Abstract
Background
A strong interdependence is known between atrial fibrillation (AF), inflammation and thrombogenesis. Monocyte–platelet aggregates (MPAs) are sensitive markers of platelets and monocyte activation. It is not known whether MPAs are associated with thrombogenicity in AF. Therefore, we examined differences in the content of MPAs and CD11b expression in patients with AF in dependence of the presence of atrial thrombus formation.
Methods
107 patients with symptomatic AF underwent transesophageal echocardiography (TEE) before planned cardioversion or pulmonary vein isolation. Flow-cytometric quantification analysis was done on the day of performed TEE to determine the content of MPAs and the expression of CD11b on monocytes and granulocytes.
Results
Compared to patients without thrombus (n = 80) those with an echocardiographic proven left atrium (LA) thrombus (n = 27) showed an increased extent of the risk factors age, diabetes and heart failure. The content of MPAs (147 ± 12 vs. 311 ± 29 cells/µl, p < 0.001) as well as the CD11b expression on monocytes (p < 0.05) and granulocytes (p < 0.05) were strongly associated with the existence of a LA thrombus. The content of MPAs and the CD11b expression remained independent predictors for LA thrombus after adjustment in logistic regression analysis and negatively correlated with left atrial appendage flow velocity. MPAs above 170 cells/µl (OR 34.2, p = 0.01) had a sensitivity of 96 % and a specificity of 73 % for predicting LA-thrombus.
Conclusions
The content of MPAs and the CD11b expression on monocytes and granulocytes are increased in AF-patients with proven thrombus formation. They seem to be appropriate biomarkers for stratification of thromboembolic risk in patients with AF.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atrial fibrillation (AF) is a main cause of morbidity and mortality due to the possibility of thromboembolic stroke [1–3]. In most cases, the point of origin of the thrombus is the left atrial appendage (LAA) [4]. For patients with known AF and a high thromboembolic risk a sufficient therapy with anticoagulants is of indisputable importance [5]. However, unresolved questions still remain [6, 7]. Is there a need for a routinely performed TEE before cardioversion, even in low risk patients? Could we dare to stop the therapy with anticoagulants after successful performed pulmonary vein isolation in patients with low risk [1]? Biomarkers which could predict an elevated thromboembolic risk would be helpful to answer these occasionally difficult questions. Increasing evidence suggests a fundamental role of inflammatory processes in atrial fibrillation (AF) [8–14]. AF itself [12] and inflammatory processes in general could be associated with markers of platelet activation. Monocyte–platelet aggregates (MPAs) are sensitive markers for both platelets and monocyte activation [15, 16] and could represent a link between inflammation and hemostasis [17]. Patients with heart failure turned out to have persistent high levels of MPAs in peripheral blood [18]. Heart failure is an established risk factor for thrombotic complications in patients with AF. An elevated content of MPAs could be further associated with worse outcome after stroke [19]. It is not known whether MPAs are increased in dependence on the extent of thrombogenicity in AF. Inflammatory circumstances or chronic inflammatory diseases can promote AF [10, 20–23]. It could be emerged that AF itself can promote inflammatory processes during atrial remodeling. AF could lead to a local infiltration of leukocytes into the atrial wall which is mediated by CD11b and CD18 integrins [24, 25]. Activated platelets are known to be able to induce activation with enhanced expression of adhesion molecules in circulating monocytes [26]. Whether AF in combination with prothrombotic conditions, like slow flow in the LAA or even an existing thrombus formation is associated with an additional increase of CD11b expression on monocytes is still unclear. In this study we examined differences in the content of MPAs and expression of CD11b on monocytes in patients with AF in dependence of their thrombogenicity.
Methods
Study population
107 patients with symptomatic and drug refractory AF underwent transesophageal echocardiography (TEE) before planned electric cardioversion or catheter ablation. In all patients clinical, laboratory and demographic data were obtained. Flow-cytometric quantification analyses were done on the day of performed TEE. Patients with a detected LA-Thrombus (n = 27) were included consecutively and then compared with patients with AF but without LA-thrombus. As the group of patients without LA thrombus would have been much larger than the group of patients with an LA thrombus we stopped the inclusion of patients without LA thrombus after 80 patients. Patients presenting an acute coronary syndrome, relevant infectious or inflammatory disorders were excluded. The patients with AF were further divided in three groups in dependency on their peak emptying blood flow velocity in the left atrial appendage. The study was performed in accordance with the Helsinki Declaration and approved by the institutional ethic committee of the Technische Universität Dresden (EK406122012). All of the participants gave their written, informed consent.
Echocardiography
All patients underwent transthoracic and transesophageal echocardiographic examinations before planned cardioversion or catheter ablation. LA diameter (from the parasternal long-axis view), evaluation of valvular heart diseases and LVEF (using biplane Simpson method) were obtained according to the American Society of Echocardiography [27]. Left atrial (LA) appendage peak emptying flow velocity (LAAEV) was determined in 99 of the 102 study participants. LAAEV below 20 cm/s was classified as a prothrombotic condition [28], blood flow >40 cm/s as non thrombogenic and LAAEV 20–40 cm/s as intermediate thrombogenic. LA-thrombus was defined as a distinct echogenic mass separate to the LA body which could be distinguished from the surrounding atrium. The presence of LA thrombus was confirmed by a second observer.
Laboratory methods
Peripheral venous blood samples were collected through non traumatic puncture from all of the study participants with minimal stasis into tubes containing sodium citrate (Sarstedt) as anticoagulants and analyzed by flow cytometry within 30 min of collection. Flow-cytometric quantification analysis of peripheral blood was performed in 102 patients. Shortly after, 50 µl blood were labeled within 10 min with CD45-FITC, CD14-APC, CD11b-PE and CD41-PE [all antibodies were purchased from Becton–Dickinson (BD), Oxford, UK]. CD11b-PE and CD41-PE were used in different tubes. As control for the determination of CD11b on monocytes tubes containing CD 45, CD14 except CD11b (fluorescence minus one) were used. Flow-cytometric measurements were performed using a BD FACSCalibur flow cytometer. Monocytes were identified by gating strategies based on CD45 expression and side scatter to select monocytes. The content of MPAs was determined by co-expression of CD41 and CD14 on monocytes. The quantification of MPAs was expressed as mean fluorescence intensity (MFI), as relative count and as absolute count. Relative counts of MPAs represent the percentage of monocytes with coexpression of the platelet marker CD41 on all monocytes. Absolute counts of monocytes (in cells per µl) were obtained by calculating the relative number of monocytes proportional to the number of the count in the standard laboratory blood test. This was performed with blood from the same puncture. Together with the ascertained percentage of monocytes, we similarly calculated the absolute number of MPAs per µl. The extent of activation on monocytes and granulocytes was determined by quantification of the cell adhesion molecule CD11b (MAC-1). The expression of CD11b was measured as mean fluorescence intensity (MFI). The values of fluorescence minus one (FMO) control were subtracted.
Statistical analysis
Statistical analysis was performed using SPSS v.18. The distribution of continuous data was examined using the Kolmogorov-Smirnov-test. Data are given as mean ± standard error of mean (SEM), unless otherwise stated. For comparison of group 1 (patients with AF, without LA-thrombus) with group 2 (AF and LA-thrombus) the data with a normal Gaussian distribution were analyzed using an unpaired Student’s t test after controlling for equality of variances with the Levene’s test. Data with a non-Gaussian distribution were analyzed using the Mann–Whitney U test. Cross-sectional comparisons among the three study populations (AF with LAAEV >40 cm/s, AF with LAAEV 20–40 cm/s and AF with LAAEV <20 cm/s) were performed with one-way ANOVA. A post hoc Tukey test was performed to assess intergroup differences. Correlation coefficients were calculated by Pearson tests. For logistic regression analysis we included predictors identified in the univariate test. A p value <0.05 was considered as statistically significant. Odds ratio and 95 % confidence interval were calculated. Receiver-operating characteristic (ROC) curve analyses were used for evaluating the optimal cutoff value, and the related sensitivity and specificity of the investigated parameters for predicting LA thrombus.
Results
Baseline characteristics
Relevant baseline clinical, laboratory and echocardiographic parameters of the study participants are summarized in Table 1. Not surprisingly, patients with detected LA thrombus showed an increased extent of already known risk factors like age, diabetes, coronary artery disease and impaired left ventricular function. In addition they had slightly elevated levels of leukocytes, D-dimer and IL-6. Other markers of systemic inflammation were only increased by trend in the LA thrombus group. The LA diameter was greater in patients with AF and detected thrombus. As expected, the CHA2DS2-VASc. score was elevated in the group with proven thrombus formation. No participant in the group with LA thrombus had a CHA2DS2-VASc.-score <1.
Monocyte–platelet aggregates
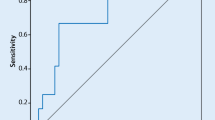
The total MPA count (147 ± 12 vs. 311 ± 29 cells/µl, p < 0.001), the relative proportion of MPAs on monocytes (34.9 ± 1.7 vs. 61.9 ± 4.7 %, p < 0.001) and the MFI of CD41 on monocytes (138 ± 13 vs. 384 ± 65, p = 0.001) were significantly increased in patients with AF and detected LA thrombus formation (Table 2). In a multivariate logistic regression analysis (Table 3) the count of MPAs remained an independent predictor of thrombus formation in patients with AF after adjustment of age, LVEF, LA diameter, diabetes, leukocyte count, D-dimer and the IL-6 level. In the ROC analysis, MPAs above 170 cells/µl predicted LA thrombus formation with a sensitivity of 93 % and a specificity of 73 % (Fig. 1). MPAs above 170 cells/µl had an odds ratio of 34.2 (95 % CI 3.6–324.7, p = 0.01) for predicting LA thrombus in patients with AF (Table 3).
CD11b expression on monocytes and granulocytes
A high expression of CD11b on all monocytes, which represents a high extent of activation, was also associated with LA thrombus compared to patients with AF but without LA thrombus formation (21.5 ± 2.6 vs. 44.4 ± 9.6 p < 0.05) (Fig. 2). The extent of CD11b expression on monocytes correlated significantly with the count of MPAs (r = 0.49, p < 0.001). In addition, a high expression of CD11b on granulocytes was seen in patients with LA thrombus (14.1 ± 1.5 vs. 29.7 ± 6.0 p < 0.05), (Fig. 2). Comparable to the MPAs, a multivariate logistic regression analysis validated both, CD11b expression on monocytes (p < 0.05) and CD11b expression on granulocytes (p < 0.01) as independent predictors for thrombus formation in patients with AF after adjustment of age, LVEF, LA-diameter, diabetes, leukocyte count, D-dimer and the IL-6 level (Table 3).
Expression of CD 11b on monocytes and granulocytes in dependence of proven LA thrombus in patients with AF. Expression of CD 11b on monocytes and granulocytes in dependence of proven LA thrombus in patients with AF. Patients with AF but without LA thrombus (no Thromb), patients with AF and attested thrombus formation (Thromb). CD11b expression is expressed as mean fluorescence intensity (MFI) as determined by flow cytometry. Results are presented as mean ± SEM. *p < 0.05
Correlation analysis of MPAs and CD 11b expression with echocardiographic parameters for thrombogenicity
Higher counts of MPAs were associated with lower LAA peak emptying flow velocity (LAAEV). There was a significant negative correlation between the content of MPAs and LAAEV (r = −0.42; p < 0.001). Also the CD11b expression on monocytes (r = −0.33; p = 0.001) and on granulocytes (r = −0.27; p = 0.006) correlated negatively with LAAEV. Although the LA-diameter correlated negatively with LAA peak emptying flow velocity (r = −0.40; p < 0.001), both the MPA count as well as the CD11b expression was only associated by trend with the LA diameter. Between the three groups of different LAA flow velocities, those patients with LAAEV <20 cm/s had an increased content of MPAs count (128 ± 13 vs. 284 ± 32 cells/µl, p < 0.001) in comparison with the group of a non thrombogenic LAAEV of greater than 40 cm/s (Fig. 3). Also the group of an intermediate LAAEV (20–40 cm/s) had a lower MPA count in comparison to patients with a thrombogenic LAAEV of <20 (184 ± 19 vs. 284 ± 32 cells/µl, p < 0.05). Both, the CD11b expression on monocytes (17 ± 3 vs. 43 ± 9 p < 0.01) and on granulocytes (12 ± 2 vs. 28 ± 5 p < 0 0.01), were elevated in the group of LAAEV of <20 compared to patients with a non thrombogenic flow of >40 cm/s (Table 4).
Monocyte–platelet aggregates in dependence on the left atrial appendage peak emptying flow velocity. Monocyte–platelet aggregates (MPAs) in dependence on the left atrial appendage (LAA) peak emptying flow velocity (LAAEV). LAAEV below 20 cm/s is regarded as a prothrombotic condition. Results are presented as mean ± SEM. *p < 0.05, ***p < 0.001
Discussion
Our study revealed three main findings. First, the content of MPAs as a marker for platelet activation was independently increased in patients with echocardiographic proven thrombus formation in the LA. Second, the expression of CD11b on monocytes as well as on granulocytes as indicator for an enhanced inflammatory activation status of these cells were significantly elevated in patients with LA thrombus, compared to those who were in AF but without a LA thrombus. Third, both, the content of MPAs and the expression of CD11b on monocytes and granulocytes correlated negatively with LAA flow velocity.
Predictors of thrombogenicity in patients with AF
Clinical scores, with their modest informative values [29], were commonly used for predicting the thrombogenic risk. Prothrombotic [30, 31] or inflammatory markers [32, 33] have been related to prognostic parameters, like stroke or mortality in AF patients. Platelet activation markers, which are already associated with AF, like P-selectin and CD40 ligand have a short detectability in peripheral blood after activation [12, 34]. In contrast, MPAs are more stable and are described to persist in an elevated state up to several months after activation [18]. Therefore, also for not immediately evolved thrombi, they seem to have advantages for individual thromboembolic risk stratification. In contrast to the modest discriminative capability of the CHA2DS2-VASc Score with a reported value of AUC of about 0.65 [35] the level of MPAs in our study adduces a respectable AUC of 0.87 (0.81–0.94). MPAs with the revealed cut off value of 170 cells/µl showed a high sensitivity (93 %) and simultaneously an acceptable high specificity (73 %) for predicting thrombus formation. A subgroup analysis of our patients without diabetes (S1) revealed a significant elevation of MPAs and CD11b expression on monocytes and granulocytes in patients with detected LA thrombus as well. The presented negative correlation of MPAs and CD11b expression with the established echocardiographic risk marker of low flow velocity in the LAA on the one hand supports the potential of these new biomarkers for individual thromboembolic risk stratification. On the other hand it could indicate a possible causal role of the degree of intra-atrial stasis on the increase of MPAs and CD11b expression. The influence of blood stasis on activation of platelets could be shown previously by Igarashi et al. when they demonstrated a negative correlation between thrombin–antithrombin III complex and the level of D-dimer with low blood flow in the LAA [36]. In our study group the level of D-dimer was increased in patients with proven LA thrombus as well. However, in contrast to the MPAs, the D-dimer did not reach statistical significance after adjustment in the regression analysis. It has to be taking into account, that despite the relatively small number of participants in our study, the translation into clinical process may not seem to be far away. Considering the fact, that TEE, prior catheter ablation, seem to be unnecessary in patients at very low risk anyway [37], the additional evaluation with suitable markers of thrombotic risk, like the content of MPAs have the potential for more safety when TEE before cardioversion or before catheter ablation is spared or when we dare to break off the therapy with anticoagulants after a successful performed pulmonary vein isolation in patients with low risk. As thrombus formation in a low risk population is a very rare event, large randomized trials would be necessary to prove the concept in clinical life adequately.
Platelets and monocytes in the pathophysiology of thrombogenicity in AF
Acute onset of AF not only increases platelet activity, but also led to monocyte–platelet interactions [38]. In platelet thrombi, monocytes constituted about 16 % of platelet thrombus-bound leukocytes, which represent an almost fourfold enrichment as compared with their proportion in circulating blood [39]. Our presented findings of the strong association of MPAs and CD11b expression of monocytes and granulocytes with thrombus formation in patients with AF support the notion of a close link between AF, inflammation and thrombogenesis. Monocytes as well as granulocytes can activate factor X and bind fibrinogen via integrin CD11b (MAC-1) [40]. In this way they are able to modulate fibrin formation, but also thrombus dissolution. Monocytes take part in haemostatic processes via secretion of procoagulant factors and promoting inflammation [41]. Furthermore, platelets are able to activate monocytes and granulocytes which react with rapid translocation of CD11b receptors from an intracellular pool to their surface [17]. The close correlation of MPAs as markers of platelet activation with CD11b expression on monocytes among themselves in our study could demonstrate further evidence of suspected interactions of monocytes and platelets during prothrombotic processes of AF. CD11b may play a key role in the link between inflammation and AF, as the detected leukocyte atrial infiltration in mice could be shown to be dependent on CD11b expression [24]. Therefore, MPA and CD11b seem to be interesting markers in two respects. They are associated with increased thrombogenicity in AF, like our study shows. Inflammation in AF consists in particular of local atrial remodeling processes and therefore biomarkers in system circulation might not detect inflammation in AF at early stages [42]. Because of its pathophysiological impact, the extent of CD11b expression could furthermore reflect a suitable marker for the level of ongoing inflammation in AF. However, this requires further studies. Between some new treatment approaches [43, 44], there is already some evidence for possible beneficial roles of anti-inflammatory therapies for prevention of the development of AF [45] as well. Colchicine was shown to be able to reduce the rate of recurrences of AF after pulmonary vein isolation [46], whereas corticosteroids significantly reduce the risk of postoperative AF [7, 47, 48]. In conclusion, inflammatory targets for modulation of thrombotic processes seem to be worthwhile approaches for further studies.
Study limitations
Several limitations have been noted. First, the study group had a relative small sample size, especially the group with detected LA thrombus. Second, because of the existed close correlation of the content of MPAs and the CD11b expression on monocytes and the concomitant problem of multicollinearity, these parameters could not be integrated together in the logistic regression analysis. Furthermore the patients with AF had a slightly, not significant increased CRP. If the finding of the elevated CRP may influence the formation of MPAs or only reflects the inflammation in AF cannot be answered with certainty.
Conclusion
Our work shows, that the content of MPAs and the level of CD11b expression on monocytes as on granulocytes is associated with echocardiographic-proven thrombogenicity in patients with AF. These findings support the interdependency between inflammation, thrombogenesis and AF. Therefore, new anti-inflammatory treatment strategies for blocking monocyte activation, or blocking monocyte–platelet interactions might be promising therapeutic targets. Both, MPAs and CD11b expression appear appropriate for thromboembolic risk stratification in patients with AF.
References
Kornej J, Hindricks G, Shoemaker MB, Husser D, Arya A, Sommer P, Rolf S, Saavedra P, Kanagasundram A, Patrick WS, Montgomery J, Ellis CR, Darbar D, Bollmann A (2015) The APPLE score: a novel and simple score for the prediction of rhythm outcomes after catheter ablation of atrial fibrillation. Clin Res Cardiol 104(10):871–876
Meinertz T, Kirch W, Rosin L, Pittrow D, Willich SN, Kirchhof P (2011) Management of atrial fibrillation by primary care physicians in Germany: baseline results of the ATRIUM registry. Clin Res Cardiol 100:897–905
Kirchhof P, Schmalowsky J, Pittrow D, Rosin L, Kirch W, Wegscheider K, Meinertz T (2014) Management of patients with atrial fibrillation by primary-care physicians in Germany: 1-year results of the ATRIUM registry. Clin Cardiol 37:277–284
Blackshear JL, Odell JA (1996) Appendage obliteration to reduce stroke in cardiac surgical patients with atrial fibrillation. Ann Thorac Surg 61:755–759
January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW (2014) 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 130:2071–2104
Calkins H, Brugada J, Packer DL, Cappato R, Chen SA, Crijns HJ, Damiano RJ Jr, Davies DW, Haines DE, Haissaguerre M, Iesaka Y, Jackman W, Jais P, Kottkamp H, Kuck KH, Lindsay BD, Marchlinski FE, McCarthy PM, Mont JL, Morady F, Nademanee K, Natale A, Pappone C, Prystowsky E, Raviele A, Ruskin JN, Shemin RJ (2007) HRS/EHRA/ECAS expert Consensus Statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation. Heart Rhythm 4:816–861
Saad EB, d’Avila A, Costa IP, Aryana A, Slater C, Costa RE, Inacio LA Jr, Maldonado P, Neto DM, Camiletti A, Camanho LE, Polanczyk CA (2011) Very low risk of thromboembolic events in patients undergoing successful catheter ablation of atrial fibrillation with a CHADS2 score</=3: a long-term outcome study. Circ Arrhythm Electrophysiol 4:615–621
Friedrichs K, Klinke A, Baldus S (2011) Inflammatory pathways underlying atrial fibrillation. Trends Mol Med 17:556–563
Greif M, Von ZF, Wakili R, Tittus J, Becker C, Helbig S, Laubender RP, Schwarz W, D’Anastasi M, Schenzle J, Leber AW, Becker A (2013) Increased pericardial adipose tissue is correlated with atrial fibrillation and left atrial dilatation. Clin Res Cardiol 102:555–562
Hu YF, Chen YJ, Lin YJ, Chen SA (2015) Inflammation and the pathogenesis of atrial fibrillation. Nat Rev Cardiol 12(4):230–243
Jiang Z, Dai L, Song Z, Li H, Shu M (2013) Association between C-reactive protein and atrial fibrillation recurrence after catheter ablation: a meta-analysis. Clin Cardiol 36:548–554
Lim HS, Willoughby SR, Schultz C, Gan C, Alasady M, Lau DH, Leong DP, Brooks AG, Young GD, Kistler PM, Kalman JM, Worthley MI, Sanders P (2013) Effect of atrial fibrillation on atrial thrombogenesis in humans: impact of rate and rhythm. J Am Coll Cardiol 61:852–860
Yao SY, Chu JM, Chen KP, Tang M, Fang PH, Wang FZ, Zhang S (2009) Inflammation in lone atrial fibrillation. Clin Cardiol 32:94–98
Yalcin MU, Gurses KM, Kocyigit D, Kesikli SA, Ates AH, Evranos B, Yorgun H, Sahiner ML, Kaya EB, Oto MA, Guc D, Ozer N, Aytemir K (2015) Elevated M2-muscarinic and beta1-adrenergic receptor autoantibody levels are associated with paroxysmal atrial fibrillation. Clin Res Cardiol 104:226–233
Burdess A, Michelsen AE, Brosstad F, Fox KA, Newby DE, Nimmo AF (2012) Platelet activation in patients with peripheral vascular disease: reproducibility and comparability of platelet markers. Thromb Res 129:50–55
Michelson AD, Barnard MR, Krueger LA, Valeri CR, Furman MI (2001) Circulating monocyte-platelet aggregates are a more sensitive marker of in vivo platelet activation than platelet surface P-selectin: studies in baboons, human coronary intervention, and human acute myocardial infarction. Circulation 104:1533–1537
Shantsila E, Lip GY (2009) The role of monocytes in thrombotic disorders. Insights from tissue factor, monocyte-platelet aggregates and novel mechanisms. Thromb Haemost 102:916–924
Wrigley BJ, Shantsila E, Tapp LD, Lip GY (2013) Increased formation of monocyte-platelet aggregates in ischemic heart failure. Circ Heart Fail 6:127–135
Lukasik M, Dworacki G, Kufel-Grabowska J, Watala C, Kozubski W (2012) Upregulation of CD40 ligand and enhanced monocyte-platelet aggregate formation are associated with worse clinical outcome after ischaemic stroke. Thromb Haemost 107:346–355
Chiang CH, Huang CC, Chan WL, Huang PH, Chen YC, Chen TJ, Lin SJ, Chen JW, Leu HB (2013) Herpes simplex virus infection and risk of atrial fibrillation: a nationwide study. Int J Cardiol 164:201–204
Hu YF, Yeh HI, Tsao HM, Tai CT, Lin YJ, Chang SL, Lo LW, Tuan TC, Tzeng CH, Huang SH, Lin YK, Chen SA (2011) Impact of circulating monocyte CD36 level on atrial fibrillation and subsequent catheter ablation. Heart Rhythm 8:650–656
Lazzerini PE, Capecchi PL, Acampa M, Galeazzi M, Laghi-Pasini F (2014) Arrhythmic risk in rheumatoid arthritis: the driving role of systemic inflammation. Autoimmun Rev
Richter B, Gwechenberger M, Socas A, Zorn G, Albinni S, Marx M, Bergler-Klein J, Binder T, Wojta J, Gossinger HD (2012) Markers of oxidative stress after ablation of atrial fibrillation are associated with inflammation, delivered radiofrequency energy and early recurrence of atrial fibrillation. Clin Res Cardiol 101:217–225
Friedrichs K, Adam M, Remane L, Mollenhauer M, Rudolph V, Rudolph TK, Andrie RP, Stockigt F, Schrickel JW, Ravekes T, Deuschl F, Nickenig G, Willems S, Baldus S, Klinke A (2014) Induction of atrial fibrillation by neutrophils critically depends on CD11b/CD18 integrins. PLoS One 9:e89307
Frustaci A, Chimenti C, Bellocci F, Morgante E, Russo MA, Maseri A (1997) Histological substrate of atrial biopsies in patients with lone atrial fibrillation. Circulation 96:1180–1184
Passacquale G, Vamadevan P, Pereira L, Hamid C, Corrigall V, Ferro A (2011) Monocyte-platelet interaction induces a pro-inflammatory phenotype in circulating monocytes. PLoS One 6:e25595
Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the american society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28:1–39
Providencia R, Trigo J, Paiva L, Barra S (2013) The role of echocardiography in thromboembolic risk assessment of patients with nonvalvular atrial fibrillation. J Am Soc Echocardiogr 26:801–812
Wasmer K, Kobe J, Dechering D, Milberg P, Pott C, Vogler J, Stypmann J, Waltenberger J, Monnig G, Breithardt G, Eckardt L (2013) CHADS(2) and CHA(2)DS (2)-VASc score of patients with atrial fibrillation or flutter and newly detected left atrial thrombus. Clin Res Cardiol 102:139–144
Heeringa J, Conway DS, van der Kuip DA, Hofman A, Breteler MM, Lip GY, Witteman JC (2006) A longitudinal population-based study of prothrombotic factors in elderly subjects with atrial fibrillation: the Rotterdam Study 1990-1999. J Thromb Haemost 4:1944–1949
Lip GY, Patel JV, Hughes E, Hart RG (2007) High-sensitivity C-reactive protein and soluble CD40 ligand as indices of inflammation and platelet activation in 880 patients with nonvalvular atrial fibrillation: relationship to stroke risk factors, stroke risk stratification schema, and prognosis. Stroke 38:1229–1237
Conway DS, Buggins P, Hughes E, Lip GY (2004) Relation of interleukin-6, C-reactive protein, and the prothrombotic state to transesophageal echocardiographic findings in atrial fibrillation. Am J Cardiol 93(1368–73):A6
Hermida J, Lopez FL, Montes R, Matsushita K, Astor BC, Alonso A (2012) Usefulness of high-sensitivity C-reactive protein to predict mortality in patients with atrial fibrillation (from the Atherosclerosis Risk In Communities [ARIC] Study). Am J Cardiol 109:95–99
Michelson AD, Barnard MR, Hechtman HB, MacGregor H, Connolly RJ, Loscalzo J, Valeri CR (1996) In vivo tracking of platelets: circulating degranulated platelets rapidly lose surface P-selectin but continue to circulate and function. Proc Natl Acad Sci U S A 93:11877–11882
Lip GY, Frison L, Halperin JL, Lane DA (2010) Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke 41:2731–2738
Igarashi Y, Kashimura K, Makiyama Y, Sato T, Ojima K, Aizawa Y (2001) Left atrial appendage dysfunction in chronic nonvalvular atrial fibrillation is significantly associated with an elevated level of brain natriuretic peptide and a prothrombotic state. Jpn Circ J 65:788–792
McCready JW, Nunn L, Lambiase PD, Ahsan SY, Segal OR, Rowland E, Lowe MD, Chow AW (2010) Incidence of left atrial thrombus prior to atrial fibrillation ablation: is pre-procedural transoesophageal echocardiography mandatory? Europace 12:927–932
Hayashi M, Takeshita K, Inden Y, Ishii H, Cheng XW, Yamamoto K, Murohara T (2011) Platelet activation and induction of tissue factor in acute and chronic atrial fibrillation: involvement of mononuclear cell-platelet interaction. Thromb Res 128:e113–e118
Kirchhofer D, Riederer MA, Baumgartner HR (1997) Specific accumulation of circulating monocytes and polymorphonuclear leukocytes on platelet thrombi in a vascular injury model. Blood 89:1270–1278
Simon DI, Ezratty AM, Francis SA, Rennke H, Loscalzo J (1993) Fibrin(ogen) is internalized and degraded by activated human monocytoid cells via Mac-1 (CD11b/CD18): a nonplasmin fibrinolytic pathway. Blood 82:2414–2422
Penn MS, Topol EJ (2001) Tissue factor, the emerging link between inflammation, thrombosis, and vascular remodeling. Circ Res 89:1–2
Kaireviciute D, Blann AD, Balakrishnan B, Lane DA, Patel JV, Uzdavinys G, Norkunas G, Kalinauskas G, Sirvydis V, Aidietis A, Lip GY (2010) Characterisation and validity of inflammatory biomarkers in the prediction of post-operative atrial fibrillation in coronary artery disease patients. Thromb Haemost 104:122–127
Hammond DA, Smotherman C, Jankowski CA, Tan S, Osian O, Kraemer D, DeLosSantos M (2015) Short-course of ranolazine prevents postoperative atrial fibrillation following coronary artery bypass grafting and valve surgeries. Clin Res Cardiol 104:410–417
Simopoulos V, Tagarakis G, Hatziefthimiou A, Skoularigis I, Triposkiadis F, Trantou V, Tsilimingas N, Aidonidis I (2015) Effectiveness of aldosterone antagonists for preventing atrial fibrillation after cardiac surgery in patients with systolic heart failure: a retrospective study. Clin Res Cardiol 104:31–37
Ozaydin M, Peker O, Erdogan D, Akcay S, Yucel H, Icli A, Ceyhan BM, Sutcu R, Uysal BA, Varol E, Dogan A, Okutan H (2014) Oxidative status, inflammation, and postoperative atrial fibrillation with metoprolol vs carvedilol or carvedilol plus N-acetyl cysteine treatment. Clin Cardiol 37:300–306
Deftereos S, Giannopoulos G, Efremidis M, Kossyvakis C, Katsivas A, Panagopoulou V, Papadimitriou C, Karageorgiou S, Doudoumis K, Raisakis K, Kaoukis A, Alexopoulos D, Manolis AS, Stefanadis C, Cleman MW (2014) Colchicine for prevention of atrial fibrillation recurrence after pulmonary vein isolation: mid-term efficacy and effect on quality of life. Heart Rhythm 11:620–628
Marik PE, Fromm R (2009) The efficacy and dosage effect of corticosteroids for the prevention of atrial fibrillation after cardiac surgery: a systematic review. J Crit Care 24:458–463
Yoo D, Vinten-Johansen J, Schmarkey LS, Whalen SP, Bone CC, Katzmark SL, Langberg J (2010) Adhesive epicardial corticosteroids prevent postoperative atrial fibrillation. Circ Arrhythm Electrophysiol 3:505–510
Acknowledgments
C.P. was supported by the Deutsche Herzstiftung (Wilhelm P. Winterstein Award). The authors want to thank Peggy Barthel and Lydia Schwab for excellent technical assistance with flow cytometry analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have conflicts of interest to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pfluecke, C., Tarnowski, D., Plichta, L. et al. Monocyte–platelet aggregates and CD11b expression as markers for thrombogenicity in atrial fibrillation. Clin Res Cardiol 105, 314–322 (2016). https://doi.org/10.1007/s00392-015-0922-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0922-4