Abstract
Purpose
Maternal nutrition during early development and paternal nutrition pre-conception can programme offspring health status. Hypothalamus adipose axis is a target of developmental programming, and paternal and maternal high-fat, high-sugar diet (HFS) may be an important factor that predisposes offspring to develop obesity later in life. This study aims to investigate Wistar rats’ maternal and paternal HFS differential contribution on the development, adiposity, and hypothalamic inflammation in male offspring from weaning until adulthood.
Methods
Male progenitors were fed a control diet (CD) or HFS for 10 weeks before mating. After mating, dams were fed CD or HFS only during pregnancy and lactation. Forming the following male offspring groups: CD—maternal and paternal CD; MH—maternal HFS and paternal CD; PH—maternal CD and paternal HFS; PMH—maternal and paternal HFS. After weaning, male offspring were fed CD until adulthood.
Results
Maternal HFS diet increased weight, visceral adiposity, and serum total cholesterol levels, and decreased hypothalamic weight in weanling male rats. In adult male offspring, maternal HFS increased weight, glucose levels, and hypothalamic NFκBp65. Paternal HFS diet lowered hypothalamic insulin receptor levels in weanling offspring and glucose and insulin levels in adult offspring. The combined effects of maternal and paternal HFS diets increased triacylglycerol, leptin levels, and hypothalamic inflammation in weanling rats, and increased visceral adiposity in adulthood.
Conclusion
Male offspring intake of CD diet after weaning reversed part of the effects of parental HFS diet during the perinatal period. However, maternal and paternal HFS diet affected adiposity and hypothalamic inflammation, which remained until adulthood.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Epidemiological studies have long demonstrated that an adverse nutritional environment during the perinatal period can cause alterations in organs during development, programming a higher susceptibility to develop obesity and metabolic diseases later in life [1,2,3]. Maternal modified diet during pregnancy and lactation, the most important period of growth and development, has been shown to programme the development of offspring [4,5,6,7]. Maternal high-fat diet during pregnancy and lactation has shown to affect neurodevelopment and somatic programming in the offspring, increasing the risk of metabolic and neuro disorders in adulthood [8, 9] and alter foetal development [10, 11]. Some studies also showed pre-conception maternal HF diet effect on the offspring, highlighting the importance of women’s diet and health before pregnancy [12, 13]. A recent study has considered the impact of maternal HFS diet from pre-conception to pregnancy. However, the effect of maternal HFS during pregnancy seems to be more pronounced [14]. Moreover, experimental studies that investigated paternal programming focused on pre-mating HFD diets known to affect sperm function [15]. Spermatogenesis disturbance by a paternal high-fat diet in rats was shown to change paternal sperm epigenetic profile, which can be transferred to offspring and confer susceptibility to altered metabolic phenotype [16]. Maternal and paternal diets can influence the epigenome of female and male mice offspring, which then determines their phenotype [17, 18].
The hypothalamic pathway to control energy homeostasis is one of the important targets of developmental programming [19]. Hypothalamus adipose tissue axis is involved in the maintenance of energy homeostasis by regulating energy intake and adiposity levels. The hypothalamus is a region in the brain that regulates hunger and satiety by communicating with peripheral organs to control energy homeostasis [20]. Hypothalamic inflammation induced by a high-fat diet has shown to disrupt normal regulatory mechanisms of energy balance and lead to weight gain and increased adiposity in male mice [21,22,23]. In female mice, high-fat diet intake during pregnancy has been shown to alter hypothalamic neurogenesis, with a preference for orexigenic neurons which cause hyperphagia and predisposed male and female offspring to obesity and metabolic disorders [24, 25]. Male rat offspring of dams fed a high-fat diet also showed lower hypothalamic anorexigenic signalling and higher expression of the orexigenic neuropeptide Y (NPY) Y1 receptors, resulting in hyperphagia that had long-lasting effects [26]. In male mice, a high-fat diet to induce obesity before mating has been shown to influence hypothalamic inflammation, but did not cause hyperphagia and weight gain in male offspring. However, the induction of maternal and paternal obesity by the consumption of 45% high-fat diet pre-mating showed that the combined maternal and paternal effects exacerbated the effect of each parent, leading to hypothalamic inflammation and disturbed hypothalamic leptin signalling and metabolic alterations in the offspring [27].
A study in humans found a stronger relationship between paternal BMI and son rather than father–daughter relationship [28]. Another study found that paternal BMI was a predictor of offspring BMI independently of the offspring’s gender [29]. An animal study in rats also associated paternal HFD with male offspring obesity, but not female offspring [30]. Paternal HFD seems to alter glucose homeostasis and programme β-cell dysfunction in females, but not in male rat offspring [31]. Some programming studies showed paternal adverse effects on male offspring, especially the additive negative impact of the paternal and maternal unbalanced diets on the offspring [32,33,34]. There are differences in the programming effect of paternal and maternal modified diets concerning offspring gender. However, our study objective is to investigate parental high-fat, high-sugar diet effect on development, adiposity, and hypothalamic inflammation of offspring from an early age until adulthood, with a focus on male offspring. We hypothesise that parental diet could programme the hypothalamus adipose tissue axis and predispose male offspring to develop obesity later in life.
Materials and methods
Animals and diet
Progenitors
Male Wistar rats (8-week-old) were treated with a modified diet prior to mating for 10 weeks, aiming to promote weight gain and affect programming as previously shown [31]. Female rats (10-week-old) were treated with a modified diet during gestation and lactation, a critical period of growth and development, sensitive to environmental cues such as maternal nutrition [35]. Males (n = 23) and females (n = 23) were randomly allocated to CD (15.5% fat and 50% carbohydrates from kcal, Nuvilab CR1, Quimtia®) or HFS (32% and 50% carbohydrates from kcal of which 25% sugar).
Males after 10 weeks of treatment (CD n = 12 and HFS n = 11) were kept with the females in the same cage overnight to mate, and copulation was verified the following morning by the presence of sperm in vaginal smears. After the confirmation of copulation, females were fed HFS (n = 12) or CD diets (n = 11) during gestation and lactation. Males were maintained in polythene cages in groups of three, and females were individually housed. They were obtained from Centro de Desenvolvimento de Modelos Experimentais (CEDEME) of Universidade Federal de São Paulo (UNIFESP). Male progenitors were euthanised after mating and female progenitors were euthanised on postnatal day 21.
Male offspring
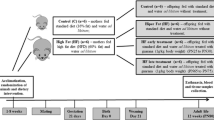
Male offspring groups were formed according to their progenitors’ diets: CD—maternal and paternal CD diet (6 litters); MH—maternal HFS and paternal CD diet (6 litters); PH-maternal CD and paternal HFS diet (5 litters); PMH—maternal and paternal HFS (6 litters) (Fig. 1). On postnatal day 1, litter size was adjusted to eight male pups for each dam; when the dam did not produce eight males, the litter size was completed with female offspring. On postnatal day 21, part of the group was euthanised, and another part was fed a control diet until 90 days old. After weaning, male offspring were housed in polythene cages in groups of four per cage.
Schematic representation of the study design. Offspring groups: CD—maternal and paternal CD diet (6 litters); MH—maternal HFS and paternal CD diet (6 litters); PH–maternal CD and paternal HFS diet (5 litters); PMH—maternal and paternal HFS (6 litters). The number of offspring per litter used in the experimental analyses were 1–2 per litter
Progenitors and offspring rats had food and water ad libitum. Animal’s food intake was measured weekly. All animals were kept on a 12:12 h light/dark cycle and controlled temperature (22 °C ± 2 °C). Animals were euthanised in the morning with inhalation of the anaesthetic isoflurane in a soaked pad, followed by decapitation as recommended by the guidelines in the practice of euthanasia of Conselho Nacional de Controle de Experimentação Animal CONCEA (2015). Anaesthetic isoflurane was chosen for its lower interference with metabolic results.
The HFS diet was made with sweetened condensed milk (Nestlè®) that is the main source of sugar, and lard as the main source of fat, adapted from Sferruzzi-Perri [10]. Additional micronutrients (vitamin and mineral mix, Rhoster®) were added to avoid micronutrient deficiencies [36]. Additional choline bitartrate and L-cysteine were added to match the amount in the CD diet, which are essential nutrients for normal animal growth [37].
Centesimal diet composition
For the centesimal composition of each diet sample (HFS and CD), the moisture, ashes, lipids, proteins, and fibre levels were quantified. The total amount of carbohydrates was determined from the difference.
To determine moisture, 10 g of sample was heated in a chamber for 2 h at 70 °C, cooled in a desiccator, and weighed.
To obtain ashes, 3 g of sample was carbonised over a Bunsen burner, placed in a muffle furnace, and heated to 500 °C for 5–6 h. Ash content was determined by weight difference.
To quantify lipid content, the dried sample was placed on a cellulose cartridge and transferred to the Soxhlet extractor with petroleum ether for 8 h. Reboiler glass was used to retain the fat extracted and heated to 105 °C for 1 h. The lipid content was calculated by the difference between the initial and final weight of the reboiler glass.
Protein measurements were carried out with 70 mg of sample, placed on vegetal paper and transferred to a Kjeldahl tube. An additional 5 mL of sulphuric acid and 0.2 g of a catalytic mixture were added. Tubes were heated in the digester to 375 °C for 3 h until the solution showed a limpid, greenish-blue colour. Tubes containing the digested sample were transferred to the Kjeldahl distillation system and added 5 mL of distilled water and 20 mL of NaOH 40%. The mixture was distilled and the distillate collected into 10 mL of 4% boric acid solution containing two to three drops of an indicator mix. The distillate was titrated with a solution of hydrochloric acid 0.02 M until the turning point characterised by a pinkish colouration was reached.
Soluble and insoluble fibres were determined with 1.000 ± 0.005 mg of sample. This method is the combination of the enzymatic action and the separation of digestible compounds under physical conditions (temperature and pH) to achieve the fraction of the non-digested food (Table 1).
Ethical approval
None of the procedures in this experimental study caused distress or suffering to the animals. All the animals were maintained according to the Conselho Nacional de Controle de Experimentação Animal CONCEA (2015). This research was approved by the ethics committee of animals use of UNIFESP, CEUA no. 822619057, which approved the use and care of all the animals in the study.
Anthropometric analysis and adiposity
Individual animal body weight (BW) and naso-anal length (NAL) were recorded weekly. Naso-anal length is a measure of the linear growth, and was measured using a ruler to assess nasal to anal distance to the nearest 1.0 mm [38]. Weight gain was calculated by the final BW subtracted from the initial BW. Metabolic efficiency was calculated by weight gain (g)/food intake (g) [39]. Male and female progenitors and 90-day-old offspring were fasted for 10–12 h prior to euthanasia and the 21-day-old offspring were not fasted to avoid weaning stress. The trunk blood was collected after decapitation to obtain serum, centrifuged at 2500 rpm, 4 °C for 15 min, frozen (− 80 °C), and stored for further analysis. Male and female progenitors’ visceral adipose tissue, retroperitoneal (RET), mesenteric (MES) and epididymal (EPI) tissue were dissected and frozen (− 80 °C) for further analysis. The relative organ weights of 21- and 90-day-old offspring were assessed following dissection at postmortem, for adipose depots (MES, RET and EPI), and hypothalamus. The organs were frozen and stored (− 80 °C) for further analysis.
Biochemical analysis
Serum glucose, total cholesterol and triacylglycerol (TAG) levels were measured by an enzymatic colorimetric method, using the commercial kits by following the manufacturer’s instructions (Labtest®, Lagoa Santa, MG, Brazil).
ELISA immunoassay
To quantify tumour necrosis factor α (TNF-α), interleukin 6 (IL-6) and interleukin 10 (IL-10) cytokines in the hypothalamus, hypothalamic protein extracts from a whole hypothalamus were used to perform commercial kit ELISA immunoassay protocol according to the manufacturer’s instructions (Duo Set ELISA, R&D Systems, Minneapolis, MN, USA). To quantify serum leptin and insulin, the commercial kit ELISA immunoassay (Millipore Corporation, St. Charles, Missouri, USA) (insulin code EZRMI-13K and leptin code EZRL-83K) were used according to the manufacturer’s instructions.
Tissue protein extraction
Whole hypothalamic samples were homogenized in buffer containing 100 mM Tris–HCl, 10% Triton X-100, 10% sodium dodecyl sulphate (SDS), 100 mM EDTA, 100 mM sodium fluoride, 10 mM sodium pyrophosphate, 10 mM sodium orthovanadate, 2 mM phenylmethylsulphonyl fluoride, and 0.1 mg/ml aprotinin. The homogenised samples were centrifuged at 14,000 rpm for 40 min at 4 °C, and the supernatant was obtained. The samples’ total protein was measured using Bradford reagent by colorimetric assessment at the wavelength of 595 nm.
Western blotting
Protein samples of whole hypothalamus were separated by electrophoresis on a 10% SDS polyacrylamide gel and transferred to a nitrocellulose membrane. To block nonspecific proteins, 5% dried skimmed milk solution was used for 1 h at room temperature. The membrane was incubated overnight with the primary antibodies MYD88 (1:10,000—Abcam, Cambridge, UK), p-IKKα + β (Abcam-1:5000), p-NFκBp65 (Abcam-1:5000), β-actin (Abcam-1:10,000), Insulin R-beta (1:1000—St Cruz Biotechnology, Inc., Santa Cruz, CA, USA), followed by incubation with horseradish peroxidase-conjugated secondary antibodies Rabbit (Abcam-1:20,000) for 1 h at room temperature. Enhanced chemiluminescence images of the membrane after adding ECL reagent (Thermo Fisher Scientific, Waltham, MA, USA) were developed through UVITec (Cambridge, UK). Bands were assessed, and their intensity was quantified by Scion Image software (Scion Image-Release Beta 3b; NIH, Frederick, MD, USA). Calculations of the target protein were normalised to β-actin levels.
Statistical analysis
Data were expressed as mean and the standard error of the mean (SEM). The normality test used was the Shapiro–Wilk test. Outliers were identified by rout test. Maternal and paternal data were analysed by t test (parametric), or by U Mann–Whitney test (non-parametric) or ANOVA for repeated measures. Offspring data were assessed by two-way ANOVA or for repeated measures for parametric data. Non-parametric data were assessed by Scheirer–Ray–Hare test. Post hoc test used was Bonferroni. Statistical analysis was performed with the software JASP 0.12.1.0 with a minimum significance level of p ≤ 0.05. Correlation analysis was conducted using Pearson’s test. Sample size was calculated using R version 3.5.0, statistical power considered was 90% and two-sided level was 0.05. Calculations of sample size considered animal weight and adiposity previously reported in Wistar rats on high-fat high-sugar and control diets. Cohen’s test was also undertaken to check the effect size. Sample size considering animal’s body weight was calculated 11 per each group with a large effect size d = 0.78.
Results
Paternal and maternal results
Paternal and maternal body parameters and diet intake
Our results showed that male progenitor fed a high-fat high-sugar diet (HFS) had significantly higher body weight (BW), compared to control (CD) group, from the second week to the end of treatment (S1A). Weight gain in the HFS group was significantly higher than that in the CD (S1B). Paternal HFS diet intake in grams was lower compared to CD (S1C). Female progenitor in the HFS group had higher body weight compared to those in the CD group, from the first week of gestation until the end of lactation (S2A). Weight gain was higher in the HFS group only during pregnancy (S2B). Maternal HFS diet intake in grams was not different compared to that in CD (S2C).
Paternal and maternal metabolic efficiency and adiposity
As shown in S1 D, paternal metabolic efficiency was significantly higher in the HFS group compared to CD, whereas maternal metabolic efficiency was higher in the HFS during pregnancy (S2 D). S1 E and F shows differences in the visceral adiposity between the paternal groups. Total visceral adipose tissue (VAT) and retroperitoneal (RET) adipose tissue depot were significantly higher in the HFS group compared to CD (S1 E and F). Maternal adiposity showed higher RET and VAT in the HFS compared to CD group (S2 E and F).
Offspring results
Body parameters, adiposity and hypothalamus weight of 21- and 90-day-old offspring, metabolic efficiency and food intake after weaning until 90 days old
Investigating the effect of paternal and maternal diet on the 21- and 90-day-old offspring, our results showed that the main effect on the body weight (BW) and naso-anal length (NAL) progress was related to maternal HFS diet and time. Offspring BW and NAL were increased by maternal HFS diet on days 14 and 21 of life and remained until adulthood (Fig. 2 A and B).
The effect of a paternal and maternal high-fat high-sugar diet on the body parameters of the 21- and 90-day-old offspring, food intake and metabolic efficiency of 90-day-old offspring. A Body weight progress from birth until adulthood (g). B Naso-anal length (NAL) (cm) from birth until adulthood. C Weight gain (final weight–initial weight) (g) of 21-day-old offspring. D Weight gain (final weight–initial weight) (g) of 90-day-old offspring. E Metabolic efficiency, weight gain (g)/food intake (g) of 90-day-old offspring. F Food intake (g) of 90-day-old offspring. G Food intake (Kcal) of 90-day-old offspring. H Profile intake in grams/rat/week from weaning until adulthood. I Profile intake in kcal/rat/week from weaning until adulthood. Data are expressed as mean ± SEM, and + p ≤ 0.05 was considered statistically significant for the effect of the maternal diet. Groups: CD—maternal and paternal CD diet; MH—maternal HFS and paternal CD diet; PH—maternal CD and paternal HFS diet; PMH—maternal and paternal HFS
Maternal HFS diet also had a significant effect on weight gain from birth until weaning (Fig. 2 C), but did not have a significant effect on weight gain post-weaning until 90-day-old (Fig. 2 D). Moreover, parental diet did not affect metabolic efficiency or food intake from weaning until adulthood (Fig. 2 E, F, G, H and I).
Moreover, maternal HFS diet had a significant effect on the accrued adipose depots of the 21-day-old offspring, such as RET, EPI and total VAT. However, the main effect on the adiposity observed on the 90-day-old offspring was the combined maternal and paternal HFS diet effect on the RET, EPI and total VAT. Parental diet did not show any significant effect on MES of 21- and 90-day-old offspring. Maternal HFS diet showed a reduction effect on the hypothalamic weight of the 21-day-old offspring, which was reversed in the 90-day-old offspring (Table 2).
Serum levels of glucose, total cholesterol and triacylglycerol, and serum levels of leptin and insulin
As shown in Table 2, serum levels of glucose were not affected by parental diet in 21-day-old offspring. However, serum triacylglycerol levels (TAG) were increased when both parents were fed HFS. Serum cholesterol levels showed a significant isolated effect of maternal HFS diet. On the other hand, isolated effects of maternal and paternal diet showed a significant effect on serum levels of glucose of 90-day-old offspring. However, serum TAG and total cholesterol were not affected by parental diet after weaning. Higher serum leptin levels in the 21-day-old offspring were affected by the combined effects of maternal and paternal HFS diet. In the 90-day-old offspring, paternal HFS diet showed an influence on lower levels of insulin. Leptin levels were shown to be rescued in the 90-day-old offspring (Table 2). The consumption of CD after weaning may have reversed the parental diet effect on the 21-day-old offspring’s serum TAG, total cholesterol and leptin levels.
Hypothalamic protein levels of TNF-α, IL-6 and IL-10 and protein content of myeloid differentiation factor (MYD88), p-IKKα + β, NFκBp65 and INSULIN RECEPTOR beta subunit (β-IR) of 21- and 90-day-old offspring
Hypothalamic cytokine levels in the 21-day-old offspring were affected by parental diet (Fig. 3). Maternal HFS influenced higher hypothalamic interleukin 6 (IL-6) levels (Fig. 3 A). The combined effects of maternal and paternal HFS showed increased levels of hypothalamic TNF-α (Fig. 3 B). Isolated effects of paternal and maternal HFS diet showed higher hypothalamic IL-10 levels in the 21-day-old offspring (Fig. 3 C). However, hypothalamic cytokines (IL-6, TNF-α and IL-10) did not show a significant parental diet effect on the 90-day-old offspring (Fig. 3 D, E and F). The consumption of CD after weaning may have reversed the parental diet effect on the 21-day-old offspring’s hypothalamic inflammation.
The effect of paternal and maternal high-fat high-sugar diet on the hypothalamic levels of cytokines in the 21- and 90-day-old offspring. A Interleukin 6 (IL-6) (pg/mg) of 21-day-old offspring. B Tumour necrosis factor α (TNF-α) (pg/mg) of 21day-old offspring. C Interleukin 10 (IL-10) (pg/mg) of 21-day-old offspring. D IL-6 (pg/mg) of 90-day-old offspring. E TNF-α (pg/mg) of 90-day-old offspring. F. IL-10 (pg/mg) of 90-day-old offspring. Data are expressed as mean ± SEM and + p ≤ 0.05 was considered statistically significant for the effect of maternal diet; §p ≤ 0.05 was considered statistically significant for the effect of paternal diet. For the interaction effect between maternal and paternal diet, *p ≤ 0.05 compared to CD; &p ≤ 0.05 compared to MH; #p ≤ 0.05 compared to PH. Groups: CD—maternal and paternal CD diet; MH—maternal HFS and paternal CD diet; PH—maternal CD and paternal HFS diet; PMH—maternal and paternal HFS
Protein expression of p-IKKα + β, NFκBp65 and MYD88 in the hypothalamus of 21-day-old offspring showed no influence of parental diet (Fig. 4 A, C and E, respectively). However, paternal HFS diet lowered the protein levels of β-IR in the hypothalamus of 21-day-old offspring (Fig. 4 G). Protein levels of p-IKKα + β, MYD88 and β-IR in the hypothalamus of 90-day-old offspring showed no influence of parental diet (Fig. 4 B, F and H, respectively). However, the isolated effect of maternal HFS diet showed higher protein levels of NFκBp65 in the hypothalamus of 90-day-old offspring (Fig. 4 D).
The effect of paternal and maternal high-fat high-sugar diet on the hypothalamic protein levels of the 21- and 90-day-old offspring. A p-IKKα + β (% of CD) of 21-day-old offspring. B p-IKKα + β (% of CD) of 90-day-old offspring. C Nuclear factor-κB subunit 65 (NFκBp65) (% of CD) of 21-day-old offspring. D NFκBp65 (% of CD) of 90-day-old offspring. E Myeloid differentiation protein (MYD88) (% of CD) of 21-day-old offspring. F. MYD88 (% of CD) of 90-day-old offspring. G Insulin receptor subunit β (β-IR) (% of CD) of 21-day-old offspring. H β-IR (% of CD) of 90-day-old offspring. Data are expressed as mean ± SEM and + p ≤ 0.05 was considered statistically significant for the effect of maternal diet; §p ≤ 0.05 was considered statistically significant for the effect of paternal diet. Groups: CD—maternal and paternal CD diet; MH—maternal HFS and paternal CD diet; PH—maternal CD and paternal HFS diet; PMH—maternal and paternal HFS
Correlations between parental and 21- and 90-day-old offspring parameters
Maternal parameters such as adiposity, RET, MES, BW gain and metabolic efficiency correlated with offspring parameters such as BW gain, RET, EPI, VAT, hypothalamus weight, hypothalamic IL-6 and TNF-α, serum TAG and total cholesterol of 21-day-old offspring (Table 3). Moreover, maternal parameters such as weight gain during pregnancy correlated with offspring VAT and hypothalamic weight, and maternal metabolic efficiency during pregnancy correlated with 90-day-old offspring adiposity (RET, MES and VAT) (Table 3). Multiple regression analysis showed that maternal metabolic efficiency during gestation is a dependent predictor of the combined effect of maternal and paternal diet on RET weight of the 90-day-old offspring (β = 1.875; p = 0.048; 95% CI = 0.021–3.729). Also, maternal weight gain during gestation is a dependent predictor of the maternal diet on total cholesterol levels of 21-day-old offspring (β = 0.361; p = 0.049; 95% CI = 0.001–0.721).
Discussion
Only a few studies have discussed the role of both maternal and paternal dietary effects on developmental programming. However, the effect of both parents could modify the phenotype of the offspring [40]. This study is novel as it is the first, to our knowledge, to discuss the effects of a parental high-fat high-sugar diet on the development, adiposity, and hypothalamic inflammation of male offspring from an early age until adulthood.
Maternal and paternal parameters, besides diet itself, could be a factor per se involved in the developmental programming [41,42,43]. Our results showed that maternal and paternal body parameters differed between groups; both parents showed higher body weight progress and higher adiposity during treatment with HFS diet, which could be explained by the higher metabolic efficiency. Metabolic efficiency is how efficiently the body gains weight per food intake [39]. Higher metabolic efficiency means one stores more substrates from the food consumed [44]. Higher metabolic efficiency favours a thrifty phenotype and can be an evolutionary advantage to aid survival over food shortage periods. However, in western societies, food is easily accessible, and higher metabolic efficiency raises the risk of developing obesity. Previous studies have also shown a relationship between fat intake and metabolic efficiency [45, 46]. Higher metabolic efficiency in adult rats fed HFD short term was associated with impaired oxidative capacity in isolated mitochondria [47]. Also, an increased metabolic efficiency might be a result of suppression in the thermogenesis due to the reduced intracellular activity of thyroid hormone triiodothyronine (T3) [48] or thyroid dysfunction induced by a high-fat diet [49]. Thyroid hormones are involved in the non-shivering thermogenesis, which involves mitochondrial uncoupling of oxidation of substrates during ATP production, dissipating energy as heat [50]. Mitochondrial respiratory efficiency is given by the coupling ratio between ATP and oxygen (ATP/O) [51]. A pivotal protein involved in the mitochondrial uncoupling of ATP/O is the uncoupling protein 1 (UCP1). The consumption of a high-fat diet showed an inverse association with the UCP-1 expression in white adipose tissue [52]. Moreover, UCP-1 deficiency has been shown to induce de novo lipogenesis in white adipose tissue [53], which could explain the increased adipose tissue in the progenitors fed the HFS diet in our study.
Maternal parameters such as RET weight, body weight gain during pregnancy and lactation, and metabolic efficiency correlated with 21- and 90-day-old offspring parameters. However, multiple regression analysis showed parental diet was an independent predictor of the offspring parameters studied, except for maternal metabolic efficiency and weight gain during gestation. Maternal metabolic efficiency during gestation showed to be a dependent predictor of the combined effects of maternal and paternal diet on RET weight of the 90-day-old offspring. Moreover, maternal body weight gain during gestation was a dependent predictor of the maternal diet on the total cholesterol levels of 21-day-old offspring.
Parental diet effect on offspring parameters showed that only maternal HFS diet influenced offspring’s higher BW progress, growth in length, and weight gain until weaning. The impact of maternal nutrition on BW progress and growth in length remained until young adulthood. Similar to our results, maternal high-fat diet during pregnancy and lactation has not been shown to affect offspring birth weight, but caused increased offspring body weight at weaning, final body weight, and increased visceral adiposity. These changes were associated especially with maternal high-fat diet during lactation [54]. However, maternal diet did not affect the offspring’s BW gain after weaning on a control diet, showing that the maternal HFS diet effect on higher BW progress after weaning could be due to an early in life programming for a higher body weight set point. There is evidence of a preset biological control of body weight to maintain weight stability. Western diet could upregulate the body weight set point, making it harder for the individual to lose weight, even eating a healthy diet [55]. Perinatal nutrition may be an important factor in the offspring’s body weight set point [20, 56]. It seems that the hypothalamus adipose axis has an essential role in maintaining body weight and adiposity levels [20].
Despite that offspring from dams fed HFS maintained a higher BW progress until adulthood, weaning on a healthy diet may have overridden the early effect of maternal HFS diet on offspring BW gain, as after weaning, offspring BW gain did not show any influence of maternal diet. Offspring food intake and metabolic efficiency after weaning on the control diet were also not affected by parental diet. Therefore, the maintenance of higher body weight after weaning could be explained by an adjustment in energy expenditure, as the animals did not consume more food to maintain higher body weight. Individuals with a thrifty phenotype have been shown to lose less weight when submitted to caloric restriction by a greater decrease in energy expenditure [57].
Increased adipose tissue mass is a characteristic of obesity and is associated with metabolic alterations and predisposition to the development of metabolic diseases. Adipocyte number (hyperplasia) is determined early in life and tends to be stable during adulthood [58]. In our study, maternal HFS diet influenced increased adipose accrual of retroperitoneal and epididymal depots early in life. The combined effects of maternal and paternal HFS diet also influenced the offspring increased adiposity at 90-day-old, which could confer a higher predisposition to the development of metabolic diseases, especially if challenged with an obesogenic environment throughout their life.
High serum TAG levels were shown to be influenced by the synergic effect of paternal and maternal HFS at weaning. Maternal HFS diet also affected increased cholesterol levels, but parental diet did not affect glycaemia of 21-day-old offspring. However, in our study, dyslipidaemia was reversed by the intake of a normal diet after weaning. The control diet used is particularly high in fibre, which has proven powerful effects on the improvement of lipaemia levels [59]. In fact, a strong negative correlation was found between control diet intake after weaning and serum TAG levels (r = − 0.506, p = 0.001). Therefore, the control diet was possibly able to reverse the negative effects of parental HFS on dyslipidaemia. However, serum glucose levels were influenced by maternal and paternal HFS diets at 90-day-old, indicating that the intake of a healthier diet after weaning was able to reverse only part of the metabolic alterations. Epigenetic alterations are a critical mechanism of programming, and early-life nutrition may permanently set epigenetic marks, such as DNA methylation, which can change the gene expression of genes involved in cell differentiation and glucose metabolism. Studies in rats and mice have previously shown maternal high-fat diet during pregnancy and lactation and alterations in the DNA methylation in male offspring. Paternal high-fat diet pre-conception has also been shown to alter DNA methylation in genes involved in glucose metabolism in female rat offspring. However, epigenetic changes may also be reversible with interventions early in life, and this may explain our results. The consumption of a healthier diet soon after weaning might have been early enough to reverse some of the alterations seen on the 21st day of life [35].
Perinatal nutrition, during pregnancy and lactation, in rodents is a critical period for the maturation of the hypothalamic neurons and accrued adiposity [20]. The higher adiposity found in the offspring may relate to the maternal HFS diet effect on lower hypothalamic weight at 21 days of age. Lower hypothalamic weight may be linked to apoptosis of hypothalamic neurons caused by the maternal HFS diet during pregnancy and lactation [22, 60]. However, a plausible explanation may be one of the mechanisms of foetal programming on changes in the organ development, structure, and tissue volume by a maternal HFS diet, which may, in turn, change organ function permanently [61]. Moreover, maternal high-fat diet during pregnancy and lactation has shown to influence hypothalamic neurogenesis, favouring orexigenic neurons, which could influence food intake and the development of obesity [24]. Indeed, a negative correlation between hypothalamic weight and weight gain has been found in the 21-day-old offspring (r = − 0.535; p < 0.001).
Dysfunction or loss of hypothalamic neurons in the arcuate nucleus (ARH) is associated with the development of obesity and metabolic disorders. Hypothalamic inflammation is a crucial mechanism linked to the overconsumption of a fatty diet [62]. ARH is close to the median eminence, a region where the blood–brain barrier is leaky, and circulating nutrients are easily accessible. Buildup of excessive amounts of fatty acids in the ARH generates microglia inflammatory response and the release of pro-inflammatory cytokines such as IL-6 and TNF-α [63]. Our results showed maternal HFS diet led to increased hypothalamic pro-inflammatory cytokine. IL-6 levels in the 21-day-old offspring were positively correlated to the visceral adiposity. In particular, a strong correlation has been found with retroperitoneal adipose tissue (r = 0.509; p = 0.002). Moreover, maternal and paternal HFS diets showed synergic effects on higher TNF-α levels in the 21-day-old offspring. As with IL-6, the hypothalamic TNF-α showed a positive relationship with visceral adiposity, particularly with RET (r = 0.483; p = 0.003). Anti-inflammatory IL-10 was also found to be higher in the offspring from the maternal and paternal HFS diets. IL-6 and TNF-α are expressed by IKKβ/NFκB activation and antagonised by the co-expression of IL-10, the primary inhibitor of IKKβ/NFκB activation [40]. In vitro study showed that after macrophages were stimulated with lipopolysaccharide (LPS), there was a surge in TNF-α after 1 h followed by IL-10 after 10 h, demonstrating that during the inflammatory process, there is also an increase in the anti-inflammatory cytokine IL-10, possibly to minimise tissue damage caused by inflammation itself [64]. However, our results showed the consumption of a control diet after weaning until young adulthood was able to reverse the early life maternal and combined parental diet effects on the hypothalamic weight and hypothalamic cytokine levels, respectively.
Transmembrane receptor toll-like receptor 4 (TLR-4) is a pattern recognition receptor (PRR) present in immune cells and has been implicated in diet-induced inflammation. A high-fat diet rich in saturated fatty acids (SFAs) may activate TLR4 signalling indirectly by affecting their recruitment to lipid rafts in the cell membrane [65]. Induction of TLR4 signalling has been implicated as one of the main pathways leading to the activation of NFκB and the expression of hypothalamic pro-inflammatory cytokines in response to a high-fat diet [23]. However, hypothalamic proteins involved in the TLR4 pathway (MYD88, NFκBp65 and IKKαβ) were not affected by parental diet in the 21-day-old offspring. Moreover, IL-10-dependent microRNA-146b is a negative switch of TLR4 and adaptor proteins such as MYD88; therefore, it inhibits TLR4 signalling [66]. Considering increased IL-10 has been found in the 21-day-old offspring when both parents were fed HFS, this could explain the normal levels of TLR4 pathway proteins in these animals. However, higher protein levels of hypothalamic NFκBp65 were shown to be influenced by maternal HFS diet in the 90-day-old offspring, even though hypothalamic MYD88, IKKαβ and hypothalamic cytokine levels were not significantly different. However, inflammation is a dynamic process, and higher hypothalamic NFκBp65 may predispose the young adult offspring to a higher inflammatory response later in life.
Hypothalamic inflammation through the activation of IKKβ/NFκB and cytokines production has been shown to cause insulin resistance in hypothalamic neurons [67]. TNF-α may inhibit insulin receptor signalling [68] and also reduce insulin receptor expression [69]. Insulin receptors are expressed in hypothalamic neurons in the arcuate nucleus, including orexigenic neurons neuropeptide Y (NPY) and agouti-related peptide (AGRP), and anorexigenic neurons proopiomelanocortin (POMC) and cocaine- and amphetamine-related transcript (CART). Insulin has a potent anorectic action stimulating POMC/CART neuropeptides expression, while it has an inhibitory effect on NPY/AGRP. A reduction in the expression of insulin receptors has been shown to increase visceral adiposity, leptinaemia, and insulinaemia, and cause insulin resistance [70]. Our results showed that paternal HFS diet influenced lower hypothalamic insulin receptor levels in the 21-day-old offspring, suggesting the onset of hypothalamic insulin resistance.
Combined effects of maternal and paternal HFS diet also showed hyperleptinaemia in the 21-day-old, suggesting hypothalamic leptin resistance. Leptin has a similar hypothalamic action as insulin to inhibit excess food intake through the stimulation of POMC/CART and inhibition of NPY/AGRP neurons. Leptin is an adipokine that reflects the amount of adipose tissue and is implicated in the negative feedback on the regulation of energy homeostasis [71]. Diet-induced hypothalamic inflammation can cause central leptin resistance, blunting leptin action [72]. Moreover, hyperleptinaemia is observed in obesity and has been shown to be deleterious as it per se can contribute to leptin resistance both centrally and peripherally [73]. Leptin resistance by high consumption of sugar increased the expression of the orexigenic hypothalamic neuropeptide NPY and increased the expression of adipogenic and lipogenic factors such as peroxisome-proliferator-activated receptor γ (PPARγ), sterol regulatory element-binding protein-1 (SREBP-1) and lipin-1 in the visceral adipose tissue, increasing adiposity [74]. Similarly, a study demonstrated that hyperleptinaemia contributes to leptin resistance in rats fed a high-fat diet, increasing the susceptibility to an exacerbated weight and fat mass gain. This escalates the vicious cycle that hyperleptinaemia contributes to leptin resistance which causes further weight gain and hyperleptinaemia, contributing to the phenotype of obesity [75]. Early life paternal diet effect on hypothalamic insulin receptor as well as the combined parental diet effect on serum leptin levels were also reversed by the consumption of a control diet after weaning until young adulthood. It may be the reason why any effect on weight gain was not found after weaning until adulthood in the offspring. In revision, the consumption of an obesogenic diet followed by a long-term healthier diet showed to rescue metabolic alterations, such as high levels of glycaemia, lipaemia, insulin and leptin during adulthood [76].
After weaning, offspring consumption of a healthier diet showed reprogramming part of the effects of parental high-fat high-sugar diet during the perinatal period. However, we could still notice a programming effect especially on the maintenance of a higher weight and adiposity, and alterations in the expression of a hypothalamic protein involved in the expression of pro-inflammatory cytokines. In this study, we observed that the paternal HFS diet had a weaker effect on the offspring than the maternal HFS diet alone, but had an additive effect. A possible explanation could be related to epigenetic changes in metabolic tissues of offspring transmitted from paternal HFD diet that was blunted by the consumption of CD diet post-weaning. A study showed that the effect of paternal HFD was exacerbated when the offspring were exposed to HFS diet, promoting greater metabolic disturbances [77]. Moreover, whether an obesogenic epigenetic profile is transmitted from paternal HFS into male offspring metabolic tissues and is further rescued by post-weaning control diet or disrupted by post-weaning HFD remains to be investigated. Our results may suggest higher susceptibility to develop obesity and metabolic disorders in the long term. The hypothalamus adipose axis seems to be involved in the programming of adiposity and weight.
Conclusions
Parental high-fat high-sugar diet was shown to affect the development, adiposity, hypothalamic inflammation and lipid metabolism of male offspring at an early age. Consumption of the control diet after weaning has managed to protect in part the young adult offspring. However, they may still have a higher susceptibility to develop obesity or metabolic disease later on, especially if challenged with an obesogenic diet. Hypothalamus adipose tissue axis may be involved in the programming of obesity and metabolic disorders. Not only the maternal and paternal diet effects alone, but also the combined effects of parental diet showed potential programming effects in the male offspring.
Change history
08 November 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00394-021-02725-7
References
Barker DJ (1991) The intrauterine environment and adult cardiovascular disease. Ciba Found Symp 156:3–6
Ravelli ACJ, Van Der Meulen JHP, Osmond C et al (1999) Obesity at the age of 50 year in men and women exposed to famine prenatally. Am J Clin Nutr 70:811–816. https://doi.org/10.1093/ajcn/70.5.811
Barker DJ, Gluckman PD, Godfrey KM et al (1993) Fetal nutrition and cardiovascular disease in adult life. Lancet (London, England) 341:938–941
Mennitti LV, Oyama LM, Santamarina AB et al (2018) Early exposure to distinct sources of lipids affects differently the development and hepatic inflammatory profiles of 21-day-old rat offspring. J Inflamm Res 11:11–24. https://doi.org/10.2147/JIR.S152326
Mennitti LV, Oyama LM, de Oliveira JL et al (2014) Oligofructose supplementation during pregnancy and lactation impairs offspring development and alters the intestinal properties of 21-d-old pups. Lipids Health Dis 13:26. https://doi.org/10.1186/1476-511X-13-26
Mennitti LV, Oyama LM, Santamarina AB et al (2018) Influence of maternal consumption of different types of fatty acids during pregnancy and lactation on lipid and glucose metabolism of the 21-day-old male offspring in rats. Prostaglandins Leukot Essent Fatty Acids 135:54–62. https://doi.org/10.1016/j.plefa.2018.07.001
Mennitti LV, Oliveira JL, Morais CA et al (2015) Type of fatty acids in maternal diets during pregnancy and/or lactation and metabolic consequences of the offspring. J Nutr Biochem 26:99–111. https://doi.org/10.1016/j.jnutbio.2014.10.001
Mendes-da-Silva C, Giriko CÁ, Mennitti LV et al (2014) Maternal high-fat diet during pregnancy or lactation changes the somatic and neurological development of the offspring. Arq Neuropsiquiatr 72:136–144. https://doi.org/10.1590/0004-282X20130220
Gawlińska K, Gawliński D, Filip M, Przegaliński E (2020) Relationship of maternal high-fat diet during pregnancy and lactation to offspring health. Nutr Rev. https://doi.org/10.1093/nutrit/nuaa020
Sferruzzi-Perri AN, Vaughan OR, Haro M et al (2013) An obesogenic diet during mouse pregnancy modifies maternal nutrient partitioning and the fetal growth trajectory. FASEB J 27:3928–3937. https://doi.org/10.1096/fj.13-234823
Musial B, Vaughan OR, Fernandez-Twinn DS et al (2017) A Western-style obesogenic diet alters maternal metabolic physiology with consequences for fetal nutrient acquisition in mice. J Physiol 595:4875–4892. https://doi.org/10.1113/JP273684
Warner MJ, Ozanne SE (2010) Mechanisms involved in the developmental programming of adulthood disease. Biochem J 427:333–347. https://doi.org/10.1042/BJ20091861
Nicholas LM, Morrison JL, Rattanatray L et al (2016) The early origins of obesity and insulin resistance: timing, programming and mechanisms. Int J Obes 40:229–238. https://doi.org/10.1038/ijo.2015.178
Xavier S, Gili J, McGowan P et al (2021) High maternal omega-3 supplementation dysregulates body weight and leptin in newborn male and female rats: implications for hypothalamic developmental programming. Nutrients. https://doi.org/10.3390/nu13010089
McPherson NO, Bell VG, Zander-Fox DL et al (2015) When two obese parents are worse than one! Impacts on embryo and fetal development. Am J Physiol Metab 309:E568–E581. https://doi.org/10.1152/ajpendo.00230.2015
Klastrup LK, Bak ST, Nielsen AL (2019) The influence of paternal diet on sncRNA-mediated epigenetic inheritance. Mol Genet Genomics 294:1–11. https://doi.org/10.1007/s00438-018-1492-8
Keleher MR, Zaidi R, Shah S et al (2018) Maternal high-fat diet associated with altered gene expression, DNA methylation, and obesity risk in mouse offspring. PLoS ONE. https://doi.org/10.1371/journal.pone.0192606
Schagdarsurengin U, Steger K (2016) Epigenetics in male reproduction: effect of paternal diet on sperm quality and offspring health. Nat Rev Urol 13:584–595
Wattez JS, Delahaye F, Lukaszewski MA et al (2013) Perinatal nutrition programs the hypothalamic melanocortin system in offspring. Horm Metab Res 45:980–990
Breton C (2013) The hypothalamus-adipose axis is a key target of developmental programming by maternal nutritional manipulation. J Endocrinol 216:R19-31. https://doi.org/10.1530/JOE-12-0157
Cesar HC, Pisani LP (2017) Fatty-acid-mediated hypothalamic inflammation and epigenetic programming. J Nutr Biochem 42:1–6. https://doi.org/10.1016/j.jnutbio.2016.08.008
Thaler JP, Yi C-X, Schur EA et al (2012) Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest 122:153–162. https://doi.org/10.1172/JCI59660
Valdearcos M, Robblee MM, Benjamin DI et al (2014) Microglia Dictate the impact of saturated fat consumption on hypothalamic inflammation and neuronal function. Cell Rep 9:2124–2138. https://doi.org/10.1016/j.celrep.2014.11.018
Lemes SF, de Souza ACP, Payolla TB et al (2018) Maternal consumption of high-fat diet in mice alters hypothalamic notch pathway, NPY cell population and food intake in offspring. Neuroscience 371:1–15. https://doi.org/10.1016/j.neuroscience.2017.11.043
Chang G-Q, Gaysinskaya V, Karatayev O, Leibowitz SF (2008) Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci 28:12107–12119. https://doi.org/10.1523/JNEUROSCI.2642-08.2008
Rajia S, Chen H, Morris MJ (2010) Maternal overnutrition impacts offspring adiposity and brain appetite markers-modulation by postweaning diet. J Neuroendocrinol 22:905–914. https://doi.org/10.1111/j.1365-2826.2010.02005.x
Ornellas F, Souza-Mello V, Mandarim-de-Lacerda CA, Aguila MB (2016) Combined parental obesity augments single-parent obesity effects on hypothalamus inflammation, leptin signaling (JAK/STAT), hyperphagia, and obesity in the adult mice offspring. Physiol Behav 153:47–55. https://doi.org/10.1016/j.physbeh.2015.10.019
Chen Y-P, Xiao X-M, Li J et al (2012) Paternal body mass index (BMI) is associated with offspring intrauterine growth in a gender dependent manner. PLoS ONE 7:e36329. https://doi.org/10.1371/journal.pone.0036329
Freeman E, Fletcher R, Collins CE et al (2012) Preventing and treating childhood obesity: time to target fathers. Int J Obes (Lond) 36:12–15. https://doi.org/10.1038/ijo.2011.198
Sanchez-Garrido MA, Ruiz-Pino F, Velasco I et al (2018) Intergenerational influence of paternal obesity on metabolic and reproductive health parameters of the offspring: male-preferential impact and involvement of kiss1-mediated pathways. Endocrinology 159:1005–1018. https://doi.org/10.1210/en.2017-00705
Ng S-F, Lin RCY, Laybutt DR et al (2010) Chronic high-fat diet in fathers programs β-cell dysfunction in female rat offspring. Nature 467:963
Masuyama H, Mitsui T, Eguchi T et al (2016) The effects of paternal high-fat diet exposure on offspring metabolism with epigenetic changes in the mouse adiponectin and leptin gene promoters. Am J Physiol Endocrinol Metab 311:E236–E245. https://doi.org/10.1152/ajpendo.00095.2016
Chowdhury S, Lecomte V, Erlich J et al (2016) Paternal high fat diet in rats leads to renal accumulation of lipid and tubular changes in adult offspring. Nutrients 8:521. https://doi.org/10.3390/nu8090521
Zhang X, Dong Y, Sun G et al (2019) Paternal programming of liver function and lipid profile induced by a paternal pre-conceptional unhealthy diet: potential association with altered gut microbiome composition. Kidney Blood Press Res 44:133–148. https://doi.org/10.1159/000497487
Zheng J, Xiao X, Zhang Q, Yu M (2014) DNA methylation: the pivotal interaction between early-life nutrition and glucose metabolism in later life. Br J Nutr 112:1850–1857. https://doi.org/10.1017/S0007114514002827
Samuelsson A-M, Matthews PA, Argenton M et al (2007) Diet-induced obesity in female mice leads to offspring hyperphagia, adiposity, hypertension, and insulin resistance. Hypertension 51:383–392. https://doi.org/10.1161/hypertensionaha.107.101477
Subcommittee on Laboratory Animal Nutrition; Committee on Animal Nutrition; Board on Agriculture; National Research Council (1995) Nutrient requirements of laboratory animals, 4th edn. The National Academies Press, Washington
Mitchell JA, Hutchins M, Schindler WJ, Critchlow V (1973) Increases in plasma growth hormone concentration and naso-anal length in rats following isolation of the medial basal hypothalamus. Neuroendocrinology 12:161–173. https://doi.org/10.1159/000122230
Nunez AA, Kannan K, Giesy JP et al (2001) Effects of bisphenol A on energy balance and accumulation in brown adipose tissue in rats. Chemosphere 42:917–922. https://doi.org/10.1016/S0045-6535(00)00196-X
Wells JCK (2018) Understanding developmental plasticity as adaptation requires an inter-generational perspective. Evol Med public Heal 2017:185–187. https://doi.org/10.1093/emph/eox023
O’Reilly JR, Reynolds RM (2013) The risk of maternal obesity to the long-term health of the offspring. Clin Endocrinol (Oxf) 78:9–16
Falcão-Tebas F, Marin EC, Kuang J et al (2020) Maternal exercise attenuates the lower skeletal muscle glucose uptake and insulin secretion caused by paternal obesity in female adult rat offspring. J Physiol. https://doi.org/10.1113/jp279582
Arentson-Lantz EJ, Buhman KK, Ajuwon K, Donkin SS (2014) Excess pregnancy weight gain leads to early indications of metabolic syndrome in a swine model of fetal programming. Nutr Res 34:241–249. https://doi.org/10.1016/j.nutres.2014.01.001
Cavalcanti-de-Albuquerque JP, Bober J, Zimmer MR, Dietrich MO (2019) Regulation of substrate utilization and adiposity by Agrp neurons. Nat Commun. https://doi.org/10.1038/s41467-018-08239-x
Carew LB Jr, Hill FW (1964) Effect of corn oil on metabolic efficiency of energy utilization by chicks. J Nutr 83:293–299. https://doi.org/10.1093/jn/83.4.293
Crescenzo R, Bianco F, Falcone I et al (2012) Hepatic mitochondrial energetics during catch-up fat with high-fat diets rich in lard or safflower oil. Obesity 20:1763–1772. https://doi.org/10.1038/oby.2011.167
Iossa S, Lionetti L, Mollica MP et al (2003) Effect of high-fat feeding on metabolic efficiency and mitochondrial oxidative capacity in adult rats. Br J Nutr 90:953–960. https://doi.org/10.1079/BJN2003000968
Calonne J, Isacco L, Miles-Chan J et al (2019) Reduced skeletal muscle protein turnover and thyroid hormone metabolism in adaptive thermogenesis that facilitates body fat recovery during weight regain. Front Endocrinol (Lausanne) 10:119. https://doi.org/10.3389/fendo.2019.00119
Zhang X, Chen W, Shao S et al (2018) A high-fat diet rich in saturated and mono-unsaturated fatty acids induces disturbance of thyroid lipid profile and hypothyroxinemia in male rats. Mol Nutr Food Res 62:1700599. https://doi.org/10.1002/mnfr.201700599
Broeders EPM, Vijgen GHEJ, Havekes B et al (2016) Thyroid hormone activates brown adipose tissue and increases non-shivering thermogenesis—a cohort study in a group of thyroid carcinoma patients. PLoS ONE 11:e0145049–e0145049. https://doi.org/10.1371/journal.pone.0145049
Bourguignon A, Rameau A, Toullec G et al (2017) Increased mitochondrial energy efficiency in skeletal muscle after long-term fasting: its relevance to animal performance. J Exp Biol 220:2445–2451. https://doi.org/10.1242/jeb.159087
Fromme T, Klingenspor M (2011) Uncoupling protein 1 expression and high-fat diets. Am J Physiol Regul Integr Comp Physiol 300:R1-8. https://doi.org/10.1152/ajpregu.00411.2010
Bond LM, Ntambi JM (2018) UCP1 deficiency increases adipose tissue monounsaturated fatty acid synthesis and trafficking to the liver. J Lipid Res 59:224–236. https://doi.org/10.1194/jlr.M078469
Ribaroff GA, Wastnedge E, Drake AJ et al (2017) Animal models of maternal high fat diet exposure and effects on metabolism in offspring: a meta-regression analysis. Obes Rev 18:673–686. https://doi.org/10.1111/obr.12524
Müller MJ, Bosy-Westphal A, Heymsfield SB (2010) Is there evidence for a set point that regulates human body weight? F100 Med Rep. https://doi.org/10.3410/M2-59
Sullivan EL, Smith MS, Grove KL (2011) Perinatal exposure to high-fat diet programs energy balance, metabolism and behavior in adulthood. Neuroendocrinology 93:1–8. https://doi.org/10.1159/000322038
Reinhardt M, Thearle MS, Ibrahim M et al (2015) A human thrifty phenotype associated with less weight loss during caloric restriction. Diabetes 64:2859–2867. https://doi.org/10.2337/db14-1881
Ghaben AL, Scherer PE (2019) Adipogenesis and metabolic health. Nat Rev Mol Cell Biol 20:242–258. https://doi.org/10.1038/s41580-018-0093-z
Reynolds AN, Akerman AP, Mann J (2020) Dietary fibre and whole grains in diabetes management: systematic review and meta-analyses. PLOS Med 17:e1003053. https://doi.org/10.1371/journal.pmed.1003053
Moraes JC, Coope A, Morari J et al (2009) High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE. https://doi.org/10.1371/journal.pone.0005045
Kim YJ (2006) What is fetal programming?: A lifetime health is under the control of in-utero health. Korean J Obs Gynecol 49:2055–2065
Lee CH, Suk K, Yu R, Kim M-S (2020) Cellular Contributors to hypothalamic inflammation in obesity. Mol Cells 43:431–437. https://doi.org/10.14348/molcells.2020.0055
Valdearcos M, Douglass JD, Robblee MM et al (2017) Microglial inflammatory signaling orchestrates the hypothalamic immune response to dietary excess and mediates obesity susceptibility. Cell Metab 26:185-197.e3. https://doi.org/10.1016/j.cmet.2017.05.015
Reynoso M, Geddis A, Mitrophanov A et al (2017) NFkB activation and cytokine output in LPS-treated RAW 264.7 macrophages. FASEB J. https://doi.org/10.1096/fasebj.31.1_supplement.lb146
Zhou H, Urso CJ, Jadeja V (2020) <p>saturated fatty acids in obesity-associated inflammation</p>. J Inflamm Res 13:1–14. https://doi.org/10.2147/JIR.S229691
Curtale G, Mirolo M, Renzi TA et al (2013) Negative regulation of toll-like receptor 4 signaling by IL-10-dependent microRNA-146b. Proc Natl Acad Sci U S A 110:11499–11504. https://doi.org/10.1073/pnas.1219852110
Clemenzi MN, Wellhauser L, Aljghami ME, Belsham DD (2019) Tumour necrosis factor α induces neuroinflammation and insulin resistance in immortalised hypothalamic neurones through independent pathways. J Neuroendocrinol 31:e12678. https://doi.org/10.1111/jne.12678
Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM (1994) Tumor necrosis factor α inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A 91:4854–4858. https://doi.org/10.1073/pnas.91.11.4854
Aljada A, Ghanim H, Assian E, Dandona P (2002) Tumor necrosis factor-α inhibits insulin-induced increase in endothelial nitric oxide synthase and reduces insulin receptor content and phosphorylation in human aortic endothelial cells. Metabolism 51:487–491. https://doi.org/10.1053/meta.2002.31339
Obici S, Feng Z, Karkanias G et al (2002) Decreasing hypothalamic insulin receptors causes hyperphagia and insulin resistance in rats. Nat Neurosci 5:566–572. https://doi.org/10.1038/nn0602-861
Richard AJ, Stephens JM (2019) Adipocyte-derived hormones. Hormonal signaling in biology and medicine: comprehensive modern endocrinology. Academic Press, Elsevier, pp 461–486
de Git KCG, Adan RAH (2015) Leptin resistance in diet-induced obesity: the role of hypothalamic inflammation. Obes Rev 16:207–224. https://doi.org/10.1111/obr.12243
Zhao S, Kusminski CM, Elmquist JK, Scherer PE (2020) Leptin: less is more. Diabetes 69:823–829. https://doi.org/10.2337/dbi19-0018
Bursać BN, Vasiljević AD, Nestorović NM et al (2014) High-fructose diet leads to visceral adiposity and hypothalamic leptin resistance in male rats—do glucocorticoids play a role? J Nutr Biochem 25:446–455. https://doi.org/10.1016/j.jnutbio.2013.12.005
Zhang Y, Scarpace PJ (2006) The role of leptin in leptin resistance and obesity. Physiol Behav 88:249–256. https://doi.org/10.1016/j.physbeh.2006.05.038
Casagrande B, Estadella D (2020) Withdrawing from obesogenic diets: benefits and barriers in the short- and long-term in rodent models. Am J Physiol Metab. https://doi.org/10.1152/ajpendo.00174.2020
Fullston T, McPherson NO, Owens JA et al (2015) Paternal obesity induces metabolic and sperm disturbances in male offspring that are exacerbated by their exposure to an “obesogenic” diet. Physiol Rep. https://doi.org/10.14814/phy2.12336
Funding
This study was supported by Fundação do Amparo à Pesquisa do Estado de São Paulo (FAPESP)—no. 2017/09646–1; 2019/09724–8, and financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001. LPP is recipient of National Council for Scientific and Technological Development (CNPq) fellowship and supported by FAPESP—no. 2019/09724–8.
Author information
Authors and Affiliations
Contributions
HC contributed with the animal care, experimental procedures, statistical analysis and writing. MNS, EAS, GJ, AS and AJ contributed to the animal care and experimental procedures. BC contributed with the statistical analysis. LPP contributed with the drafting and revising critical intellectual content. All authors have approved the final article.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
394_2021_2690_MOESM1_ESM.tif
Supplementary file1 (TIF 571 kb) Paternal differences in body measurements, metabolic efficiency and adiposity between control diet (CD) and high-fat high-sugar (HFS) groups. A. Paternal body weight progress (g); B. Paternal weight gain (g) (Final weight – Initial weight); C. Paternal diet intake (g/week); D. Paternal metabolic efficiency weight gain (g)/ diet intake (g); E. Paternal Retroperitoneal Adipose Tissue (RET) relative weight (%); F. Paternal Total Visceral Adipose Tissue (VAT) relative weight (%); Data are expressed as mean ± SEM and *p≤0.05 was considered statistically significant compared to CD. Statistical test: ANOVA for repeated measures was used for A; U-Mann Whitney test (non-parametric data) was used for B and C, and unpaired T-test (parametric data) for D, E and F
394_2021_2690_MOESM2_ESM.tif
Supplementary file2 (TIF 604 kb) Maternal differences in body measurements, metabolic efficiency and adiposity between control diet (CD) and high-fat high-sugar (HFS) groups. A. Maternal body weight progress (g) during pregnancy and lactation; B. Maternal Weight gain (g) (Final weight – Initial weight) during pregnancy and lactation; C. Maternal diet intake (g) during pregnancy and lactation; D. Maternal metabolic efficiency (weight gain (g)/ diet intake (g) during pregnancy and lactation; E. Maternal Retroperitoneal Adipose Tissue (RET) relative weight (%); F. Maternal Total Visceral Adipose Tissue (VAT) relative weight (%); Data are expressed as mean ± SEM and *p≤0.05 was considered statistically significant compared to CD. Statistical test: ANOVA for repeated measures was used for A; U-Mann Whitney test (non-parametric data) was used for E and F, and unpaired T-test (parametric data) for B, C and D.
Rights and permissions
About this article
Cite this article
César, H., Sertorio, M.N., de Souza, E.A. et al. Parental high-fat high-sugar diet programming and hypothalamus adipose tissue axis in male Wistar rats. Eur J Nutr 61, 523–537 (2022). https://doi.org/10.1007/s00394-021-02690-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-021-02690-1