Abstract
Purpose
Preventive health effects of coffee could have a widespread impact on public health. Green coffee has more phenols than roasted, and thus is healthier, although with less acceptable organoleptic properties. Therefore, the effects of regularly consuming a green/roasted coffee blend (35/65) on the main components of MetS in humans were evaluated.
Methods
A crossover, randomized, controlled study was performed in 25 normocholesterolaemic and 27 hypercholesterolaemic men and women aged 18–45 years with BMI 18–25 kg/m2. Three servings/day of the blend, providing 510.6 mg hydroxycinnamic acids and 121.2 mg caffeine/day, were consumed versus a control drink, during 8 weeks each. Polyphenol and methylxanthine-rich foods were restricted along the study. At the beginning (baseline) and end of the control and coffee interventions, blood samples were collected and glucose, HDL-cholesterol, triglycerides, insulin, leptin, plasminogen activator inhibitor-1 (PAI-1), resistin and visfatin were analysed; waist circumference, %body fat, and blood pressure were measured and dietary records and physical activity questionnaires completed.
Results
Systolic and diastolic blood pressure decreased (p = 0.001 and p < 0.001, respectively) in both groups as well as %body fat (p = 0.001) which may be related to the lower leptin (p = 0.001), PAI-1 (p < 0.001) and resistin (p = 0.034) levels in the two groups after coffee consumption. Glucose concentration (p = 0.030) and insulin resistance (p = 0.011; HOMA-IR) also decreased, as well as triglyceride levels (p = 0.017), so that the reduction was much greater in the hypercholesterolaemics (group effect, p = 0.027).
Conclusion
Regular consumption of the green/roasted coffee blend may be recommended to healthy and hypercholesterolaemic subjects to prevent MetS, as it produces positive effects on blood pressure, glucose and triglyceride levels.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The prevalence of metabolic syndrome (MetS) has risen during the last decade due to unhealthy lifestyle and dietary habits [1]. MetS is characterized as a combination of underlying risk factors that when occurring together culminate in adverse outcomes, including type 2 diabetes mellitus, cardiovascular disease and an approximately 1.6-fold increase in mortality [2]. A single precise definition and the contributions of the underlying components of MetS have been of much debate. According to the National Heart, Lung and Blood Institute/American Heart Association, MetS is diagnosed if any three of the five following criteria is met: central obesity, raised triglycerides, reduced high-density lipoprotein cholesterol (HDL-C), raised blood pressure and elevated fasting plasma glucose [3, 4]. The complex interactions between these risk factors contribute to chronic organ damage. Obesity, hypertension and dyslipidaemia lead to the remodelling of the heart and blood vessels, producing chronic cardiovascular complications in which a variety of bioactive substances secreted by the adipose tissue, known as adipokines, is involved [5]. Adipokines play a key role in the development of metabolic syndrome.
Health protective effects of coffee could have a widespread impact on the population health, considering the high intake of this beverage, particularly in industrialized countries where it is the largest source of dietary antioxidants [6]. The potential impact of coffee on health has been recently reviewed in Ludwing et al. [7]. Most recent studies observed a 30–60 % reduction in diabetes risk, whereas coffee seems to have a mix of beneficial and harmful cardiovascular effects, as the undesirable influence of diterpenes and caffeine may be compensated by the beneficial effects of the phenolic components, mainly chlorogenic acid (CGA). CGA, which also shows positive effects on glucose metabolism [8], may reduce fat accumulation in numerous tissues via inhibition of enzyme regulators involved in lipogenesis [9], although recently such effect was not observed in mice fed a high-fat diet [10]. On the other hand, caffeine, with lipolytic and thermogenic activities, increases metabolic rate, energy expenditure and lipid oxidation, and so it has potential as an aid in weight loss and reducing the overall risk for developing MetS [11]. Balancing all these effects, a positive beneficial relationship between coffee consumption and MetS could be expected, attributed mainly to the bioactive compounds polyphenols and caffeine.
However, studies aimed at evaluating the association between long-term coffee consumption and the components of MetS are scarce and have not reached a consensus. Studies carried out in a Japanese population demonstrated a significant inverse correlation between coffee intake and MetS [12–14]. Other studies conducted in a Dutch population described that after adjustment for physical activity, energy intake, smoking behaviour and alcohol consumption, the relationship between coffee consumption and MetS or its components was not significant [15, 16]. In contrast, a study conducted in a Mediterranean population described a positive and inverse association [1]. The differences in the outcome of the coffee-MetS association may be attributed, to a certain extent, to the overall diet quality of the population studied. In this sense, in Japan and Mediterranean countries a healthier diet is consumed than in Central Europe. In fact, subjects more adherent to the Mediterranean diet are less likely to suffer MetS or its components [17].
In view of the foregoing, this work looks into the preventive effects of regularly consuming three cups of a green/roasted coffee blend per day, within a Mediterranean diet, on MetS components and certain serum adipokine levels in healthy and cardiovascular risk subjects.
Subjects
The present study was approved by the Clinical Research Ethics Committee of Hospital Puerta de Hierro (Madrid, Spain) and therefore has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Volunteers’ recruitment was carried out by giving short talks and placing advertisements at Complutense University campus.
The inclusion criteria was as follows: men and women with body mass index (BMI) under 25 kg/m2, 18–45 years old, non-smokers, non-vegetarian, non-pregnant women, not-having vitamins or dietary supplements, not-having taken antibiotics 6 months before the start of the study and not suffering chronic disorders or pathologies, apart from hypercholesterolaemia. Participants were separated in two groups attending to their total cholesterol (TC) levels: normocholesterolaemic (TC < 200 mg/dL) and hypercholesterolaemic (TC > 200–240 mg/dL) subjects.
Fifty-four subjects accepted to participate in the study and gave informed written consent; however, two withdrew due to personal and professional reasons. Of the remaining volunteers, twenty-five were normocholesterolaemic and twenty-seven were hypercholesterolaemic. Baseline characteristics of the fifty-two volunteers who finally participated in the study are presented in Table 1.
Study design
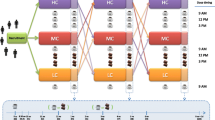
A randomized, controlled, crossover study was carried out in free-living subjects (Fig. 1). After a run-in stage (2 weeks), subjects were randomly distributed in 2 groups, so that half the participants firstly consumed the coffee product and the other half had the control drink for 8 weeks. Then, after a 3 week washout stage, subjects changed to drink the other beverage during the same time (8 weeks). During the coffee intervention, volunteers consumed three times a day a 2 g serving of the coffee blend dissolved in 200 mL of hot water, preferably at breakfast, midday and after lunch, without milk or sugar. At the control intervention, instead of coffee, a control drink consisting in water or an isotonic caffeine- and polyphenol-free drink was consumed three times a day. Blood samples, blood pressure and anthropometric measurements were taken at the end of the run-in (baseline), control and coffee stages. Blood samples were collected in BD Vacutainer® tubes (Becton, Dickinson and Company, NJ, USA) with or without EDTA to separate plasma and serum, respectively, and aliquots were separated and stored at −80 °C until analysis.
Coffee composition
The soluble coffee product used was a commercial green/roasted mixture (35:65, w/w) that was provided and packed explicitly for the study in blind 2-g sachets by the manufacturing company. Total soluble polyphenols in the green-roasted coffee product were analysed by high-performance liquid chromatography with diode array detection (HPLC–DAD) as described elsewhere [18]. Methylxanthine content in the infusions was analysed by HPLC–DAD in an Agilent 1200 series (Agilent Technologies, CA, USA) following a method previously described [19]. The coffee contained 85.1 mg/g of total hydroxycinnamic acids (57.4 mg/g corresponded to caffeoylquinic acids, mainly 5-O-caffeoylquinic acid) and 20.5 mg/g of total methylxanthines (20.2 mg/g was caffeine). Therefore, the daily consumption of hydroxycinnamic acids and methylxanthines was 510.6 and 123 mg, respectively.
Dietary control and compliance
Along the whole study, participants were asked to maintain their habitual lifestyle and dietary habits, except for consuming other coffee products, certain fruits and vegetables rich in polyphenols, particularly those rich in hydroxycinnamic acids (artichokes, oranges, grapes, aubergines or soya among others) as well as products like tea, yerba mate, whole wheat products and caffeine-containing drinks. The consumption of potatoes, apples, pomegranates, rice and corn was reduced to once a week.
Before starting the study, volunteers were instructed on how to fill in the dietary records. In each stage, they were asked to fill out a 72-hour food intake report. To estimate the energy intake and dietary composition, the programme DIAL (Department of Nutrition and Bromatology I, School of Pharmacy, Complutense University of Madrid, Spain) was used. Study compliance was controlled by calling the volunteers every week and counting the number of coffee servings returned after the intervention.
Physical activity, body fat and waist circumference
Participants were asked to maintain their usual physical activity throughout the study. They filled out questionnaires before the start of the study and at each intervention to evaluate their physical activity which was estimated using the programme ADN (Department of Nutrition and Bromatology I, School of Pharmacy, Complutense University of Madrid, Spain).
At baseline and the end of each stage of the study, volunteer’s percentage of total body fat and waist circumference were measured using a Tanita Segmental Body Composition Analyzer BC-418 MA (Tanita Corp. Tokyo, Japan) and a SECA 203 flexible tape (SECA Ltd, UK), respectively.
Blood pressure parameters
At the end of each stage, before blood sampling, systolic and diastolic blood pressure (BP) were measured using an automatic arm sphygmomanometer (Pic Indolor Diagnostic, BS 150, Artsana, Italy) in triplicate, waiting for 3 min between measurements. Readings were compared and accepted if in agreement within 10–15 mmHg.
Biochemical analysis and insulin resistance calculations
Triglycerides and HDL-C were determined in serum samples following reference methods or methods recommended by Sociedad Española de Bioquimica Clinica y Patologia Molecular (SEQC) using a Roche Cobas Integra 400 Plus Analyser (Roche Diagnostics, Mannheim, Germany). Fasting glucose was analysed using a colorimetric kit (Spinreact). Fasting insulin concentration was analysed using the Bio-Rad Multiplex Diabetes Kit on Bio-Plex MAGPIX system. Using fasting glucose and insulin data, homoeostasis model assessment index was calculated to estimate insulin resistance (HOMA-IR) according to the equation by Matthews et al. [20]: HOMA-IR = [Glucose (mg/dL) × Insulin (mU/L)]/405.
Adipokine biomarker analysis
Leptin, resistin, PAI-1 and visfatin were analysed in plasma samples using the Bio-Plex Pro Human Diabetes Kit. All analytes were measured in duplicate on a MAGPIX™ Multiplex reader, and software Bio-Plex Manager™ MP (Luminex Corporation, Austin, USA) was used for data processing. Intra-assay %CV were 3, 3, 4 and 5 and inter-assay %CV were 4, 4, 4 and 3 for leptin, resistin, PAI-1 and visfatin, respectively. Results were expressed as pg/mL plasma.
Statistical analysis
Setting the statistical power at 80 %, the significance level at 0.05, and taking total cholesterol as the main variable, a sample size of 23 subjects per group was calculated based on the assumption that the within-patient standard deviation of the response variable was 10 and the difference between treatments was 6 mg/dL. Statistical analyses were carried out using the program SPSS version 20.0 (IBM Company, NY, USA). Normality of distribution and homogeneity of variance of all variables studied were evaluated using the Kolmogorov–Smirnov and Levene tests, respectively. A general linear model of variance for repeated measures was used to assess differences due to consuming coffee, and the group was considered an inter-individual factor. Furthermore, differences within each group were studied using the paired Bonferroni test. Significance level was established at p < 0.05.
Results
In all the studied parameters, the normocholesterolaemic and hypercholesterolaemic subjects exhibited the same behaviour after the consumption of coffee and thus the effect of the group was not significant except for waist circumference (p = 0.007) and triglycerides (p = 0.027). There was no significant coffee × group interaction in any of the biomarkers studied.
Effects on food intake
Protein intake was significantly lower at the end of the coffee intervention (Table 2), whereas none of the other dietary intake parameters showed statistical differences along the study. There was no influence due to the group.
Effects on physical activity, body fat and waist circumference
Volunteers did not show changes in their physical activity along the study (data not shown). A significant decrease (p = 0.001) in the percentage of body fat was observed in both groups after coffee consumption (normocholesterolaemics: 22.8 ± 1.4, 23.7 ± 1.4 and 21.5 ± 1.2 and hypercholesterolaemics: 25.4 ± 1.3, 24.4 ± 1.2 and 23.6 ± 1.3, at baseline, control and coffee interventions, respectively). Regarding waist circumference, there was a significant difference between the two groups; in contrast to the normocholesterolaemic subjects, among the hypercholesterolaemic volunteers there was a tendency to reduce their waist circumference, although the reduction was not statistically significant (Table 3).
Effects on blood pressure
According to the general linear model of variance for repeated measures analysis, systolic and diastolic BP significantly decreased in both the healthy and hypercholesterolaemic group (Table 3) after coffee consumption (p = 0.001 and p < 0.001, respectively). Furthermore, when the differences within each group were studied using the paired Bonferroni test, in the latter group both parameters were statistically lower after the coffee intervention compared to baseline, although not to control values.
Effects on triglycerides and HDL-cholesterol
Regarding triglyceride levels, a significant reduction (p = 0.017) was observed after coffee consumption as well as a significant difference due to the group (p = 0.027), so that the reduction in the lipid biomarker was much greater in the hypercholesterolaemic subjects compared to the healthy. In contrast, HDL-C levels did not change.
Effects on glucose levels and insulin resistance
At the end of the coffee intervention, glucose concentration significantly decreased (Table 3, p = 0.030). In contrast, insulin data did not show changes (normocholesterolaemics: 8.60 ± 0.39, 9.14 ± 0.31 and 8.01 ± 0.37 and hypercholesterolaemics: 8.95 ± 0.45, 8.80 ± 0.36 and 8.56 ± 0.42 mU/L at baseline, control and coffee interventions, respectively). Insulin resistance, calculated according to the HOMA-IR model, decreased significantly (p = 0.011, Fig. 2).
Effects on adipokines
Leptin (p = 0.001), PAI-1 (p < 0.001) and resistin (p = 0.034) significantly decreased in the two study groups (Table 4). According to the Bonferroni test, after coffee consumption leptin and resistin levels were statistically lower with respect to their corresponding baseline values in both groups of volunteers. Similarly, PAI-1 levels after the coffee intervention were significantly lower in both groups of intervention with respect to the baseline, and in the healthy volunteers also with respect to the control.
Discussion
The prevalence of MetS in the Mediterranean regions is estimated to be 15–20 % in men and 19–25 % in women, with an increase as high as 50 % in subjects over 70 years in certain countries [1]. These rates have further risen during the last decade due to unhealthy lifestyle and dietary habits [1], and thus it is pertinent to identify foodstuffs or beverages that can help to counteract MetS. In this sense, coffee could play an important role, given its high rate of consumption and its composition, rich in polyphenols. In the present study, the intake of three cups of coffee per day was established in order to reproduce a realistic consumption rate and pattern (morning, midday and after lunch) of coffee consumers in Spain. Apart from setting the intake of coffee and restricting the consumption of certain foods rich in polyphenols and methylxanthines, the study was carried out in free-living subjects who kept their lifestyle unchanged, as far as the dietary and physical activity questionnaires filled out showed. Attending to the dietary records, their energy and macronutrient intakes were slightly below the dietary recommendations established by Moreiras et al. [21] for the Spanish population, except those for proteins, lipids and saturated fatty acids that were slightly above recommended intakes. Overall, it may be considered that volunteers consumed a healthy Mediterranean diet.
The results of the present study show that the consumption of a green/roasted coffee blend is inversely related with three components of MetS: blood pressure, blood glucose and triglyceride levels, which is in agreement with the cross-sectional study by Hino et al. [12]. It is noticeable that the positive effect on triglycerides was more pronounced in the hypercholesterolaemic group. On the other hand, in this study no effects were observed on central obesity or HDL-C, in contrast to the results described in another cross-sectional study when more than two cups/day of coffee were consumed [22]. Again the hypercholesterolaemic group showed a better response to coffee consumption as waist circumference tended to decrease in these subjects.
Our study supports that fasting plasma glucose and triglycerides are among the components of MetS more susceptible to the protective effect of coffee [23]. CGA is the major bioactive compound in coffee likely to be responsible for these health benefits, as the hypoglycaemic and antidiabetic effects of polyphenols are well known, stimulating glucose uptake in both insulin-sensitive and insulin-resistant adipocytes. CGA also improves glucose tolerance and insulin resistance, acting as an active principle in glucose metabolism regulation (reviewed in Meng et al. [8]). In addition, CGA has shown to induce positive effects on lipid metabolism, lowering serum and hepatic cholesterol and triglyceride levels, inhibiting fat absorption, activating fat metabolism in the liver, and improving obesity-related hormone levels among other effects [8]. Relating to the bioavailability and pharmaceutical studies in humans carried out in our group using the green/roasted coffee [24, 25], the biological effects here observed should be mainly attributed to the content in phenolic compounds, without discarding the contribution of methylxanthines. Considering that in the study three doses of coffee were consumed distributed along the day (breakfast, midday and after lunch), it is likely that during the study the levels of phenolic metabolites remained in the blood stream at relatively high concentrations for a long time [24], in contrast, to caffeine and its derived methylxanthines and methyluric which circulated in plasma for a shorter period [25]. However, the transformation of the CGA during roasting and soluble coffee manufacture/brewing is complex. Drastic roasting conditions may produce losses of up to 95 % of CGAs with 8–10 % being lost for every 1 % loss of dry matter [7]. The content of CGA in the coffee blend studied was twice as high as that reported in regular instant coffees (decaffeinated and caffeinated) which ranged between 30 and 40 mg/g [26].
This study has looked into certain adipokines as the regulators of systemic glucose and lipid homoeostasis, attempting to further understand the effects on glucose and triglycerides observed. Moreover, we endeavoured to shed light in the association between coffee consumption and adipokines, with evidences scarce and inconsistent so far. Regarding the relationship between coffee consumption and circulating leptin, an inverse association [27–29] or no change in serum leptin at higher levels of coffee consumption [30] has been reported. Similarly, an inverse association between caffeine consumption and plasma leptin has been described [28, 31]. As for PAI-1, one clinical study has reported an increase in plasma PAI-1 in heavy coffee drinkers compared to light coffee drinkers [32], whereas another intervention observed a decrease in serum levels of PAI-1 with higher consumption of coffee and caffeine [28]. These authors reported no changes on resistin or visfatin levels in a cross-sectional study. Our results support an inverse association between regular coffee consumption and serum concentrations of leptin, PAI-1 and resistin. The possible mechanism underlying the observed adipokine decrease may be related to the effect of phenolic compounds and/or caffeine in the coffee blend decreasing body fat mass, thereby decreasing the number of adipocytes and the subsequent secretion of the studied adipokines. This would be in agreement with the results of an experiment on the efficacy of CGA altering body fat in high-fat diet-induced obese mice in which CGA significantly lowered body weight, visceral fat mass and plasma leptin compared to the high-fat control group that consumed caffeic acid instead of CGA [33]. Leptin is a hormone exclusively excreted by adipocytes. Although the pathophysiological significance of blood leptin levels is not fully understood, it is positively correlated with obesity and inflammatory markers [29]. In contrast, resistin is produced in adipocytes and macrophages, and elevated circulating resistin has been associated with higher risk of type 2 diabetes, inflammation and atherosclerosis [34]. It cannot be ruled out that the observed beneficial effect of coffee consumption on glucose levels has operated through an improved adipokine profile. Visfatin concentrations did not show changes along the study, in agreement with the lack of changes in the visceral fat (waist circumference) where this adipokine is synthesized.
It is noteworthy that according to the results of the present study, the component of MetS most susceptible to the protective effect of coffee was blood pressure. The reduction in systolic and diastolic BP was −5.2 and −5.6 mmHg in the hypercholesterolaemic group, and −3.4 and −2.3 mmHg in the normocholesterolaemic subjects, respectively, which is highly significant considering that volunteers had normal BP values at baseline (Table 1). The magnitude of the reduction in BP is comparable to that in systolic BP (9.2 mmHg) in mild-hypertensive subjects after the intake of 300 mg/d of hydroxycinnamic acids during 8 weeks reported by Ochiai et al. [35]. Considering this, the intake of the green/roasted coffee might be a promising strategy to reduce both systolic and diastolic BP.
Caffeine has an acute pressor effect, particularly in hypertensive subjects, which may be regulated through many biological mechanisms, including binding to the adenosine receptor, activating the sympathetic nervous system via elevated plasma concentration of catecholamines and stimulating the pituitary-adrenocortical response to increase cortisol production. However, changes in the caffeine-induced pressor effect develop in habitual coffee drinkers, as there are a complex set of counterregulatory hormones that maintain blood pressure and may cause tolerance to the humoral and hemodynamic effects of caffeine [36]. Nevertheless, other ingredients in coffee may also have blood pressure control effects, mainly CGA. A group of studies support that CGA in coffee counteracts the effect of caffeine. Among these, a placebo-controlled, randomized clinical trial carried out in patients with mild hypertension showed that CGA (140 mg/day) reduced blood pressure (systolic and diastolic) after 12 week consumption [37]. The same research group previously demonstrated that the short-term ingestion of a green coffee extract (GCE) had hypotensive effects and reported a dose–response relationship from 70 to 280 mg CGA/day [38]. Similarly, in spontaneous hypertensive rats, the ingestion of GCE reduced blood pressure in a dose-dependent manner [39]. Contrarily, in normotensive subjects the consumption of a drink containing green coffee bean extract (140 CGA mg/day) for 4 months did not induce hypotensive effects, although improved vasoreactivity was observed through an increase in the vasodilative response in ischemic reactive hyperaemia [40]. Therefore, the hypotensive effect of CGA in normotensive subjects is not clear, although the present study supports that 345 mg/d of CGA does induce such positive effect in healthy subjects as well as a pilot crossover study carried out in healthy subjects who consumed a green coffee rich in CGA compared to black coffee [41]. Other components in coffee formed during roasting may play a role in coffee’s effect on blood pressure, such as hydroxyhydroquinone (HHQ). Yamaguchi et al. [42] compared the effects of ordinary coffee to HHQ-free coffee: the former had no effect on blood pressure after 4 weeks; in contrast the HHQ-free coffee reduced blood pressure in subjects with mild hypertension which points to HHQ inhibiting the effect of CGA. Once again, in hypertensive rats, HHQ inhibited the hypotensive effects of CGA [43]. In addition, quinides in coffee may counteract caffeine-induced adenosine receptor antagonism by inhibiting the adenosine receptor transporter [44].
Limitations of this study: the study population was healthy or relatively healthy, being the prevalence of metabolic syndrome null. Strengths of the study: Lifestyle characteristics and social status of the volunteers were homogenous. Other background features were controlled such as volunteers’ physical activity and dietary habits. Intake of the coffee was well controlled.
Conclusion
Regular consumption of a green/roasted coffee blend may be recommended to healthy and hypercholesterolaemic subjects to prevent MetS, as it produces positive effects on blood pressure, blood glucose and triglyceride levels, being particularly interesting for hypercholesterolaemic subjects as coffee improved the lipid profile.
Abbreviations
- BMI:
-
Body mass index
- BP:
-
Blood pressure
- CGA:
-
Chlorogenic acid
- FA:
-
Fatty acids
- HDL-C:
-
High-density lipoprotein cholesterol
- HOMA-IR:
-
Homoeostasis model assessment-insulin resistance index
- MetS:
-
Metabolic syndrome
- PAI-1:
-
Plasminogen activator inhibitor-1
- TC:
-
Total cholesterol
References
Grosso G, Marventano S, Galvano F, Pajak A, Mistretta A (2014) Factors associated with metabolic syndrome in a Mediterranean population: role of caffeinated beverages. J Epidemiol 24:327–333
O’Neill S, O’Driscoll L (2015) Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev 16:1–12
Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C (2004) Definition of metabolic syndrome: report of the national heart, lung and blood institute/American heart association conference on scientific issues related to definition. Arterioscler Thromb Vasc Biol 24:e13–e18
Sadikot S, Hermans M (2010) Here we go again … The metabolic syndrome revisited! Diabetes Metab Syndr: Clin Res Rev 4:111–120
Pala L, Monami M, Ciani S, Dicembrini I, Pasqua A, Pezzatini A, Francesconi P, Cresci B, Mannucci E, Rotella CM (2012) Adipokines as possible new predictors of cardiovascular diseases: a case control study. J Nutr Metab 2012:253428
Tunnicliffe JM, Shearer J (2008) Coffee, glucose homeostasis, and insulin resistance: physiological mechanisms and mediators. Appl Physiol Nutr Metab 33:1290–1300
Ludwig IA, Clifford MN, Lean ME, Ashihara H, Crozier A (2014) Coffee: biochemistry and potential impact on health. Food Funct 5:1695–1717
Meng S, Cao J, Feng Q, Peng J, Hu Y (2013) Roles of chlorogenic acid on regulating glucose and lipids metabolism: a review. Evid Based Complement Altern Med 801457
Murase T, Hattori T, Ohtake M, Abe M, Amakusa Y, Takatsu M, Murohara T, Nagata K (2012) Cardiac remodeling and diastolic dysfunction in DahlS.Z-Lepr(fa)/Lepr(fa) rats: a new animal model of metabolic syndrome. Hypertens Res 35:186–193
Li Kwok Cheong JD, Croft KD, Henry PD, Matthews V, Hodgson JM, Ward NC (2014) Green coffee polyphenols do not attenuate features of the metabolic syndrome and improve endothelial function in mice fed a high fat diet. Arch Biochem Biophys 559:46–52
Heckman MA, Weil J, Gonzalez de Mejia E (2010) Caffeine (1,3,7-trimethylxanthine) in foods: a comprehensive review on consumption, functionality, safety, and regulatory matters. J Food Sci 75:R77–R87
Hino A, Adachi H, Enomoto M, Furuki K, Shigetoh Y, Ohtsuka M, Kumagae S, Hirai Y, Jalaldin A, Satoh A, Imaizumi T (2007) Habitual coffee but not green tea consumption is inversely associated with metabolic syndrome: an epidemiological study in a general Japanese population. Diabetes Res Clin Pract 76:383–389
Matsuura H, Mure K, Nishio N, Kitano N, Nagai N, Takeshita T (2012) Relationship between coffee consumption and prevalence of metabolic syndrome among Japanese civil servants. J Epidemiol 22:160–166
Takami H, Nakamoto M, Uemura H, Katsuura S, Yamaguchi M, Hiyoshi M, Sawachika F, Juta T, Arisawa K (2013) Inverse correlation between coffee consumption and prevalence of metabolic syndrome: baseline survey of the Japan Multi-Institutional Collaborative Cohort (J-MICC) Study in Tokushima, Japan. J Epidemiol 23:12–20
Balk L, Hoekstra T, Twisk J (2009) Relationship between long term coffee consumption and components of the metabolic syndrome: the Amsterdam Growth and Health Longitudinal Study. Eur J Epidemiol 24:203–209
Driessen MT, Koppes LL, Veldhuis L, Samoocha D, Twisk JW (2009) Coffee consumption is not related to the metabolic syndrome at the age of 36 years: the Amsterdam Growth and Health Longitudinal Study. Eur J Clin Nutr 63:536–542
Kastorini CM, Panagiotakos DB (2010) The role of the mediterranean diet on the development of the metabolic syndrome. Front Biosci (Elite Ed) 2:1320–1333
Baeza G, Amigo-Benavent M, Sarria B, Goya L, Mateos R, Bravo L (2014) Green coffee hydroxycinnamic acids but not caffeine protect human HepG2 cells against oxidative stress. Food Res Int 62:1038–1046
Martínez-López S, Sarriá B, Baeza G, Mateos R, Bravo-Clemente L (2014) Pharmacokinetics of caffeine and its metabolites in plasma and urine after consuming a soluble green/roasted coffee blend by healthy subjects. Food Res Int 64:125–133
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Moreiras O, Carbajal A, Cabrera L, Cuadrado C (2009) In Tablas de composición de alimentos, ed. Pirámide. 13th edn. Ediciones Pirámide, Madrid, Spain
Grosso G, Stepaniak U, Micek A, Topor-Madry R, Pikhart H, Szafraniec K, Pajak A (2015) Association of daily coffee and tea consumption and metabolic syndrome: results from the Polish arm of the HAPIEE study. Eur J Nutr 54:1129–1137
Grosso G, Pajak A, Mistretta A, Marventano S, Raciti T, Buscemi S, Drago F, Scalfi L, Galvano F (2014) Protective role of the Mediterranean diet on several cardiovascular risk factors: evidence from Sicily, southern Italy. Nutr Metab Cardiovasc Discov 24:370–377
Mateos R, Gómez-Juaristi M, Martínez-López S, Baeza G, Amigo-Benavent M, Sarriá B, Bravo-Clemente L (2015) Bioavailability of hydroxycinnamate derivatives after consuming a soluble green/roasted coffee blend by healthy humans. Poster presented in the International Congress: EuroFoodChem XVIII, Madrid
Martínez-López S, Sarriá B, Baeza G, Mateos R, Bravo-Clemente L (2014) Pharmacokinetics of caffeine and its metabolites in plasma and urine after consuming a soluble green/roasted coffee blend by healthy subjects. Food Res Int 64:125–133
Johnston KL, Clifford MN, Morgan LM (2003) Coffee acutely modifies gastrointestinal hormone secretion and glucose tolerance in humans: glycemic effects of chlorogenic acid and caffeine. Am J Clin Nutr 78:728–733
Lagiou P, Signorello LB, Mantzoros CS, Trichopoulos D, Hsieh CC, Trichopoulou A (1999) Hormonal, lifestyle, and dietary factors in relation to leptin among elderly men. Ann Nutr Metab 43:23–29
Pham NM, Nanri A, Yasuda K, Kurotani K, Kuwahara K, Akter S, Sato M, Hayabuchi H, Mizoue T (2015) Habitual consumption of coffee and green tea in relation to serum adipokines: a cross-sectional study. Eur J Nutr. doi:10.1007/s00394-014-0701-4
Yamashita K, Yatsuya H, Muramatsu T, Toyoshima H, Murohara T, Tamakoshi K (2012) Association of coffee consumption with serum adiponectin, leptin, inflammation and metabolic markers in Japanese workers: a cross-sectional study. Nutr Diabetes 2:e33
Kempf K, Herder C, Erlund I, Kolb H, Martin S, Carstensen M, Koenig W, Sundvall J, Bidel S, Kuha S, Tuomilehto J (2010) Effects of coffee consumption on subclinical inflammation and other risk factors for type 2 diabetes: a clinical trial. Am J Clin Nutr 91:950–957
Westerterp-Plantenga MS, Lejeune MP, Kovacs EM (2005) Body weight loss and weight maintenance in relation to habitual caffeine intake and green tea supplementation. Obes Res 13:1195–1204
Tsioufis C, Dimitriadis K, Vasiliadou C, Taxiarchou E, Vezali E, Tsiamis E, Stefanadis C, Kallikazaros I (2006) Heavy coffee consumption in conjunction with smoking is accompanied by increased inflammatory processes and impaired thrombosis/fibrinolysis system in essential hypertensive subjects. J Hum Hypertens 20:470–472
Cho AS, Jeon SM, Kim MJ, Yeo J, Seo KI, Choi MS, Lee MK (2010) Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem Toxicol 48:937–943
Cao H (2014) Adipocytokines in obesity and metabolic disease. J Endocrinol 220:T47–T59
Ochiai R, Chikama A, Kataoka K, Tokimitsu I, Maekawa Y, Ohishi M, Rakugi M, Mikami H (2009) Effects of hydroxyhydroquinone-reduced coffee on vasoreactivity and blood pressure. Hypertens Res 32:969–974
Zhang Z, Hu G, Caballero B, Appel L, Chen L (2011) Habitual coffee consumption and risk of hypertension: a systematic review and meta-analysis of prospective observational studies. Am J Clin Nutr 93:1212–1219
Watanabe T, Arai Y, Mitsui Y, Kusaura T, Okawa W, Kajihara Y, Saito I (2006) The blood pressure-lowering effect and safety of chlorogenic acid from green coffee bean extract in essential hypertension. Clin Exp Hypertens 28:439–449
Saito I, Tsuchida T, Watanabe T, Arai Y, Mitsui Y, Okawa W, Kajihara Y (2002) Effect of coffee bean extract in essential hypertension. Jpn J Med Pharm Sci 47:67–74
Suzuki A, Yamamoto N, Jokura H, Yamamoto M, Fujii A, Tokimitsu I et al (2006) Chlorogenic acid attenuates hypertension and improves endothelial function in spontaneously hypertensive rats. J Hypertens 24:1065–107336
Ochiai R, Jokura H, Suzuki A, Tokimitsu I, Ohishi M, Komai N, Rakugi H, Ogihara T (2004) Green coffee bean extract improves human vasoreactivity. Hypertens Res 27:731–737
Revuelta-Iniesta R, Al-Dujaili EA (2014) Consumption of green coffee reduces blood pressure and body composition by influencing 11β-HSD1 enzyme activity in healthy individuals: a pilot crossover study using green and black coffee. Biomed Res Int 2014:482704
Yamaguchi T, Chikama A, Mori K, Watanabe T, Shioya Y, Katsuragi Y, Tokimitsu I (2008) Hydroxyhydroquinone-free coffee: a double blind, randomized controlled dose-response study of blood pressure. Nutr Metab Cardiovasc Dis 18:408–414
Suzuki A, Fujii A, Yamamoto N, Yamamoto M, Ohminami H, Kameyama A, Shibuya Y, Nishizawa Y, Tokimitsu I, Saito I (2006) Improvement of hypertension and vascular dysfunction by hydroxyhydroquinone-free coffee in a genetic model of hypertension. FEBS Lett 580:2317–2322
de Paulis T, Schmidt DE, Bruchey AK, Kirby MT, McDonald MP, Commers P, Lovinger DM, Martin PR (2002) Dicinnamoylquinides in roasted coffee inhibit the human adenosine transporter. Eur J Pharmacol 442:215–223
Acknowledgments
Project AGL2010-18269 from the Spanish Ministry of Economy and Competitivity is acknowledged. We want to thank the volunteers who participated in the study and M. Jimenez and L. T. Cayuelas for their assistance in dietary records analysis. S.M.-L. thanks the Spanish National Research Council for her pre-doctoral fellowship under the JAE-Pre programme funded by the European Social Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
Sarriá, B., Martínez-López, S., Sierra-Cinos, J.L. et al. Regularly consuming a green/roasted coffee blend reduces the risk of metabolic syndrome. Eur J Nutr 57, 269–278 (2018). https://doi.org/10.1007/s00394-016-1316-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-016-1316-8