [23]. Thus, the generalizability of our study findings may be limited according to eligibility and exclusion criteria applied.
In conclusion, we found indications of lower rates of ASCVD and acute major ischemic events in subjects with a high intake of EPA and/or DHA, while no associations were found for intake of ALA.
Data availability
Data are available from the Diet, Cancer and Health Institutional Data Access (https://www.cancer.dk/research/dcrc-research/diet-cancer-and-health/). To access data, an application must be approved by the Scientific Board. Furthermore, as data contain potentially identifying or sensitive information, access to data has to be registered and approved by The Danish Data Protection Agency and/or a Health Research Ethics Committee.
References
Baker E, Miles E, Burdge G, Yaqoob P, Calder P (2016) Metabolism and functional effects of plant-derived omega-3 fatty acids in humans. Prog Lipid Res 64:30–56. https://doi.org/10.1016/j.plipres.2016.07.002
Dyerberg J, Bang H, Stoffersen E, Moncada S, Vane J (1978) Eicosapentaenoic acid and prevention of thrombosis and atherosclerosis? Lancet 2:117–119. https://doi.org/10.1016/s0140-6736(78)91505-2
Mozaffarian D, Wu J (2011) Omega-3 fatty acids and cardiovascular disease: effects on risk factors, molecular pathways, and clinical events. J Am Coll Cardiol 58:2047–2067. https://doi.org/10.1016/j.jacc.2011.06.063
Innes JK, Calder PC (2020) Marine omega-3 (N-3) fatty acids for cardiovascular health: an update for 2020. Int J Mol Sci 21:1362. https://doi.org/10.3390/ijms21041362
De Caterina R (2011) N-3 fatty acids in cardiovascular disease. N Engl J Med 364:2439–2450. https://doi.org/10.1056/NEJMra1008153
Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, May HT, Shaikh K, Shekar C, Roy SK et al (2020) Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J 4:3925–3932. https://doi.org/10.1093/eurheartj/ehaa652
Thies F, Garry J, Yaqoob P, Rerkasem K, Williams J, Shearman C, Gallagher P, Calder P, Grimble R (2003) Association of n-3 polyunsaturated fatty acids with stability of atherosclerotic plaques: a randomised controlled trial. Lancet 361:477–485. https://doi.org/10.1016/S0140-6736(03)12468-3
de Lorgeril M, Salen P (2007) Mediterranean diet and n-3 fatty acids in the prevention and treatment of cardiovascular disease. J Cardiovasc Med 8:38–41. https://doi.org/10.2459/01.JCM.0000289268.90482.7b
Estruch R, Ros E, Salas-Salvadó J, Covas M, Corella D, Arós F, Gómez-Gracia E, Ruiz-Gutiérrez V, Fiol M, Lapetra J et al (2018) Primary Prevention of cardiovascular disease with a mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med 378:e34. https://doi.org/10.1056/NEJMoa1800389
Rajaram S (2014) Health benefits of plant-derived alpha-linolenic acid. Am J Clin Nutr 100:443–448. https://doi.org/10.3945/ajcn.113.071514
Rimm E, Appel L, Chiuve S, Djoussé L, Engler M, Kris-Etherton P, Mozaffarian D, Siscovick D, Lichtenstein A (2018) Seafood long-chain n-3 polyunsaturated fatty acids and cardiovascular disease: a science advisory from the American heart association. Circulation 138:e35-47. https://doi.org/10.1161/CIR.0000000000000574
Macartney M, Peoples G, McLennan P (2020) Cardiac arrhythmia prevention in ischemia and reperfusion by low-dose dietary fish oil supplementation in rats. J Nutr 150:3086–3093. https://doi.org/10.1093/jn/nxaa256
Schmidt E, Calder P (2020) Marine n-3 fatty acids, sudden cardiac death, and ischemic heart disease: fish or supplements? J Nutr 12:3055–3057. https://doi.org/10.1093/jn/nxaa319
Venø S, Schmidt E, Bork C (2019) Polyunsaturated fatty acids and risk of ischemic stroke. Nutrients 11:E1467. https://doi.org/10.3390/nu11071467
Bork CS, Venø SK, Lasota AN, Lundbye-Christensen S, Schmidt EB (2020) Marine and plant-based n-3 PUFA and atherosclerotic cardiovascular disease. Proc Nutr Soc 79:22–29. https://doi.org/10.1017/S0029665119000582
Ascherio A, Rimm E, Giovannucci E, Spiegelman D, Stampfer M, Willett W (1996) Dietary fat and risk of coronary heart disease in men: cohort follow up study in the United States. BMJ 313:84–90. https://doi.org/10.1136/bmj.313.7049.84
Mozaffarian D, Ascherio A, Hu F, Stampfer M, Willett W, Siscovick D, Rimm E (2005) Interplay between different polyunsaturated fatty acids and risk of coronary heart disease in men. Circulation 111:157–164. https://doi.org/10.1161/01.CIR.0000152099.87287.83
Vedtofte M, Jakobsen M, Lauritzen L, O’Reilly E, Virtamo J, Knekt P, Colditz G, Hallmans G, Buring J, Steffen L et al (2014) Association between the intake of alpha-linolenic acid and the risk of CHD. Br J Nutr 112:735–743. https://doi.org/10.1017/S000711451400138X
Hu F, Stampfer M, Manson J, Rimm E, Wolk A, Colditz G, Hennekens C, Willett W (1999) Dietary intake of alpha-linolenic acid and risk of fatal ischemic heart disease among women. Am J Clin Nutr 69:890–897. https://doi.org/10.1093/ajcn/69.5.890
Dolecek T (1992) Epidemiological evidence of relationships between dietary polyunsaturated fatty acids and mortality in the multiple risk factor intervention trial. Proc Soc Exp Biol Med 200:177–182. https://doi.org/10.3181/00379727-200-43413
Koh A, Pan A, Wang R, Odegaard A, Pereira M, Yuan J, Koh W (2015) The association between dietary omega-3 fatty acids and cardiovascular death: the Singapore Chinese health study. Eur J Prev Cardiol 22:364–372. https://doi.org/10.1177/2047487313517576
Albert C, Oh K, Whang W, Manson J, Chae C, Stampfer M, Willett W, Hu F (2005) Dietary alpha-linolenic acid intake and risk of sudden cardiac death and coronary heart disease. Circulation 112:3232–3238. https://doi.org/10.1161/CIRCULATIONAHA.105.572008
Tjønneland A, Olsen A, Boll K, Stripp C, Christensen J, Engholm G, Overvad K (2007) Study design, exposure variables, and socioeconomic determinants of participation in diet, cancer and health: a population-based prospective cohort study of 57,053 men and women in Denmark. Scand J Public Health 35:432–441. https://doi.org/10.1080/14034940601047986
Willett W, Stampfer M (1986) Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 124:17–27. https://doi.org/10.1093/oxfordjournals.aje.a114366
Tjønneland A, Overvad K, Haraldsdóttir J, Bang S, Ewerts M, Jensen O (1991) Validation of a semiquantative food frequency questionnaire developed in Denmark. Int J Epidemiol 20:906–912. https://doi.org/10.1093/ije/20.4.906
Schmidt M, Schmidt S, Sandegaard J, Ehrenstein V, Pedersen L, Sørensen H (2015) The Danish national patient registry: a review of content, data quality, and research potential. Clin Epidemiol 7:449–490. https://doi.org/10.2147/CLEP.S91125
Juel K, Helweg-Larsen K (1999) The Danish registers of causes of death. Dan Med Bull 46:354–357
Joensen A, Jensen M, Overvad K, Dethlefsen C, Schmidt E, Rasmussen L, Tjønneland A, Johnsen S (2009) Predictive values of acute coronary syndrome discharge diagnoses differed in the Danish national patient registry. J Clin Epidemiol 62:188–194. https://doi.org/10.1016/j.jclinepi.2008.03.005
Adams HJ, Bendixen B, Kappelle L, Biller J, Love B, Gordon D, Marsh E (1993) Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 24:35–41. https://doi.org/10.1161/01.str.24.1.35
Lühdorf P, Overvad K, Schmidt E, Johnsen S, Bach F (2017) Predictive value of stroke discharge diagnoses in the Danish national patient register. Scand J Public Health 45:630–636. https://doi.org/10.1177/1403494817716582
Lammie G (2000) Pathology of small vessel stroke. Br Med Bull 56:296–306. https://doi.org/10.1258/0007142001903229
Lasota A, Overvad K, Eriksen H, Tjønneland A, Schmidt E, Grønholdt M (2017) Validity of peripheral arterial disease diagnoses in the Danish national patient registry. Eur J Vasc Endovasc Surg 53:679–685. https://doi.org/10.1016/j.ejvs.2016.12.031
Regression HF, Strategies M (2015) With applications to linear models, logistic and ordinal regression, and survival analysis. Springer, Second Edi. New York
Greenland S, Senn S, Rothman K, Carlin J, Poole C, Goodman S, Altman D (2016) Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol 31:337–350. https://doi.org/10.1007/s10654-016-0149-3
Rhee J, Kim E, Buring J, Kurth T (2017) Fish consumption, omega-3 fatty acids, and risk of cardiovascular disease. Am J Prev Med 52:10–19. https://doi.org/10.1016/j.amepre.2016.07.020
Siscovick DS, Barringer TA, Fretts AM, Wu JHY, Lichtenstein AH, Costello RB, Kris-Etherton PM, Jacobson TA, Engler MB, Alger HM et al (2017) Omega-3 polyunsaturated fatty acid (fish oil) supplementation and the prevention of clinical cardiovascular disease: a science advisory from the American heart association. Circulation 135:e867–e884. https://doi.org/10.1161/CIR.0000000000000482
Bork C, Mortensen L, Hjelmgaard K, Schmidt E (2020) Marine n-3 fatty acids and CVD: new insights from recent follow-up studies and clinical supplementation trials. Proc Nutr Soc. https://doi.org/10.1017/S0029665120006886d
Kalstad A, Myhre P, Laake K, Tveit S, Schmidt E, Smith P, Nilsen D, Tveit A, Fagerland M, Solheim S et al (2021) Effects of n-3 fatty acid supplements in elderly patients after myocardial infarction: a randomized controlled trial. Circulation 143:528–539. https://doi.org/10.1161/CIRCULATIONAHA.120.052209
Nicholls S, Lincoff A, Garcia M, Bash D, Ballantyne C, Barter P, Davidson M, Kastelein J, Koenig W, McGuire D et al (2020) Effect of high-dose omega-3 fatty acids vs corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA 324:2268–2280. https://doi.org/10.1001/jama.2020.22258
Hu Y, Hu FB, Manson JE (2019) Marine omega-3 supplementation and cardiovascular disease: an updated meta-analysis of 13 randomized controlled trials involving 127 477 participants. J Am Heart Assoc 8:e013543. https://doi.org/10.1161/JAHA.119.013543
Bhatt D, Steg P, Miller M, Brinton E, Jacobson T, Ketchum S, Doyle R, Juliano R, Jiao L, Granowitz C et al (2019) Cardiovascular Risk reduction with icosapent ethyl for hypertriglyceridemia. N Engl J Med 380:11–22. https://doi.org/10.1056/NEJMoa1812792
Manson JE, Cook NR, Lee I-M, Christen W, Bassuk SS, Mora S, Gibson H, Albert CM, Gordon D, Copeland T et al (2019) Marine n-3 fatty acids and prevention of cardiovascular disease and cancer. N Engl J Med 380:23–32. https://doi.org/10.1056/NEJMoa1811403
Calder PC (2012) The role of marine omega-3 (n-3) fatty acids in inflammatory processes, atherosclerosis and plaque stability. Mol Nutr Food Res 56:1073–1080. https://doi.org/10.1002/mnfr.201100710
Funding
The Danish Cancer Society funded the Diet, Cancer and Health cohort. The current study was financially supported by Health Research Foundation of North Denmark Region. The funding agency had no role in the current research.
Author information
Authors and Affiliations
Contributions
The authors’ responsibilities were as follows; AT and KO: conceived the study concept and contributed to the data acquisition; CSB: contributed to the study design and planning of the statistical analyses, interpretation of data and conducted the statistical analyses, prepared the tables and figures, and wrote the manuscript; SKV, ANL, SLC, EBS, and KO: contributed to the study design and planning of the statistical analyses, interpretation of data, and writing of the manuscript; SLC: supervised the conduct of the statistical analyses; AT: contributed to the critical interpretation of the manuscript. CSB: had the primary responsibility for the final content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Bork, C.S., Lundbye-Christensen, S., Venø, S.K. et al. Intake of marine and plant-derived n-3 fatty acids and development of atherosclerotic cardiovascular disease in the Danish Diet, Cancer and Health cohort. Eur J Nutr 62, 1389–1401 (2023). https://doi.org/10.1007/s00394-022-03081-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-022-03081-w