Abstract
Purpose
Cordyceps sinensis has been regarded as a precious tonic food and herbal medicine in China for thousands of years. The exopolysaccharide (EPS) from an anamorph of Cordyceps sinensis was found to have antitumor immunomodulatory activity. Mature dendritic cells play a role in initiating antitumor immunity, so we try to investigate the effects of EPS on the murine dendritic cell line DCS.
Methods
Flow cytometry was used to assay the expression levels of cell surface molecules including major histocompatibility complex (MHC)-II, CD40, CD80, and CD86 of DCS cells and their ability to take up antigens. The ability of DCS cells to activate the proliferation of CTLL-2 T cells was measured by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) method. IL-12 and TNF-α levels were detected using ELISA. Western blotting was performed to estimate the levels of phosphorylated Janus kinase 2 (p-JAK2), phosphorylated signal transducer and activator of transcription 3 (p-STAT3), nuclear factor-κB (NF-κB) p65 and p105.
Results
EPS increased the expressions of MHC-II, CD40, CD80, and CD86 of DCS cells and up-regulated their ability to take up antigens. EPS also enhanced their ability to activate the proliferation of CTLL-2 T cells. IL-12 and TNF-α secreted from DCS cells were up-regulated after EPS treatment. Furthermore, EPS significantly caused the decline of p-JAK2 and p-STAT3, significantly increased levels of NF-κB p65 in the nucleus and decreased levels of NF-κB p105 in the cytoplasm.
Conclusions
EPS may induce DCS cells to exhibit mature characteristics, and the mechanism involved is probably related to the inhibition of the JAK2/STAT3 signal pathway and promotion of the NF-κB signal pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Polysaccharides isolated from various medicinal plants play important roles in the regulation of the human immune system. Polysaccharides from Ganoderma lucidum affect immune cells including B lymphocytes, T lymphocytes, dendritic cells (DCs), macrophages, and natural killer cells. Both in vitro and in vivo studies suggested that the antitumor activities of the polysaccharides are mediated by their immunomodulatory, anti-angiogenic, and cytotoxic effects [1]; polysaccharides from the seed of P. asiatica L. had significant immunity-enhancing activity by inducing the maturation of murine bone marrow-derived dendritic cells (BMDCs) from BALB/c mice [2]; polysaccharide-rich root fraction of Echinacea pupurea may activate the maturation of BMDCs from C57BL/6 [3]. The immunity-regulating EPS is extracted from an anamorph of Cordyceps sinensis, which has been regarded as a precious tonic food and herbal medicine in China for thousands of years.
Our previous research has indicated that the crude exopolysaccharide fraction (EPSF) can inhibit metastasis of melanoma cells in the lungs and the liver of melanoma-bearing mice [4]. The EPSF also significantly enhanced spleen lymphocyte proliferation in tumor-bearing mice and stimulate mouse thymus lymphocytes proliferation both in vivo and in vitro [4, 5]. Levels of Bcl-2, c-Myc, c-Fos, and vascular endothelial growth factor (VEGF) expression in the lungs and livers of melanoma-bearing mice were decreased [4, 6]. These results were confirmed in another kind of animal experiment: EPSF significantly inhibited the H22 tumor growth in ICR mice and elevated the activity of immunocytes. It also enhanced the proliferation and cytotoxicity of spleen lymphocytes and elevated their levels of TNF-α and IFN-γ mRNA expression [7]. These results suggest that the polysaccharide has an immunomodulatory function and antitumor activity, making it a possible adjuvant in cancer therapy.
In order to determine the immunomodulatory mechanism of this EPS, EPSF was further chromatographed by DEAE-32 and Sephadex G-200 column. The components of EPS were analyzed by gas chromatography, and the molecular weight was analyzed by gel chromatography. It consists of mannose, glucose, and galactose in a ratio of 23:1:2.6 and its molecular weight is about 1.04 × 105 [8]. Subsequently, our further investigation revealed that it triggers activation and maturation in murine bone marrow-derived DCs and human DC from peripheral blood [9, 10]. DCs, which are known sentinel component of the immune system, play an important role in the initiation of antitumor immune response [11]. Along with DCs maturation, they increasingly express major MHC and co-stimulatory molecules (CD40, CD80, and CD86) on the surface. These molecules assist DCs to submit co-stimulatory signals and presenting antigens to naive T cells and then activate immune reaction. Thus, high levels of MHC and co-stimulatory molecules of DCs are related to their state of maturation.
To verify our discovery and obtain consistent results, DCS was chosen for further investigation. DCS is the first established DC cell line. Researchers often choose it as a model for DC studies because it has characteristics similar to DCs, but it is easier to propagate and store, providing more pure population of DCs that closely resemble DC progenitors [12]. In this study, we attempted to evaluate the effects of EPS on the phenotype and function of DCS. We also explored parts of the mechanisms involved.
Materials and methods
Reagents
RPMI 1640 (Gibco) supplemented with 50 IU/ml penicillin, 50 IU/ml streptomycin and 2 ml l-glutamine (Sigma), 25 μM Hepes (Amresco), and 10 % heat-inactivated fetal bovine serum (Gibco) was used as a culture medium. Fluorescein isothiocyanate (FITC)-labeled anti-MHC-II/CD80 and phycoerythrin (PE)-labeled anti-CD86/CD40 antibodies were obtained from eBioscience.
Antibodies of phosphorylated signal transducer and activator of transcription 3 (p-STAT3, Tyr705), phosphorylated Janus kinase 2 (p-JAK2) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Cell Signaling Technology (Beverly, MA, USA); antibodies against STAT3, JAK2, NF-κBp65, and p105 and horseradish peroxidase (HRP)-IgG were purchased from Bioworld Technology (Atlanta, GA, USA). Protein inhibitor cocktails were obtained from Amresco. EPS was prepared as previously described [8].
Cell culture and treatment
DCS cell line was obtained from the Cell Culture Center of Peking Union Medical College, and CTLL-2 cell line was purchased from American Type Culture Collection (ATCC), and it is a clone of cytotoxic T cells derived from a C57BL/6 mouse. They were cultured at 37 °C in a 5 % CO2 atmosphere in RPMI 1640 complete culture medium as previous described. EPS was diluted in the medium before being applied to the cultures at a final concentration of 12.5, 25, 50, 100 μg/ml. Lipopolysaccharide (LPS) (Sigma) at 1 μg/ml was used as a positive control. After a 2-day treatment with different concentrations of EPS, cells were collected to analyze their changes in phenotype and detect the stimulatory function on T-cell proliferation.
Phenotype analysis
To confirm the effects of EPS on the phenotype of DCS cells, the surface molecule expression was analyzed by flow cytometry. Cells (1 × 105) were harvested and washed with phosphate-buffered saline (PBS) containing 0.5 % fetal calf serum (FCS) and 0.05 % sodium azide after 2 days of treatment with different concentrations (12.5, 25, 50, and 100 μg/ml) of EPS. Then, they were stained with FITC-labeled anti-MHC-II/CD80 and PE-labeled anti-CD86/CD40 antibodies, respectively, at 4 °C for 30 min. The fluorescence was measured using flow cytometry (FACS Aria II, Becton–Dickinson, U.S.). Forward and side scatter parameters were used to gate live cells. At least 10,000 cells were analyzed per sample.
Lymphoproliferation assay
T-cell proliferation after co-culture with the EPS-treated DCS cells was evaluated to determine the stimulatory capacity of DCS cells. They were collected after 2 days of treatment in different concentrations of EPS (12.5, 25, 50, and 100 μg/ml) and were treated with mitomycin C (50 μg/ml) at 37 °C for 60 min. Then, DCS and CTLL-2 T cells were seeded in the U-bottom 96-well plate at the ratio of 1:10 or 1:1, simultaneously. The untreated cells were used as the control group. Cells were cultured at 37 °C in a 5 % CO2 atmosphere for 3 days. T-cell proliferation was measured by conventional 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay.
Determination of antigen uptake ability
For the antigen uptake assay, DCS cells (5 × 105/well) were incubated with FITC-dextran (1 mg/ml RPMI 1640) at 37 °C for 30 min after treatment with EPS for 48 h. They were then washed three times with ice-cold PBS containing 0.5 % FCS and resuspended in PBS containing 1 % paraformaldehyde. FITC-positive cells were detected using flow cytometry. The background fluorescence of each sample was subtracted from the mean value of fluorescence.
ELISA assay for IL-12 and TNF-α secretion
DCS cells were treated with different concentrations of EPS (12.5, 25, 50, 100 μg/ml) or LPS. Supernatants were collected 24 h after treatment to assess cytokine secretion (IL-12 and TNF-α) using ELISA according to the manufacturer’s recommendations (R&D Systems, Minneapolis, MN, USA).
Preparation of protein
For isolation of total cell extracts, DCS cells (2 × 105/well) were cultured with EPS for 30 min or for 12 h. They were then were collected and washed with PBS. The collected cells were lysed with RIPA Reagent (Beyotime Biotech, China) containing protease inhibitor (1 %) and phosphatase inhibitor (1 %) on ice for 60 min. After centrifugation at 12,000 g/min for 20 min, supernatants were harvested and served as total cell extracts.
To obtain nuclear extracts, DCS cells (2 × 105/well) were cultured with EPS for 12 h, nuclear extracts were isolated according to the protocol described as the previous study [13]. Protein concentration was measured by BCA kit (KeyGen Biotech, Nanjing, China).
Western blot analysis
Equal amounts of protein (50 μg) were boiled in loading buffer (20 mM Tris–HCl with pH 6.8, 10 % glycerol, 4 % sodium dodecyl sulfate (SDS), 100 mM DTT and 0.04 % bromophenol blue) for 5 min and then fractionated with 10 % SDS–polyacrylamide gel electrophoresis (PAGE) and transferred onto nitrocellulose membranes. These membranes were blocked with 5 % bovine serum albumin (BSA, Roche) (prepared in PBS containing 0.1 % Tween 20, PBST) at 37 °C for 1 h and then incubated with an appropriate dilution of primary antibody (p-STAT3 (Tyr705), STAT3, p-JAK2, JAK2, NF-κB p65, NF-κB p105, GAPDH) in 5 % BSA at 37 °C for 1 h. Then, these membranes were washed three times with PBST. Membranes were incubated with HRP-conjugated rat anti-mouse IgG (1:3000) at room temperature for another 2 h. Specific bands were visualized using an enhanced chemiluminescence (ECL) Western Blotting Kit (Beyotime Biotech. Co. Ltd., China) and were quantitated with FluorChem FC2 ImageQuant. Results were analyzed with IPP 6.0 software. p-STAT3 and p-JAK2 were normalized to total STAT3 and JAK2; NF-κB p65 and p105 were normalized to GAPDH.
Statistical analysis
Data represent mean ± standard deviation (SD). Student’s test was used to compare various experimental groups. Significance was set at P-values < 0.05.
Results
Effects of EPS on the phenotype of DCS cells
To determine whether EPS affects the phenotypes of DCS cells, we treated them with EPS for 48 h. Flow cytometry analysis indicated that the basal levels of CD40, CD80, and CD86 expression in DCS cells were much lower. Treatment with EPS greatly increases the levels of CD40, CD80, and CD86 over those of untreated groups (Fig. 1a, b, c) (P < 0.05) with dose dependence (CD40: r = 0.9664, P < 0.01; CD80: r = 0.9490, P < 0.05; CD86: r = 0.9819, P < 0.01). It also causes a significant increase in MHC-II expression in a dose-dependent manner (r = 0.9525, P < 0.05). This was observed in almost every EPS-treated group (Fig. 1d) (P < 0.05).
Phenotypic changes in DCS cells. Representative histograms showing the expression of a CD40, b CD80, c CD86, d MHC-II. After treatment with EPS in different concentrations (12.5, 25, 50, 100 μg/ml) or LPS (1 μg/ml) for 48 h, cells were stained with PE-CD40, FITC-CD80, PE-CD86, or FITC-MHC-II antibodies, fluorescence-labeled cells were gated by flow cytometry. Data are mean of percentage of fluorescence-positive cells ± SD (n = 3). *P < 0.05, ***P < 0.001 compared with the control group
Effects of EPS on the allostimulatory activity of DCS cells
To examine the allostimulatory activity of DCS on T-cell proliferation, DCS cells and CTLL2 T cells were co-cultured. T-cell proliferation response to EPS-treated DCs is presented in Fig. 2. After CTLL-2 T cells were co-cultured for 3 days at a ratio of 1:1 with DCS cells that had been pretreated with EPS, the number of T cells in the EPS-treated group became higher than that of the control group (P < 0.05) in a dose-dependent manner (r = 0.9808, P < 0.01). This indicated that EPS-treated DCs had developed enhanced allostimulatory activity. When DCS cells and T cells were co-cultured at a ratio of 1:10, this effect was not significant (P > 0.05).
Effects of DCS cells on proliferation of T cells. DCS were treated with different concentrations of EPS for 48 h. The indicated numbers of DCS cells were co-cultured with CTLL-2 T cells for another 72 h. The proliferation of the responding T cells was evaluated by MTT. OD value at 570 nm was used to represent the proliferation of T cells. Data are means of OD value (570 nm) ± SD (n = 6). *P < 0.05, **P < 0.01, ***P < 0.001 relative to the group of T cells co-cultured with untreated DCS
Effects of EPS on antigen uptake ability
The ability of DCs to take up antigens was analyzed with dextran-FITC by flow cytometry. After 48 h of treatment with EPS, DCS cells presented dose dependently decreased FITC-dextran uptake ability when compared with the untreated group (25, 50 μg/ml, P < 0.05; 100 μg/ml, P < 0.01; r = 0.9947, P < 0.001). The percentage of FITC-positive cells was reduced to 21.7 % from 53.9 % when treated with 100 μg/ml of EPS (Fig. 3).
Ability of DCS cells to take up antigens as determined by flow cytometry after cells had been stimulated with EPS for 2 days. a Flow cytometry analysis of dextran uptake by DCS. The first curve represents nonspecific fluorescence and the second represents the endocytosis of FITC-dextran. b Statistical results of uptake ability, n = 3. *P < 0.05, **P < 0.01 versus control (0 μg/ml)
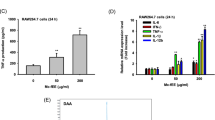
Secretion of IL-12 and TNF-α
DC may secrete cytokines and chemokines that, in turn, attract different immune cells, which are intrinsic to mature DCs. After 24-h treatment with EPS, levels of IL-12 and TNF-α in the culture supernatants were measured with ELISA. The secretion of IL-12 and TNF-α by DCS cells was found to be dose dependently up-regulated in the EPS-treated groups (IL-12, r = 0.9282, P < 0.05; TNF-α, r = 0.9349, P < 0.05) (Fig. 4).
Effects of EPS on JAK2/STAT3 signal pathway in DCS cells
The effect of EPS on phosphorylation of JAK2 and STAT3 in DCS cells was examined by Western blotting. The yields of p-STAT3 and p-JAK2 in EPS-treated cells gradually decreased in a dose-dependent manner (Fig. 5) (p-STAT3, r = 0.9984, P < 0.001; p-JAK2, r = 0.9788, P < 0.01). No significant difference in expression of STAT3 or JAK2 was detected in any group.
Activation of NF-κB signal pathway in DCS cells by EPS
The effect of EPS on the NF-κB signal pathway of DCs was further examined. After 12 h of treatment with EPS, levels of NF-κB p65 and p105 were also detected by Western blotting. P65 translocation into the nucleus was analyzed in the nuclear protein of DCS cells, while the level of NF-κB p105 was analyzed with respect to total cellular protein. Relative levels of NF-κB p65/GAPDH and NF-κB p105/GAPDH were determined to confirm the activation of NF-κB signal pathway. As shown in Fig. 6, EPS treatment significantly and dose dependently increased NF-κB p65 levels in the nucleus (r = 0.9634, P < 0.01) and decreased NF-κB p105 levels (r = 0.9453, P < 0.05) in the cytoplasm in all groups except the EPS12.5 group. This means that EPS markedly promoted both translocation of NF-κB p65 into the nucleus and degradation of p105 in the cytoplasm.
Discussion
The maturation of DCs is a critical step in antitumor immunity. These cells are characterized by their high expression of co-stimulatory molecules and MHC-II, their ability to induce lymph proliferation, and their high cytokine production [14]. The DCs found in tumors usually represent immature and are defective in these respects. This causes them to lose their ability to prime naïve T cells and so impairs antitumor activity [15–17].
EPS has several kinds of biological activity and performs immunoregulatory functions, some of which have been studied in our previous investigation. We here aimed to investigate the effect of EPS on the phenotypes of DCS cells and on their ability to take up antigens, secrete cytokines, and stimulate T cells. The results showed that, after EPS treatment, DCS cells expressed co-stimulatory molecules and MHC-II at a higher rate, gained an enhanced ability to activate T cells, attenuated DC endocytic ability, and secreted cytokines (IL-12 and TNF-α), which are characteristics of mature DCs. All of these results indicated that EPS can stimulate the maturation of DCs.
These results raise questions regarding the mechanisms underlying this process. Several studies have provided evidence of hyperactivation of STAT3 in DCs in tumor-filtered tissues [18, 19]. STAT3 hyperactivation is associated with defective DC maturation mediated by tumor cells and tumor-derived factors [20, 21]. JAK2/STAT3 hyperactivation has also been observed in myeloid cells and tumor cells. Our previous studies have shown that EPS can decrease STAT3 activation in murine and human DCs. Here, we investigated the phosphorylation of STAT3 in DCS and results demonstrated a reduced p-STAT3 expression in DCS that had been exposed to EPS. The reduction of p-STAT3 does not conclusively indicate the mechanism underlying this process. In our present study, we detected the phosphorylation of JAK2, a Janus kinase that in turn phosphorylates tyrosine residues on receptors [22]. These receptors ultimately serve as docking sites for STATs. Our findings indicated that the p-JAK2 in DCS cells was also down-regulated by EPS. These results suggest that EPS may decrease the tumor character of DCS and promote maturation through blockage of the JAK2/STAT3 pathway.
Nuclear factor-κB (NF-κB) plays a pivotal role in developmental and immunological processes [23]. This signal pathway had previously been studied in antigen presentation cell (APC) activation. Its activation is usually associated with a series of up-regulated expression of pro-inflammatory mediators including cytokines, adhesion molecules and co-stimulatory molecules, which is usually also involved in the process of DC maturation [24, 25]. The mammalian NF-κB family includes RelA (p65), RelB, and c-Rel as well as p50 and p52 [26]. Knockouts of any of these subunits can be embryonically lethal [27]. IκB family members usually exist in the cytoplasm and block the NF-κB p65/p50 complex to translocate into nucleus. It can then be phosphorylated by the activation of IκB kinase (IKK) complex, leading to ubiquitination and subsequent degradation, releasing NF-κB subunits into the nucleus to activate the expression of a number of target genes. p105 is also considered as one of the members of the IκB family members. Its degradation may be triggered by the activated IKK complex [28–30]. In this study, we detected the levels of NF-κB p65 in the nucleus and of p105 in the cytoplasm. The results showed profoundly increased accumulation of p65 in the nucleus and degradation of p105 in the cytoplasm of DCS cells after EPS treatment. In this way, we primarily concluded that EPS could induce NF-κB activation in DCs.
In conclusion, our findings clearly show that EPS can effectively cause DCs to express MHC-II, CD40, CD80, and CD86; secrete IL-12 and TNF-α; and exhibit enhanced stimulatory capacity for T cells, and weakened endocytic ability. We also observed JAK2/STAT3 inhibition and NF-κB activation in EPS-treated DCS cells. The immunomodulatory functions of EPS on DCS may be corporately mediated through these two signal pathways. This may partially account for the antitumor function of the EPS from Cordyceps sinensis anamorph.
References
Xu Z, Chen X, Zhong Z, Chen L, Wang Y (2011) Ganoderma lucidum polysaccharides: immunomodulation and potential anti-tumor activities. Am J Chin Med 39(1):15–27
Huang DF, Tang YF, Nie SP, Wan Y, Xie MY, Xie XM (2009) Effect of phenylethanoid glycosides and polysaccharides from the seed of Plantago asiatica L. on the maturation of murine bone marrow-derived dendritic cells. Eur J Pharmacol 620:105–111
Benson JM, Pokorny AJ, Rhule A, Wenner CA, Kandhi V, Cech NB, Shepherd DM (2010) Echinacea purpurea extracts modulate murine dendritic cell fate and function. Food Chem Toxicol 48:1170–1177
Zhang W, Yang J, Chen J, Hou Y, Han X (2005) Immunomodulatory and antitumour effects of an exopolysaccharide fraction from cultivated Cordyceps sinensis (Chinese caterpillar fungus) on tumor-bearing mice. Biotechnol Appl Biochem 42:9–15
Yang J, Zhang W, Shi P, Ling L, Hou Y, Wu J (2004) Effects of exopolysaccharide from Tolypocladium sinense on murine immunocytes in vitro. Zhong Yao Cai 27:930–932
Yang J, Zhang W, Shi P, Chen J, Han X, Wang Y (2005) Effects of exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis fungus on c-Myc, c-Fos and VEGF expressions in B16 melanoma bearing mice. Pathol Res Pract 201:745–750
Zhang W, Li J, Qiu S, Chen J, Zheng Y (2008) Effects of the exopolysaccharide fraction (EPSF) from a cultivated Cordyceps sinensis on immunocytes of H22 tumor bearing mice. Fitoterapia 79:168–173
Sheng L, Chen J, Li J, Zhang W (2011) An exopolysaccharide from cultivated Cordyceps sinensis and its effects on cytokine expressions of immunocytes. Appl Biochem Biotechnol 163:669–678
Song D, Lin J, Yuan F, Zhang W (2011) Ex vivo stimulation of murine dendritic cells by an exopolysaccharide from one of the anamorph of Cordyceps sinensis. Cell Biochem Funct 29:555–561
Huang J, Song D, Yang A, Yin H, Zhang W (2011) Differentiation and maturation of human dendritic cells modulated by an exopolysaccharide from a cultivated Cordyceps sinensis. Biomed Prev Nutr 1:126–131
Dieu MC, Vanbervliet B, Vicari A, Bridon JM, Oldham E, Aït-Yahia S, Brière F, Zlotnik A, Lebecque S, Caux C (1998) Selective recruitment of immature and mature dendritic cells by distinct chemokines expressed in different anatomic sites. J Exp Med 188:373–386
Zhou SX, Liu YQ, Weng JH (2005) Expression profiles of mouse dendritic cell sarcoma are similar to those of hematopoietic stem cells or progenitors by clustering and principal component analyses. Biochem Biophys Res Commun 331:194–202
Chen W, Zhang W, Shen W, Wang K (2010) Effects of the acid polysaccharide fraction isolated from a cultivated Cordyceps sinensis on macrophages in vitro. Cell Immunol 262:69–74
Tang Z, Saltzman A (2004) Understanding human dendritic cell biology through gene profiling. Inflamm Res 53:424–441
Cheng F, Wang HW, Cuenca A, Huang M, Ghansah T, Brayer J, Kerr WG, Takeda K, Akira S, Schoenberger SP, Yu H, Jove R, Sotomayor EM (2003) A critical role for Stat3 signaling in immune tolerance. Immunity 19(3):425–436
Sombroek CC, Stam AG, Masterson AJ, Lougheed SM, Schakel MJ, Meijer CJ, Pinedo HM, van den Eertwegh AJ, Scheper RJ, de Gruijl TD (2002) Prostanoids play a major role in the primary tumor-induced inhibition of dendritic cell differentiation. J Immunol 168:4333–4343
Bharadwaj U, Li M, Zhang R, Chen C, Yao Q (2007) Elevated interleukin-6 and G-CSF in human pancreatic cancer cell conditioned medium suppress dendritic cell differentiation and activation. Cancer Res 67:5479–5488
Haura EB, Turkson J, Jove R (2005) Mechanisms of disease: insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol 2:315–324
Nefedova Y, Huang M, Kusmartsev S, Bhattacharya R, Cheng P, Jove R, Salup R, Gabrilovich D (2004) Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J Immunol 172:464–474
Nefedova Y, Nagaraj S, Rosenbauer A, Muro-Cacho C, Sebti SM, Gabrilovich DI (2005) Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res 65:9525–9535
Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H (2004) Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med 10:48–54
Wang L, Kurosaki T, Corey SJ (2007) Engagement of the B-cell antigen receptor activates STAT through Lyn in a Jak-independent pathway. Oncogene 26:2851–2859
Ghosh S, Karin M (2002) Missing pieces in the NF-κB puzzle. Cell 109(Suppl):S81–S96
Neumann M, Fries H, Scheicher C, Keikavoussi P, Kolb-Mäurer A, Bröcker E, Serfling E, Kämpgen E (2000) Differential expression of Rel/NF-kappaB and octamer factors is a hallmark of the generation and maturation of dendritic cells. Blood 95:277–285
Kim E, Kim SH, Kim S, Kim TS (2006) The novel cytokine p43 induces IL-12 production in macrophages via NF-kappaB activation, leading to enhanced IFN-gamma production in CD4+T cells. J Immunol 176:256–264
Verma IM, Stevenson JK, Schwarz EM, Van Antwerp D, Miyamoto S (1995) Rel/NF-kappa B/I kappa B family: intimate tales of association and dissociation. Genes Dev 9:2723–2735
Gerondakis S, Grossmann M, Nakamura Y, Pohl T, Grumont R (1999) Genetic approaches in mice to understand Rel/NF-κB and IκB function: transgenics and knockouts. Oncogene 18:6888–6895
Kar S, Ukil A, Das PK (2011) Cystatin cures visceral leishmaniasis by NF-κB-mediated proinflammatory response through co-ordination of TLR/MyD88 signaling with p105-Tpl2-ERK pathway. Eur J Immunol 41:116–127
Karin M, Ben-Neriah Y (2000) Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol 18:621–663
Nicholas C, Batra S, Vargo MA, Voss OH, Gavrilin MA, Wewers MD, Guttridge DC, Grotewold E, Doseff AI (2007) Apigenin blocks lipopolysaccharide-induced lethality in vivo and proinflammatory cytokines expression by inactivating NF-{kappa}B through the suppression of p65 phosphorylation. J Immunol 179:7121–7127
Acknowledgments
This study was financed by the National Nature Science Foundation of China (No. 30873188) and the Fundamental Research Funds for the Central Universities (No. 1112021402).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Song, D., He, Z., Wang, C. et al. Regulation of the exopolysaccharide from an anamorph of Cordyceps sinensis on dendritic cell sarcoma (DCS) cell line. Eur J Nutr 52, 687–694 (2013). https://doi.org/10.1007/s00394-012-0373-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-012-0373-x