Abstract
Background
Few studies have reported a possible involvement of pleiotrophin (PTN) in the pathophysiology of osteoarthritis (OA) and very little is known about its role in rheumatoid arthritis (RA). This study is to measure PTN in the sera and synovial fluids in RA and OA and to assess its relation to activity, functional class and radiological staging.
Subjects and methods
Serum and synovial fluid samples were collected from 35 RA patients and 40 knee OA patients and serum samples were withdrawn from 20 healthy controls. Demographic, clinical and serological data were prospectively assessed. Functional and radiographic grades were also assessed. Serum and synovial fluid PTN levels were measured using enzyme-linked immunosorbent assay (ELISA).
Results
There was no statistical significant differences (p > 0.05) on comparing the mean PTN level in sera of RA, OA patients and healthy controls. However the mean synovial fluid level of PTN in both patient groups was significantly higher than mean serum level (p < 0.001). Significant correlations between the serum PTN level and both morning stiffness duration (p = 0.008) and mHAQ score (p = 0.039) were only observed in RA patients.
Conclusion
Our results point to a possible important role of PTN in RA and OA. We firstly report a serological pattern of PTN in the sera and synovial fluids of RA patients. However its implementation as a disease marker or a potential target therapy in both diseases awaits larger studies and further investigations.
Zusammenfassung
Hintergrund
In einigen wenigen Studien wurde über eine mögliche Beteiligung von Pleiotrophin (PTN) an den pathophysiologischen Prozessen der Arthrose berichtet. Nur wenig ist über die Rolle bei der rheumatoiden Arthritis (RA) bekannt. Zweck der vorliegenden Studie war es, die PTN-Spiegel in Serum und Synovia bei RA und Arthrose zu bestimmen. Zudem wurde der Zusammenhang mit der Aktivität, dem Funktionsstatus und dem radiologischen Stadium untersucht.
Probanden und Methoden
Serum- und Synoviaproben wurden von 35 Patienten mit RA und 40 Patienten mit Kniegelenksarthrose gewonnen, Serumproben auch von 20 gesunden Kontrollen. Demografische, klinische und serologische Daten wurden prospektiv erfasst. Die funktionellen und röntgenologischen Stadien wurden ebenfalls ermittelt. Die PTN-Konzentrationen in Serum und Synovia wurden mithilfe eines „enzyme-linked immunosorbent assay“ (ELISA) bestimmt.
Ergebnisse
Es fand sich kein statistisch signifikanter Unterschied (p > 0,05) bei Vergleich der durchschnittlichen PTN-Spiegel im Serum von Patienten mit RA, Patienten mit Arthrose und gesunden Kontrollen. Die durchschnittliche Konzentration in der Synovia war dagegen in beiden Patientengruppen signifikant höher als der durchschnittliche Serumspiegel (p < 0,001). Eine signifikante Korrelation fand sich nur zwischen den Serum-PTN-Spiegeln und der Dauer der Morgensteifigkeit (p = 0,008) sowie dem mHAQ-Score (p = 0,039) bei Patienten mit RA.
Schlussfolgerungen
Die Ergebnisse deuten darauf hin, dass PTN eine wichtige Rolle bei RA und Arthrose spielen könnte. Dies ist der erste Bericht über ein serologisches PTN-Muster in Serum und Synovia von Patienten mit RA. Bevor aber PTN als Erkrankungsmarker etabliert oder als potenzieller Ansatzpunkt für eine zielgerichtete Therapie beider Erkrankungen herangezogen werden kann, müssen größere Studien und weitere Untersuchungen durchgeführt werden.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cartilage loss and degradation are found in both rheumatoid arthritis (RA) and osteoarthritis (OA) [1]. Concurrent to degradation of the articular cartilage, there is invasion of the cartilage by blood vessels derived from the subchondral bone [2], rendering osteochondral plate angiogenesis a novel goal in treating chronic arthritis.
RA represents the most famous form of chronic inflammatory joint disease resulting in bone and cartilage destruction [3]. The inflammatory process causes diffuse hyperplasia of the rheumatoid synovium [4]. Leukocytes emigrate into the synovium through the vascular endothelium resulting in synovial inflammation with subsequent joint destruction. RA synovial tissue is rich in newly formed vessels where angiogenesis promotes leukocyte extravasation into the synovium [5].
In OA, the mechanisms underlying cartilage angiogenesis are unclear but may involve hypertrophic chondrocyte differentiation. Blood vessels from the subchondral bone could invade the osteoarthritic cartilage with subsequent extracellular matrix mineralization, chondrocyte hypertrophy and cartilage innervation. Cartilage neo-innervation associated with vascular invasion has an important role in the mechanical pain that is a hallmark of OA [6]. OA is now regarded not only as a degenerative disease of the joints but also as an inflammatory disease [7].
It is well established nowadays that chronic joint inflammation in both rheumatoid arthritis and osteoarthritis includes a wide expression of inflammatory cytokines and synthesis of proangiogenic factors [1]. This concept is vital for diagnosis, prevention and treatment. We are still unaware of the full spectrum of the cytokines that play a role in the pathogenesis. Various cytokines (e. g. TNF-α, IL-1, IL-6, IL-8, IL-15, IL17, TFG-β and PDGF) have already been established to be involved in the inflammatory reaction. However, several studies suggest that pleiotrophin (PTN) and midkine (MK) should be included in this list, although their precise role is not completely revealed [8].
PTN is a growth factor formed of 136 amino acids, which form with MK, a distinct family of heparin-binding growth factors. It is called “pleiotrophin” because of its function as a differentiation factor and growth factor for a variety of cell types [9].
PTN is a potent angiogenic factor that has a role in survival of endothelial cells as well as its proliferation, migration and capillary-like structure formation [9]. PTN also has been discovered to enhance the expression of inflammatory cytokines including TNF-α, IL-1b and IL-6 in quiescent human peripheral blood mononuclear cells (PBMC) which emphasize its important role in the control of inflammatory processes [10].
In the adult cartilage, PTN expression is limited to specific pathological conditions which includes RA and OA [6] where developmentally regulated factors often show again during disease [9]. However, little is known about their soluble concentrations in synovial fluid and serum and its association with clinical and radiological disease parameters. Accordingly, our study aimed to investigate whether PTN could be found in the sera and synovial fluid in patients with RA and OA and its relation to disease activity, functional class and radiological staging.
Patients and methods
This study was carried on 35 adult RA patients (21 female, mean age of 42.23 ± 9.32 years) fulfilling the ACR/EULAR 2010 classification criteria of RA [11] and 40 adult patients with knee OA (25 female, mean age 52.82 ± 7.33 years) diagnosed according to 1986 ACR criteria for knee osteoarthritis [12].
All patients were recruited from the outpatient clinic of the Rheumatology and Rehabilitation Department, Faculty of Medicine, Cairo University. Patients were taken consecutively, provided that knee effusion was evident. Exclusion criteria of the present study included arthritis of other etiologies, preceding fractures, preceding infection in joint, systemic inflammatory or autoimmune diseases (except RA), malignancies, intra-articular administration of steroids for at least 3 months before joint aspiration. The study also included 20 healthy subjects who served as the normal control group.
All patients were subjected to full history taking, complete physical examination as well as assessment of disease activity and functional ability.
For RA patients, disease activity was assessed by using the Disease Activity Score 28 (DAS 28) [13]. RA functional ability was assessed by using the modified version of the health assessment questionnaire (mHAQ) which was previously validated [14].
Plain radiographs for hands, wrists and feet were taken for each RA patient at the time of blood sampling. Radiographic joint damage was assessed according to the Larsen score [15], with the number and size of bone erosions and the extent of joint space narrowing related to the cartilage damage being evaluated. Treatment modalities were determined by combining information provided by the patients and the medical records. A maximum daily dose of 7.5 mg of prednisolone was accepted. The presence of the extra-articular manifestations and previous joint surgery were recorded.
For OA patients plain radiographs for both knees were obtained and evaluated according to Kellgren and Lawrence grading [16]. The functional ability in OA patients were measured using the Western Ontario and McMaster Universities osteoarthritis index (WOMAC) questionnaire [17].
The study was approved by the local ethics committee and informed consent according to the Declaration of Helsinki was obtained from all patients and normal subjects.
Laboratory investigations
Routine biochemistry tests were registered from the patients’ records. Complete blood count (CBC) was performed using a Coulter counter (T660) and erythrocyte sedimentation rate (ESR) was detected by the Westergren method. Rheumatoid factor (RF) was determined by the latex fixation method. Anti-citrullinated cyclic peptide (anti-CCP) was measured using the microparticle enzyme immunoassay (MEIA) method with the Abbott AxSym (Chicago, IL, USA).
Serum and synovial fluid pleiotrophin
Serum samples were obtained from all patients and volunteers. Synovial fluid samples were obtained from effused knees in both RA and OA patients. Serum and synovial fluid pleiotrophin was assayed using the quantitative enzyme immunoassay technique (ELISA). A monoclonal antibody specific for pleiotrophin had been precoated onto a microplate. Standards and samples were pipetted into the wells and any pleiotrophin present was bound by the immobilized antibody. After washing away unbound substances, an enzyme-linked monoclonal antibody reagent specific for pleiotrophin was added to the well, then unbound antibody enzyme reagent was washed away. The substrate solution was then added to the wells and color developed in proportion to the amount of pleiotrophin bound in the initial step and then the intensity of colour was measured. The PTN concentrations were analyzed in accordance with the manufacturer’s instructions and with an ELISA reader at 450 nm. Both standards and samples were evaluated in duplicate and the interassay variations were shown to be within the range given by the manufacturers.
Statistical analysis
Results are expressed as mean ± standard deviation (SD) or number (%). Comparison between categorical data was performed using the χ2 test. Comparison between values of different variables in the two studied groups was performed using either the unpaired t test or Mann–Whitney test whenever it was appropriate while the Wilcoxon matched paired test was used for matched serum and synovial fluid level of PTN in the two studied groups. Comparison between values of PTN in serum in the three studied groups was performed using the Kruskal–Wallis test. Correlation between PTN either in serum or in synovial fluid and different variables in RA and OA groups was tested using theSpearman rho correlation test. The Statistical Package for Social Sciences (SPSS) computer program (version 19 windows) was used for data analysis. A probability value (P value) less than 0.05 was considered statistically significant.
Results
RA and OA patients’ characteristics are displayed in Table 1. Ten RA patients (28.5%) had extra-articular manifestations. Subcutaneous nodules were found in 4 patients (11.4%) and 6 patients had secondary Sjogren syndrome (17.1%). RF was positive in 28 RA patients (82%) with a mean of 38.62 ± 41.62 IU/ml. Anti-CCP antibody was positive in 13 RA patients (37.1%) with a mean of 54.6 ± 44.8 U/ml. Regarding medical treatment, all our RA patients were on methotrexate (100%), 25 patients were on steroids (71.4%), 9 patients on leflunomide (25.7%) and 10 on antimalarial therapy (28.5%).
Comparison between serum PTN levels in RA patients, OA patients and in healthy controls
There was no statistical significant differences (p > 0.05) on comparing the mean PTN level in sera of RA, OA patients and healthy controls (22.51 ± 18.49, 23.14 ± 23.79, and 22.44 ± 2.82 ng/ml respectively; Fig. 1).
Comparison between serum and synovial fluid PTN levels in RA and OA patients
As shown in Fig. 2, PTN levels were significantly higher (P < 0.001) in synovial fluid (34.30 ± 31.75 ng/ml) than serum levels in RA patients (22.51 ± 18.49 ng/ml). It was also found significantly higher in synovial fluid than serum of OA patients (37.30 ± 28.80 and 23.14 ± 23.79 ng/ml respectively; P < 0.001).
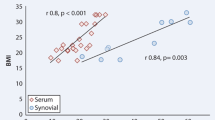
Confirming the above results by using Wilcoxon matched paired test for matched serum and synovial fluid level of PTN in the two studied groups, it was found that the PTN level in synovial fluid was significantly higher than that in serum where p < 0.001 in both patients group (Fig. 3). Serum PTN levels correlated positively with its synovial fluid levels in RA patients (r = 0.0378, p = 0.025) and OA patients (r = 0.0378, p = 0.016).
Association of serum and synovial fluid PTN level with RA disease characteristics
There were significant correlations between serum PTN level with morning stiffness duration (r = −0.443, p = 0.008) and mHAQ score (r = −0.351, p = 0.039). However, no other significant correlations were found between PTN level in either serum or synovial fluid of RA patients and other parameters (p > 0.05; Table 2). PTN levels were similar in both males and females and were not dependent on the age of the patients or on the duration of arthritis.
Furthermore, there was no statistically significant difference on comparing mean PTN level in serum and synovial fluid level of RA patients who received and those who did not receive steroids, leflunomide and antimalarial medications as shown in Table 3.
Association of serum and synovial fluid PTN level with OA disease characteristics
No significant correlation was found between serum and synovial fluid level of PTN with age, disease duration, WOMAC functional score and Kellgren and Lawrence radiological score (p > 0.05). Furthermore no significant correlation was found between PTN serum and synovial fluid level with the different laboratory data in OA patients (Table 4).
Comparison between serum and synovial fluid PTN levels in RA and OA patients
As shown in Fig. 2, PTN levels were significantly higher (P < 0.001) in synovial fluid (34.30 ± 31.75 ng/ml) than serum levels in RA patients (22.51 ± 18.49 ng/ml). It was also found to be significantly higher in synovial fluid than serum of OA patients (37.30 ± 28.80 and 23.14 ± 23.79 ng/ml respectively; P < 0.001).
Confirming the above results by using the Wilcoxon matched paired test for matched serum and synovial fluid level of PTN in the two studied groups, it was found that PTN level in synovial fluid was significantly higher than that in serum, where p < 0.001 in both patient groups (Fig. 3). Serum PTN levels correlated positively with its synovial fluid levels in RA patients (r = 0.0378, p = 0.025) and OA patients (r = 0.0378, p = 0.016).
Association of serum and synovial fluid PTN level with RA disease characteristics
There was significant correlations between serum PTN level with morning stiffness duration (r = −0.443, p = 0.008) and mHAQ score (r = −0.351, p = 0.039). However, no other significant correlations were found between PTN level in either serum or synovial fluid of RA patients and other parameters (p > 0.05; Table 2). PTN levels were similar in both males and females and were not dependent on the age of the patients or on the duration of arthritis.
Furthermore, there was no statistically significant difference on comparing mean PTN level in the serum and synovial fluid level of RA patients who received and those who did not receive steroids, leflunomide and antimalarial medications as shown in Table 3.
Association of serum and synovial fluid PTN level with OA disease characteristics
No significant correlations were found between serum and synovial fluid level of PTN with age, disease duration, WOMAC functional score and Kellgren and Lawrence radiological score (p > 0.05). Furthermore no significant correlations were found between PTN serum and synovial fluid level with the different laboratory data in OA patients (Table 4).
Discussion
Current disease-modifying therapies for rheumatic diseases sometimes fail or produce only partial responses. Reliable biomarkers of therapeutic response, toxicity and prognosis are lacking. It is difficult to achieve sustained remission and ongoing pharmacologic therapy is required [18].
Targeted therapies have revolutionized treatment of many complex diseases. Indeed, targeted therapy became one of the major modalities of medical treatment for cancer [19]. Application of targeted therapies in immune-mediated inflammatory diseases (IMIDs) is another area of interest based on several facts as the effective implementation of anticytokine therapy in many orphan inflammatory conditions [20].
Several reports have focused on the PTN effect in cancer angiogenesis [21]. Accordingly, a bulk of research has studied PTN as an attractive target for tumour therapy and data suggest that PTN may hold promise for breast and prostate cancers [22, 23]. In addition to cancer, this embryonic growth and differentiation factor was found to be expressed in adults with inflammatory diseases, with a proposed role in the angiogenesis and growth of synovial cells [9]and hence could be a promising applicant to develop targeted therapeutic regimens.
As far as we know, the concept of the current study was investigated only once in RA by Pufe et al., who determined the PTN expression in the synovial membranes of patients with RA [9]. However, the basic premise of the ease and rapidity of the serological tests had urged us to assess the PTN concentrations in the sera and synovial fluids of a group of RA patients.
On studying the difference between the serum and synovial fluid levels of PTN in the RA patients group, we found statistically significant higher PTN levels in the synovial fluid compared to that of the serum (p < 0.001). This is consistent with the results reported by Pufe et al. who found that PTN mRNA expression and PTN protein were considerably up-regulated in the synovial tissues of patients with RA [9].
However, we did not find any statistical difference between the serum levels of PTN in RA patients group and controls (p > 0.05). This insignificant difference between serum PTN in RA and healthy controls was not expected. Nevertheless, it is known that PTN can act in a paracrine manner as it is released and consumed locally, close to the site where the immune reaction occurs [9]. Similarly, a number of inflammatory cytokines related to the pathogenesis of RA were also not detected in the serum. Of which that have been reported are the TNF-α, IL-1b and IL-6 [24–26]. Multiple explanations for the inability to detect a cytokine, when actually it is expected to be found, have been proposed. The presence of specific or nonspecific inhibitors or excessive consumption of a cytokine or diurnal variations are among causes [18]. From another aspect, blood may not be the appropriate material of choice. The half-life of many cytokines is less than 10 min; hence, the time lapse between the collection and the processing of samples may be a significant factor limiting the use of levels of cytokines as biomarkers [18].
In the present research, we studied the association of PTN levels in serum and synovial fluid with some clinical parameters and disease activity score in RA. We found a significant correlation between serum PTN level with morning stiffness duration and mHAQ score. On the other hand, no correlation was found with age, disease duration, DAS 28 score or the presence of extra articular manifestations.
Therefore, we investigated PTN level in the sera and synovial fluid of OA. We also found that the serum levels in OA patients were not statistically different from normal healthy controls. In contrast to our findings, Kaspiris et al. assessed serum level of PTN in 16 OA patients and they found that it was significantly higher than in controls [6]. This may be attributed to different causes. Firstly, their study differs from ours that the patients were with hip and knee OA, while ours were only knee OA; also they classified their knee OA patients using different radiological scoring system making it difficult to compare both patients groups.
On comparing serum and synovial fluid levels in our OA patients, we found statistically significant difference where it was higher in the synovial fluid than serum (p < 0.001).
Synovial fluid samples were measured in previous studies in OA. Pufe et al. studied PTN mRNA and protein expression in the synovial fluids of OA patients and reported that PTN concentrations were elevated in earlier OA stages, but rarely in late OA stages [27]. The local production of PTN in OA cartilage is emphasized by our findings; however it was difficult to classify our OA patients as early or late as most of them were nearly the same OA stage.
In our study, serum and synovial fluid PTN levels did not correlate with Womac functional score nor with Kellgren and Lawrence radiological score in the OA patients group (p < 0.05). Since no other studies correlated PTN level with OA disease parameters, we can say that further studies including a larger number of patients with different disease stages should be carried out.
As a matter of fact, the role of PTN in OA is still obscure and another concept was adopted by Kaspiris et al. For the first time, they showed expression of PTN in subchondral bone osteocytes of OA patients, which also play a role in tissue remodeling during disease progression [6]. Previous studies showed expression of PTN in osteocytes also increased after local bone damage and in cases of fracture healing in experimental models [28], and its over-expression presents a mean reinforcing effect on the growth of long bones in rats [29]. In vitro, PTN stimulates the adhesion, migration, and differentiation of osteoblasts, and the creation of the intercellular matrix [30], further supporting the notion that PTN expression in osteocytes of OA patients may have a role in tissue remodeling.
In conclusion, the results of the present study emphasize the role of PTN in both RA and OA. We were the first to report a serological study of this cytokine in RA and to correlate its level with different functional and radiological parameters of both diseases.
However, it is important to bear in mind that there are several limitations in our study. Firstly, the number of patients was relatively small. Secondly, the cross sectional pattern of our study make it difficult to assess the effect of treatment and medications taken by the patients and PTN levels in serum and synovial fluid.
Accordingly, further studies on PTN and rheumatic diseases and prolonged follow-up are needed to reach reliable conclusions and unveil its exact role in the disease process.
References
Jiao K, Zhang J, Zhang M, Wei Y, Wu Y (2013) The identification of CD163 expressing phagocytic chondrocytes in joint cartilage and its novel scavenger role in cartilage degradation. PLOS ONE 8(1):e53312
Ashraf S, Walsh DA (2008) Angiogenesis in osteoarthritis. Curr Opin Rheumatol 20(5):573–580
Takeuchi T, Yamanaka H, Ishiguro N, Miyasaka N, Mukai M, Matsubara T, Uchida S (2014) Adalimumab, outcome study for the prevention of joint damage in Japanese patients with early rheumatoid arthritis: the HOPEFUL 1 study. Ann Rheum Dis 73(3):536–543
Tanaka S (2013) Regulation of bone destruction in rheumatoid arthritis through RANKL-RANK pathways. World J Orthop 4(1):1–6
Szekanecz Z, Koch AE (2008) Targeting angiogenesis in rheumatoid arthritis. Curr Rheumatol Rev 4(4):298–303
Kaspiris A, Mikelis C, Heroult M, Khaldi L, Grivas TB, Kouvaras I, Dangas S, Vasiliadis E, Lioté F, Courty J, Papadimitriou E (2013) Expression of the growth factor pleiotrophin and its receptor protein tyrosine phosphatase beta/zeta in the serum, cartilage and subchondral bone of patients with osteoarthritis. Joint Bone Spine 80(4):407–413
Houard X, Goldring MB, Berenbaum F (2013) Homeostatic mechanisms in articular cartilage and role of inflammation in osteoarthritis. Curr Rheumatol Rep 15(11):375
Mentlein R (2007) Targeting pleiotropin to treat osteoarthritis. Expert Opin Ther Targets 11(7):861–867
Pufe T, Bartscher M, Petersen W, Tillmann B, Mentlein R (2003) Expression of pleiotrophin, an embryonic growth and differentiation factor, in rheumatoid arthritis. Arthritis Rheum 48(3):660–667
Achour A, M’bika JP, Baudouin F, Caruelle D, Courty J (2008) Pleiotrophin induces expression of inflammatory cytokines in peripheral blood mononuclear cells. Biochimie 90(11-12):1791–1795
Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, Combe B, Costenbader KH, Dougados M, Emery P, Ferraccioli G, Hazes JM, Hobbs K, Huizinga TW, Kavanaugh A, Kay J, Kvien TK, Laing T, Mease P, Ménard HA, Moreland LW, Naden RL, Pincus T, Smolen JS, Stanislawska-Biernat E, Symmons D, Tak PP, Upchurch KS, Vencovský J, Wolfe F, Hawker G (2010) Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism Collaborative Initiative. Arthritis Rheum 62(9):2569–2581
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, Christy W, Cooke TD, Greenwald R, Hochberg M (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic References and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29(8):1039–1049
Prevoo ML, van ’t Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL (1995) Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 38(1):44–48
Pincus T, Summey JA, Soraci SA Jr, Wallston KA, Hummon NP (1983) Assessment of patient satisfaction in activities of daily living using a modified Stanford Health Assessment Questionnaire. Arthritis Rheum 26(11):1346–1353
Rau R, Herborn G (1995) A modified version of Larsen’s scoring method to assess radiologic changes in rheumatoid arthritis. J Rheumatol 22(10):1976–1982
Kellgren JH, Lawrence JS (1957) Radiological assessment of Osteo-Arthrosis. Ann Rheum Dis 16(4):494–502
Bellamy N (1982) Osteoarthritis – An evaluative index for clinical trials. MSc Thesis, McMaster University, Hamilton, Canada.
Burska A, Boissinot M, Ponchel F (2014) Cytokines as biomarkers in rheumatoid arthritis. Mediators Inflamm. doi:10.1155/2014/545493
National Cancer Institute (2014) Targeted cancer therapies. www.cancer.gov/cancertopics/factsheet/Therapy/targeted
Kuek A, Hazleman BL, Östör AJK (2007) Immune-mediated inflammatory diseases (IMIDs) and biologic therapy: a medical revolution. Post Grad Med J 83(978):251–260
Souttou B, Carvalho NB, Raulais D, Vigny M (2001) Activation of anaplastic lymphoma kinase receptor tyrosine kinase induces neuronal differentiation through the mitogen-activated protein kinase pathway. J Biol Chem 276(12):9526–9531
Tsirmoula S, Dimas K, Hatziapostolou M, Lamprou M, Ravazoula P, Papadimitriou E (2012) Implications of pleiotrophin in human PC3 prostate cancer cell growth in vivo. Cancer Sci 103(10):1826–1832
Lynn KD, Roland CL, Brekken RA (2010) VEGF and pleiotrophin modulate the immune profile of breast cancer. Cancers (Basel) 2(2):970–988
Straub RH, Cutolo M (2007) Circadian rhythms in rheumatoid arthritis: implications for pathophysiology and therapeutic management. Arthritis Rheum 56(2):399–408
Ebrahimi AA, Noshad H, Sadreddini S et al (2009) Serum levels of TNF-alpha, TNF-alpha RI, TNF-alpha RII and IL-12 in treated rheumatoid arthritis patients. Iran J Immunol 6(3):147–153
Goëb V, Aegerter P, Parmar R et al (2013) Progression to rheumatoid arthritis in early inflammatory arthritis is associated with low IL-7 serum levels. Ann Rheum Dis 72(6):1032–1036
Pufe T, Bartscher M, Petersen W, Tillmann B, Mentlein R (2003) Pleiotrophin, an embryonic differentiation and growth factor, is expressed in osteoarthritis. Osteoarthr Cartil 11(4):260–264
Peterson WJ, Tachiki KH, Yamaguchi DT (2004) Serial passage of MC3T3-E1 cells down-regulates proliferation during osteogenesis in vitro. Cell Prolif 37(5):325–336
Li G, Cui Y, Mcilmurray L, We A, Wang H (2005) rhBMP-2, rhVEGF (165), rhPTN and thrombin related peptide, TP508 induce chemotaxis of human osteoblasts and microvascular endothelial cells. J Orthop Res 23:680–685
Yang X, Tare RS, Partridge KA (2003) Induction of human osteoprogenitor chemotaxis, proliferation, differentiation, and bone formation by osteoblast stimulating factor-1/pleiotrophin:osteoconductive biomimetic scaffolds for tissue engineering. J Bone Miner Res 18:47–57
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
S. M. H. Fadda, I. H. Bassyouni, R. H. Khalifa and N. Y. Elsaid declare that they have no competing interests.
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Redaktion
U. Müller-Ladner, Bad Nauheim
U. Lange, Bad Nauheim
Rights and permissions
About this article
Cite this article
Fadda, S.M.H., Bassyouni, I.H., Khalifa, R.H. et al. Pleiotrophin, the angiogenic and mitogenic growth factor: levels in serum and synovial fluid in rheumatoid arthritis and osteoarthritis. Z Rheumatol 77, 322–329 (2018). https://doi.org/10.1007/s00393-016-0234-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00393-016-0234-8