Abstract
Purpose
To assess vitamin D status in children aged 2–220 months in northeastern Italy (latitude 46°). Serum 25-hydroxyvitamin D (25OHD) concentration was assessed in 93 children afferent to the Pediatric Department of the Hospital of Udine.
Methods
Vitamin D status was defined as follows: sufficient with serum 25OHD between 50 and 250 nmol/l (level 4); insufficient between 37.5 and 50 nmol/l (level 3); deficient less than 37.5 nmol/l (level 2); severely deficient if less than 12.5 nmol/l (level 1). We investigated the potential risk factors of vitamin D deficit.
Results
We found that six children (6.4%) had level 1, 36 (38.7%) had level 2, 9 (9.7%) had level 3, and only 45.2% had sufficient level of 25OHD. Immigrate children had a higher risk for vitamin D deficiency if compared with Italians: 75% of non-Italian children had an insufficient 25OHD level compared with 47.0% of Italian children (p = 0.0036). There was a marked seasonal effect on 25OHD level: when plasma sample was withdrawn between November and May, only 29.4% of children showed sufficient vitamin D level, while 70.5% was insufficient (p < 0.0001). Among the obese children, 9.0% had sufficient level of 25OHD with 90% being deficient (p = 0.01). We did not find any significant difference in vitamin D status among children in different age groups.
Conclusion
Vitamin D deficiency is common in children living in northeastern Italy. The risk factors were winter season for blood withdrawal, non-Caucasian race, and obesity. These high-risk groups should be targeted for screening and educated about the need of sunlight exposure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Nutritional rickets is known to be reemerged in certain pediatric populations such as exclusively breast-fed and dark-skinned infants due to the low vitamin D content of breast milk (range: 15–50 international units (IU)/l in a vitamin D sufficient mother) and to the reduced vitamin D synthesis of dark skin especially at high latitude with poor sun exposition [1]. In fact, vitamin D can be synthesized endogenously and factors affecting its cutaneous synthesis include age, season, latitude, time of day, skin pigmentation, amount of skin exposed, and use of sunscreen. For this reason in 2003, the American Academy of Pediatrics (AAP) recommended prophylaxis with vitamin D (200 UI/day) in exclusively breast-fed infants or infants who did not consume at least 500 ml of vitamin D fortified formula [2]. Rickets, however, is not limited to infancy and early childhood, as evidenced by cases of rickets caused by nutritional vitamin D deficiency being reported in adolescents, so the last AAP guideline to prevent vitamin D deficiency recommends an intake of 400 IU/day in infants, children, and adolescents [3]. It should be noted that Canadian Pediatric Society recommends a higher supplementation (800 IU/day) in breast-fed infants during the winter months [4].
Many recent studies demonstrate that hypovitaminosis D is common in the general US pediatric population, also among children and adolescents probably due to the predominantly indoor lifestyle, the low dietary vitamin D intake, associated obesity, and extensive use of sunscreen [5]. Few foods naturally contain vitamin D, and in Italy, there are actually few fortified foods. Vitamin D insufficiency may have important health consequences because of its role in the maintenance of normal bone mass turnover. Two studies conducted in adolescent girls in Finland suggested that lower vitamin D status correlated with smaller gains in lumbar spine area bone mineral density [6, 7]. Other studies have demonstrated that vitamin D may confer protection against diabetes mellitus type 1, hypertension, multiple sclerosis, and cancer [5].
The serum 25-hydroxyvitamin D (25OHD) is the most common measured indicator of vitamin D status because it reflects dietary intake from vitamin D2 and D3 together with cutaneous synthesis of vitamin D3 [8].
To date, few studies have assessed vitamin D status among Italian children, especially among those living at a high latitude or immigrated [9]. The purpose of the present study is to assess serum 25OHD in children living in the Italian province of Udine, northeastern Italy, (latitude 46°) and to identify risk factors for vitamin D deficiency in different age groups.
Methods
We conducted a prevalence study of vitamin D status among children of the province of Udine (northeastern Italy). We enrolled 93 children (aged 2–220 months) afferent to the Pediatric Department (Day Hospital and Ward) of the University Hospital of Udine from July 21, 2009, to June 14, 2010. Children were enrolled if they attended our Day Hospital and Ward and had to undergo a blood withdrawal for reasons other than the present survey. Parents provided written consent to participate in the study.
All children underwent physical examination and performed laboratory test of plasma 25OHD level. Their parents completed a standardized interview recording: race, religion, country of birth, birthweight, type of feeding during the first year (breast, formula milk, or mixed), mother’s use of vitamin supplementation during pregnancy, child’s use of vitamin D supplementation, and daily intake of cow milk (categorized as more or less than 200 ml per day).
Concentration of 25OHD in EDTA was determined by an immunoassay implemented on a Liaison automatic analyzer (The Liaison 25OH Vitamin D Total, DiaSorin Inc., Stillwater, MN, USA). The Liaison total 25OH assay is a direct competitive chemiluminescence immunoassay (CLIA) for quantitative determination of total 25OHD in human serum (SRM 972, Level 1, standard reference material from the National Institute of Standards and Technology, NIST) [10]. Test within-batch precision (CV%) is <5.5 and between-batch precision (CV%) is <12.9. Vitamin D status was defined as follows: sufficient with 25OHD plasma concentration between 50 and 250 nmol/l (20–100 ng/ml), or level 4; insufficient between 37.5 and 50 nmol/l (15–20 ng/ml), or level 3; deficient less than 37.5 nmol/l (15 ng/ml), or level 2; severely deficient if less than 12.5 nmol/l (5 ng/ml), or level 1 (Table 1). These reference ranges were used in previous pediatric literature, on the basis of biomarkers such as changes in alkaline phosphatase (ALP), bone density, calcium absorption at varying vitamin D level, and the presence of rickets [1].
Race was categorized as black, Caucasian, Indian, and Hispanic-American. Regarding seasonality, although solar winter comprises the period from November to February, for the purpose of the study, winter was defined as the period November–May and summer as June–October.
Children were divided into the following age groups: infants (1–23 months), children (2–12 years), and adolescents (13–18 years).
Obesity was categorized on the basis of body mass index (over 95°p) and ideal body weight (over 140%), defined by using age and gender CDC centile curves on the basis of pooled international data [11].
Statistical analyses
The characteristics of the study population were described through frequency distributions for categorical variables and through means and standard deviations (SD), medians, and range for continuous variables. To allow for a computation of those measures, 25OHD concentrations below the detection limit of 10 nmol/l were assumed to be equal to 10 nmol/l. The crude association of vitamin D status and the other categorical variables was assessed through chi-square test and Fisher’s exact test when the expected number of counts in any cell of the contingency tables was <5. The crude association of vitamin D status and continuous variables was assessed through Wilcoxon’s rank sums test (with 25OHD concentration dichotomized into sufficient (50–250 nmol/l) and insufficient (<50 nmol/l)) and Kruskal–Wallis test (with 25OHD concentration divided in four levels) since all continuous variables resulted not normally distributed according to Shapiro–Wilk’s test. All p-values <0.05 were considered statistically significant. Multivariate logistic regression analyses were performed to investigate factors associated with insufficient vitamin D status, after adjusting for potential confounders. The association between 25OHD concentration and each variable included in the logistic model was expressed in terms of odds ratio (OR) and 95% confidence interval (95% CI).
Results
The participants’ characteristics and 25OHD level are presented in Table 1. Among those 93 children, the mean 25OHD concentration was 55.6 nmol/l (SD 39.1, median 44.0, range 10–184 nmol/l). Six children (6.4%) had level 1 (severe deficiency), 36 (38.7%) had level 2 (deficiency), 9 (9.7%) had level 3 (insufficiency), and 42 (45.2%) had level 4 (sufficiency). Among those with sufficient level, mean concentration was 90.9 nmol/l (SD 32.3, median 84, range 51–184 nmol/l).
Race and country of birth
Among all children with 25OHD level under 50 nmol/l, 15.7% were black, 76.4% were Caucasian, 5.8% were Indian, and 1.9% were Hispanic.
Vitamin D deficiency was found in all Indian children (n = 3), in 88% (n = 8) of dark-skinned children (with 44.4% having severe insufficiency, n = 4), compared with 48.7% (n = 39) of Caucasian children. Dark-skinned infants had significantly lower plasma 25OHD level than light-skinned infants and, after adjusting for potential confounders, were significantly more likely to have severe deficiency (Table 2).
Immigrate children had a higher risk for vitamin D deficiency if compared with Italians: 75% of non-Italian children had an insufficient level of 25OHD (20% with level 1, 44% level 2, and 12% level 3) compared with 47.0% of Italian children (p = 0.0036).
Among children with 25OHD in range of severe deficiency, one 14-month-old infant from Morocco showed clinical and radiological finding of rickets.
Season of blood withdrawal
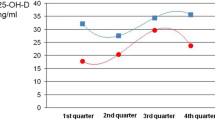
There was a marked seasonal effect on 25OHD levels, in fact when the plasma sample was withdrawn between November and May, only 29.4% (n = 20) of children showed sufficient 25OHD level, while 70.5% (n = 48) was insufficient (6.4% level 1, 36.6% level 2, and 8.6% level 3). In the period June–October, 88% (n = 22) of children had normal levels and only 12% (n = 3) were insufficient, none having severe deficiency. Ninety-four percent of children (n = 48) whose sample was recorded between November and May had level of plasma 25OHD under 50 nmol/l (Table 2, Fig. 1).
We found 25OHD in the range of severe insufficiency only during winter months.
Using 75 nmol/l as the cut off value for Vitamin D sufficiency, we found that 28 children of our sample (30.1%) were above this level (64% were sufficient during summer period and only 17.6% during winter, p < 0.001).
Age groups
We did not find any significant difference regarding the level of 25OHD comparing children in different age groups.
Obesity
Among all the obese children, only 9.0% (n = 1) had sufficient level of 25OHD with 90% (n = 10) being deficient (p = 0.01). Dividing the sample of obese children by 25OHD levels: 9.0% (n = 1) had level 1, 72.7% (n = 8) had level 2, and 9.0% (n = 1) had level 3 (p = 0.02).
Cow milk
The amount of cow milk consumption per day was inversely correlated with 25OHD levels, with lower milk intake in children with 25OHD in the range of severe insufficiency (see Table 3), in particular, only 13.5% of children with severe insufficiency (n = 5) consumed more than 200 ml of cow milk per day versus 35.1% of children (n = 13) with sufficient 25OHD level (p = 0.03). This difference is no longer statistically significant when considering only two categories of 25OHD level—sufficient/insufficient.
No significant associations were observed between plasma 25OHD (neither as sufficient/insufficient nor by four levels) and the following variables: sex, religion, pregnancy (normal or pathological), gestational age, iron deficiency, and the presence of causes of vitamin D malabsorption (hepatic disease, drugs, or celiac disease).
In multivariate analysis, we confirm that the factors significantly associated with vitamin D deficit are winter season for blood withdrawal, non-Caucasian race, and obesity (see Table 4).
Quartiles
Using 25OHD levels in quartiles (level 1: <25 nmol/l, level 2: 25–43 nmol/l, level 3: 44–82 nmol/l, level 4: >83 nmol/l), we confirm a significant association between low 25OHD levels and non-Caucasian ethnicity (p = 0.04), obesity (p = 0.02), and blood withdrawal in winter season (p < 0.001). We found a significant association with pathologic pregnancy and low 25OHD levels (p = 0.03), but no more with cow milk consumption (p = 0.35).
Discussion
This study provides data on the prevalence of 25OHD status and its correlates in a sample of children 2–220 months of age afferent to our Department of Pediatric (Udine, Italy). It was expected to find some vitamin D deficiency among children knowing to be at high risk (dark-skinned, exclusively breast-fed infants that not receive preformed vitamin D), but it was surprising the extent of the deficiency in children and adolescent. We found a high prevalence of vitamin D deficiency (>50% of our population) in our sample at different age groups.
Consistent with previous studies [12, 13], we demonstrate that the risk of vitamin D deficiency is higher in dark-skinned population, living at high latitudes, as the immigrates from India and Africa if compared with the white children. Increased melanin in the skin has been shown to decrease the production of precursors of vitamin D per the same dose of exposure to UV radiation.
In our study, season strongly influences vitamin D status, as observed in some studies performed in countries at northern latitudes and in different age groups [14–18]. These data are the result of less skin production of vitamin D during winter months and less sunlight exposure of the children. In fact in winter months and with increasing latitude, the number of ultraviolet (UV) radiation reaching the earth’s atmosphere is decreased, because the rays of the sun enter the atmosphere at an oblique angle so that UV–B photons pass through a greater distance of the atmosphere and were more efficiently absorbed by ozone. As a consequence, little vitamin D is produced in the skin [19]. Moreover, during winter months, children spend more time indoors and the amount of skin exposed to the sun is lower.
We did not find any difference in the prevalence of 25OHD in range of deficiency between infants, children, and adolescent (15.6, 60.7 and 23.5%, respectively, p = 0.3). Prescription of vitamin D supplementation by Pediatricians did not appear to be a protective factor. Only 33.3% of our sample (n = 30) received vitamin D supplementation, and this was limited to the first 12 months of life. These data confirm the need to extend vitamin D prophylaxis in all children until after the pubertal growth spurt as suggested by the new AAP recommendations [4] particularly in high-risk population.
Possible reasons for the prevalence of hypovitaminosis D in children and adolescent include the following: increased urbanization and air pollution, increased time spent indoors, and extensive use of sunscreens—all these factors leading to reduction in vitamin D skin synthesis—but also less dietary intake of calcium and vitamin D (unusual diet such as macrobiotic, use of beverages alternative to cow milk) [20].
We demonstrate an inverse association between cow milk intake and 25OHD deficit, although of limited statistical significance, concordant with other studies in which the nutritional deficiency was attributed to the intake of milk alternative (such as soy milk, cereals) that provide little vitamin D and interfere with calcium uptake [21, 22]. A recent study found that American women with low milk intake during childhood had a lower bone mass during adulthood and a higher risk of fracture, suggesting skeletal implication of this trend [23]. In Italy, the content of vitamin D in cow milk is 0.01–0.03 μg/100 ml in partially skimmed milk and not skimmed milk, respectively [24].
As shown in recent studies, we confirm that obese children were at higher risk of vitamin D deficit. These are likely due to the decreased vitamin D bioavailability from cutaneous and dietary sources because of its deposition in body fat, and because obese children may have a more sedentary, indoor lifestyle [25, 26].
A recent study [11] have demonstrated that 25OHD deficiency (defined as 25OH level <15–20 ng/ml) was associated with elevated PTH hormone level (>65 pg/ml), thus confirming the importance of a sufficient vitamin D status to maintain a normal bone metabolism, and with other cardiovascular risk factors (hypertension and HDL cholesterol level).
Consistent with other studies, non-white race and non-Caucasian ethnicity was a strong predictor of 25OHD deficiency. This association was present even after adjustment for potentially modifiable risk factors including season, milk intake, and breast feeding. Increased melanin in the skin decreases the production of vitamin D for the same dose of exposure to UV radiation.
We found no difference in vitamin D status based on age groups confirming that risk of vitamin D deficiency is not only a problem of infants but also children and adolescence should be supplemented especially in winter months and if conducting an extremely sedentary lifestyle. Race and season were the strongest predictors of 25OHD status.
This study has several important limitations: we did not measure calcium, phosphorus, ALP, and PTH, which might have been useful to assess the biochemical consequences of low 25OHD levels. We collected only one sample of 25OHD level, without repetition of the blood sample. Previous studies, however, have observed suppression of PTH at 25OHD concentration between 30 and 50 nmol/l in children aged 7–10 years and between 40 and 60 nmol/l in adolescent [27, 28], so under our cut off of 50 nmol/l, we would expect an elevation in PTH level. In addition, we lack information about sun exposure, weekly outdoor activities, sunscreen use, and use of milk alternative such as soft drinks and juice. Finally, we performed a prevalence study of children afferent to our Department of Pediatrics, but the results may not be generalizable to the general population living in the same area.
Conclusion
In conclusion, low 25OHD status was more common in children living in Northern Italy during winter months. Vitamin D deficiency is a problem spanning the age spectrum, particularly among immigrates, dark-skinned children resident in northern latitude, obese children, and children with low cow milk consumption. These high-risk groups should be targeted for screening for hypovitaminosis D. Further studies are needed to document the effects of screening for and treatment of hypovitaminosis D and to assess the importance of vitamin D prophylaxis also in school children and adolescents. It is also important to educate the general population about the need of sunlight exposure and the minimal amount of time required to synthesize vitamin D.
References
Misra M, Pacaud D, Petryk A, Collett-Solberg PF, Kappy M (2008) Drug and Therapeutics Committee of the Lawson Wilkins Pediatric Endocrine Society. Vitamin D deficiency in children and its management: review of current knowledge and recommendations. Pediatrics 122(2):398–417
Gartner LM, Greer FR (2003) Section on Breastfeeding and Committee on Nutrition. American Academy of Pediatrics. Prevention of rickets and vitamin D deficiency: new guidelines for vitamin D intake. Pediatrics 111(4 Pt 1):908–910
Wagner CL, Greer FR (2008) American Academy of Pediatrics Section on Breastfeeding; American Academy of Pediatrics Committee on Nutrition. Prevention of rickets and vitamin D deficiency in infants, children, and adolescents. Pediatrics 122(5):1142–1152
Canadian Paediatric Society Position Statement (2002) Vitamin D supplementation in northern native communities. Paediatr Child Health 7(7):459–472 (English, French)
Rovner AJ, O’Brien KO (2008) Hypovitaminosis D among healthy children in the United States: a review of the current evidence. Arch Pediatr Adolesc Med 162(6):513–519
Cheng S, Tylavsky F, Kröger H, Kärkkäinen M, Lyytikäinen A, Koistinen A, Mahonen A, Alen M, Halleen J, Väänänen K, Lamberg-Allardt C (2003) Association of low 25-hydroxyvitamin D concentrations with elevated parathyroid hormone concentrations and low cortical bone density in early pubertal and prepubertal Finnish girls. Am J Clin Nutr 78(3):485–492
Lehtonen-Veromaa M, Möttönen T, Irjala K, Kärkkäinen M, Lamberg-Allardt C, Hakola P, Viikari J (1999) Vitamin D intake is low and hypovitaminosis D common in healthy 9- to 15-year-old Finnish girls. Eur J Clin Nutr 53(9):746–751
Heaney RP (1999) Lessons for nutritional science from vitamin D. Am J Clin Nutr 69(5):825–826
Lippi G, Montagnana M, Targher G (2007) Vitamin D deficiency among Italian children. CMAJ 177(12):1529–1530 author reply 1530
Wallace AM, Gibson S, De La Hunty A, Lamberg-Allardt C, Ashwell M (2010) Measurement of 25-hydroxyvitamin D in the clinical laboratory: current procedures, performance characteristics and limitations. Steroids 75:477–488
Kumar J, Muntner P, Kaskel FJ, Hailpern SM, Melamed ML (2009) Prevalence and associations of 25-hydroxyvitamin D deficiency in US children: NHANES 2001–2004. Pediatrics 124(3):e362–e370 [Epub 2009 Aug 3]
Kreiter SR, Schwartz RP, Kirkman HN Jr, Charlton PA, Calikoglu AS, Davenport ML (2000) Nutritional rickets in African American breast-fed infants. J Pediatr 137(2):153–157
Callaghan AL, Moy RJ, Booth IW, Debelle G, Shaw NJ (2006) Incidence of symptomatic vitamin D deficiency. Arch Dis Child 91(7):606–607 [Epub 2006 Apr 4]
Specker BL, Valanis B, Hertzberg V, Edwards N, Tsang RC (1985) Sunshine exposure and serum 25-hydroxyvitamin D concentrations in exclusively breast-fed infants. J Pediatr 107(3):372–376
Gessner BD, Plotnik J, Muth PT (2003) 25-Hydroxyvitamin D levels among healthy children in Alaska. J Pediatr 143(4):434–437
Markestad T (1983) Effect of season and vitamin D supplementation on plasma concentrations of 25-hydroxyvitamin D in Norwegian infants. Acta Paediatr Scand 72(6):817–821
El Hayek J, Egeland G, Weiler H (2010) Vitamin D status of Inuit preschoolers reflects season and vitamin D intake. J Nutr 140(10):1839–1845 [Epub 2010 Aug 11]
Huotari A, Herzig KH (2008) Vitamin D and living in northern latitudes—an endemic risk area for vitamin D deficiency. Int J Circumpolar Health 67(2–3):164–178
Holick MF (2004) Sunlight and vitamin D for bone health and prevention of autoimmune diseases, cancers, and cardiovascular disease. Am J Clin Nutr 80(6 Suppl):1678S–1688S
Wharton B, Bishop N (2003) Rickets. Lancet 362(9393):1389–1400
Carvalho NF, Kenney RD, Carrington PH, Hall DE (2001) Severe nutritional deficiencies in toddlers resulting from health food milk alternatives. Pediatrics 107(4):E46
Edidin DV, Levitsky LL, Schey W, Dumbovic N, Campos A (1980) Resurgence of nutritional rickets associated with breast-feeding and special dietary practices. Pediatrics 65(2):232–235
Kalkwarf HJ, Khoury JC, Lanphear BP (2003) Milk intake during childhood and adolescence, adult bone density, and osteoporotic fractures in US women. Am J Clin Nutr 77(1):257–265
Banca Dati di Composizione degli Alimenti per Studi Epidemiologici in Italia a cura di Gnagnarella P, Salvini S, Parpinel M. Versione 1.2008. http://www.ieo.it/bda
Gordon CM, DePeter KC, Feldman HA, Grace E, Emans SJ (2004) Prevalence of vitamin D deficiency among healthy adolescents. Arch Pediatr Adolesc Med 158(6):531–537
Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF (2000) Decreased bioavailability of vitamin D in obesity. Am J Clin Nutr 72(3):690–693
Harkness L, Cromer B (2005) Low levels of 25-hydroxy vitamin D are associated with elevated parathyroid hormone in healthy adolescent females. Osteoporos Int 16(1):109–113 [Epub 2004 Jun 2]
Docio S, Riancho JA, Pérez A, Olmos JM, Amado JA, González-Macías J (1998) Seasonal deficiency of vitamin D in children: a potential target for osteoporosis-preventing strategies? J Bone Miner Res 13(4):544–548
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Marrone, G., Rosso, I., Moretti, R. et al. Is vitamin D status known among children living in Northern Italy?. Eur J Nutr 51, 143–149 (2012). https://doi.org/10.1007/s00394-011-0200-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-011-0200-9