Abstract
Background
New oral anticoagulants are increasingly used in women of childbearing age, but apart from one case report there is no published experience with rivaroxaban exposure during pregnancy.
Methods
From October 2008 to December 2014, the German Embryotox Pharmacovigilance Centre identified 63 exposed pregnancies among 94 requests concerning rivaroxaban use during childbearing age. Follow-up included paediatric checks until 6 weeks after birth.
Results
All pregnancies with completed follow-up were exposed at least during the first trimester. Treatment indications included venous thromboembolism, knee surgery, and atrial fibrillation. 37 pregnancies were prospectively ascertained and resulted in six spontaneous abortions, eight elective terminations of pregnancy, and 23 live births. All women had discontinued rivaroxaban after recognition of pregnancy, mostly in the first trimester, but in one woman treatment continued until gestational week 26. There was one major malformation (conotruncal cardiac defect) among the 37 prospectively ascertained pregnancies in a woman with complex medication and a previous foetus with cardiac malformation without exposure to rivaroxaban. Only one case of bleeding concerning a retrospective report of surgery for missed abortion was observed in our case series.
Conclusion
Our results might give reassurance to those women, who were inadvertently exposed to rivaroxaban in early pregnancy. However, our limited cohort size does not allow ruling out an increased malformation risk and does not support the use of rivaroxaban during pregnancy. In all cases of (inadvertent) rivaroxaban exposure during 1st trimester, anticoagulation regimen should be reconsidered and a detailed ultrasound assessment recommended to confirm normal foetal development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The risk of venous thromboembolism (VTE) during pregnancy is increased by factor 4–5 compared to non-pregnant women of the same age and VTE is a major cause of maternal morbidity and mortality during pregnancy or after delivery [9]. Indications for treatment with anticoagulants during pregnancy include women with a high risk for VTE, valvular atrial fibrillation, or mechanical prosthetic heart valves [7, 9, 20, 24, 33, 40, 44]. The use of vitamin K antagonists (VKA) as established oral anticoagulants is limited during pregnancy due to their teratogenic effects. Consequently, low molecular weight heparins (LMWH) are the preferred treatment option in pregnancy today and use of VKAs is limited to high risk situations (e.g., mechanical heart valves) [4, 10, 19, 33, 39]. However, heparins are parenteral drugs requiring daily s.c. injections and delayed-type hypersensitivity skin reactions are frequent during pregnancy [37, 38]. One study reported that in almost every second pregnancy women had to switch at least once to another anticoagulant due to the development of hypersensitivity skin reactions on LMWH [37]. In most cases (77 %) skin reactions did not recur on the second preparation of LMWH but for the remaining patients there are limited treatment options [37, 38].
During the last years non-vitamin K antagonist oral anticoagulants (NOACs) as selective and direct inhibitors of either thrombin (factor IIa) or factor Xa have been developed as an alternative to the established anticoagulants that are also considered during pregnancy, e.g., VKA, unfractionated heparins (UFH) and LMWH [9, 33]. Meanwhile NOACs have been approved in many countries for prophylaxis of VTE in adult patients after hip or knee replacement surgery, for the treatment of acute deep vein thrombosis (DVT) and acute pulmonary embolism (PE), and for the prevention of recurrent DVT and PE [21]. In addition, in Europe the direct factor Xa inhibitor rivaroxaban has been approved for the prevention of atherothrombotic events in adult patients with elevated cardiac biomarkers after an acute coronary syndrome (ACS) [1]. NOACs were considered to be similarly effective as VKAs with a lower risk for intracranial bleedings [43]. Therefore, they have been recommended as an alternative to VKAs for patients with VTE and non-valvular atrial fibrillation [6, 14, 17, 18, 27, 31, 35, 41]. However, experience so far is largely derived from clinical studies and has to be confirmed in the “real world” [45].
Due to their physical and chemical properties, mostly based on the rather low molecular weight and data on placental transfer in rats, NOACs including the direct thrombin inhibitor dabigatran and the factor Xa inhibitors apixaban, edoxaban and rivaroxaban are expected to cross the placenta [9]. Except for one case report on rivaroxaban [26], there are no published studies on use of NOACs in pregnancy. The increase in prescription rates of NOACs in many countries around the world [11] will probably cause an increase in women with unplanned pregnancy while being on NOACs. Accordingly, raising numbers of information requests on NOACs during pregnancy are received by the German Embryotox Pharmacovigilance Centre. Therefore, we decided to present our observations of the outcome of 39 pregnancies exposed to rivaroxaban at least during the first trimester.
Materials and methods
The German Embryotox Pharmacovigilance Centre offers risk assessment on drug use in pregnancy to health care professionals of all specialities and pregnant women. On behalf of the German Federal Institute for Drugs and Medical Devices, more than 10,000 exposed pregnancies per year are documented through risk consultation. Using a structured questionnaire or phone interview at the first contact, all relevant data with respect to drugs, exposures to other agents, maternal characteristics as well as obstetric and family history are documented with the permission of the patient. Approximately 8 weeks after the expected date of delivery, follow-up is conducted by a questionnaire mailed to the woman or her physician and covers at least the first three paediatric examinations from birth up to the age of 4–6 weeks. In addition, information on complications during pregnancy such as infections, gestational diabetes, pre-eclampsia, details in case of pregnancy loss, gestational age at birth, sex, birth weight, length, head circumference, pH, and Apgar scores are obtained. On average, 85 % of our clients return their follow-up questionnaires. The main reasons for non-response are relocation, change of the physician, and very rarely refusal to answer.
Weeks of gestation are calculated from ultrasound during the first trimester or, if not available, from last menstrual period. Pregnancies are classified as prospective when the first contact to our Institute occurred before the outcome of pregnancy or prenatal diagnostic findings were known. Pregnancies that were reported because of pathological findings or after birth are considered as retrospective and evaluated separately. Further details of patients and methods have been described elsewhere [28].
Our survey includes all requests on rivaroxaban to our Institute from October 2008 (approval of the drug in Germany) until December 2014. All requests were analysed with regard to indication for treatment and pregnancy outcomes with special focus on miscarriage and on major malformations, defined as structural abnormalities of medical, surgical or cosmetic relevance [29, 32].
Results
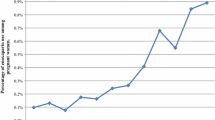
From October 2008 to December 2014 our Institute received 94 requests for information on rivaroxaban. Most requests were on maternal exposure during pregnancy (86 %) followed by requests on use during lactation (11 %) or paternal exposure (3 %); further details are given in Fig. 1. In comparison 427 requests with 273 (64 %) concerning maternal exposure during pregnancy were received for phenprocoumon, the preferred VKA in Germany, during the same period. The number of requests on rivaroxaban increased over time, especially after its approval for treatment and prophylaxis of DVT and PE in Germany in November 2012. Treatment indications among our requests in relation to time of approval are shown in Fig. 2. Of interest, before the respective approval rivaroxaban was already used off-label for treatment or secondary prophylaxis of VTE and other indications such as minor surgery procedures and thrombophlebitis (Fig. 2). Moreover, inappropriate dosing was observed in at least 12 (13 %) of the 94 requests. Nine patients received a daily dose of only 10 mg, one patient received 5 mg daily after an initial 3 weeks regimen of 10 mg daily, and two patients were treated with higher doses (40 and 50 mg daily); all for prophylaxis of recurrent VTE.
Flow chart of requests for information on rivaroxaban exposure to the German Embryotox Pharmacovigilance Centre from October 2008 until December 2014 with number of completed follow-ups. Asterisk indicates that two pregnancies with elective termination have been reported before the scheduled follow-up date. Double asterisk indicates that the estimated date of birth of all pending pregnancies is in 2015, follow-up at 8 weeks after estimated date of birth is not yet available
Treatment indication for rivaroxaban and date of treatment start in 94 patients in relation to the approval status for rivaroxaban indications in Germany over time. For each patient the reported time (month and year) of treatment start is shown. (ACS acute coronary syndrome with elevated biomarkers)
The outcome of rivaroxaban exposed pregnancies will be presented separately for prospectively ascertained cases and retrospective case reports.
Outcome of prospective cases (n = 37)
Follow-up was completed in 37 prospectively ascertained pregnancies with treatment at least during the first trimester. Maternal characteristics are summarised in Table 1. Further details of maternal and obstetric history are presented as supplementary material (Supplementary material online File Table 1). In 34 patients, treatment indication was prophylaxis of recurrent VTE, among these were one woman with systemic lupus erythematosus, one with antiphospholipid syndrome and one with homocystinuria (#2, #14 and #25 in Fig. 3 and Supplementary material online File Table 1). Further indications were prophylaxis of VTE after knee surgery (#1) and long distance flight after previous DVT (#4 and #16). The median pre-pregnancy BMI was 24.7, while eight patients had a BMI > 30. Median gestational age at study enrolment was 7 weeks. Rivaroxaban treatment had been initiated before pregnancy in 33 (89 %) of the 37 women and median treatment discontinuation in these women was in week 7. Of interest, in one woman (#19) treatment was continued until her pregnancy was recognised in week 26. In four cases therapy was started before pregnancy recognition, in gestational week 4, 5 and 7 (#1, #4, #6, and #22), respectively. Rivaroxaban exposure intervals and pregnancy outcomes are shown in Fig. 3. Median rivaroxaban dose was 20 mg/day (range 5–50 mg).
After discontinuation of rivaroxaban, all 26 women in whom anticoagulant treatment was continued were switched to LMWH (Fig. 3). However, in four patients there was a gap of up to 5 weeks (#6, #10, #17, and #26), before treatment with LMWH was initiated and in three patients rivaroxaban and LMWH were given concomitantly for 1–3 days. Two patients had to switch from one LMWH to another, one in week 10 and the other in week 29 (#25 and #26). One patient received low dose acetyl salicylic acid for VTE prophylaxis after discontinuation of rivaroxaban (#34). Five patients did not receive further anticoagulation: one patient after knee surgery, two patients for VTE prophylaxis because of long distance flights, two patients with a history of PE, four patients after DVT.
Pregnancy outcomes were six spontaneous abortions, eight elective terminations of pregnancy and 23 live births. The group of pregnant women with spontaneous abortions included one woman with antiphospholipid syndrome (#14; week 10), one with a prosthetic heart valve and opiate co-medication (#11; week 15), one with premature rupture of membranes and histologically confirmed ascending infection (#10; week 16), one with a twin pregnancy (#27, missed abortion in wk 10), one in a women with bicornuate uterus (#32) and only one without known risk factors (#9; week 8). Six pregnancies were terminated for social reasons, one for fear of malformations after rivaroxaban exposure and one after diagnosis of a complex foetal heart defect (#2, see below).
Overall, 23 pregnancies were carried to term; in eight of these pregnancies Caesarean section was performed (#15–19, #24, #26, #30, #31). Mild thrombocytopenia was observed in two women (#5, #17) and interpreted as pregnancy related. Neonatal characteristics are summarised in Table 2.
There was only one case with a congenital malformation among the 37 prospectively reported pregnancies: a conotruncal cardiac defect leading to termination of pregnancy (case #2). The first pregnancy of this woman 1 year before was not exposed to rivaroxaban, but had also been terminated after prenatal diagnosis of tetralogy of Fallot. She had systemic lupus erythematosus with recurrent VTE necessitating treatment with multiple drugs: tacrolimus, hydroxychloroquine, prednisolone, pantoprazole, ramipril, torasemide, simvastatin, ezetimibe, acetyl salicylic acid, and salbutamol were used throughout her current pregnancy. In addition, she took metamizole and aliskiren during the first trimester. Rivaroxaban was discontinued because of suspected inefficiency and replaced by LMWH, when inferior vena cava and renal thrombosis were diagnosed in week 5 of the—at that time—unknown pregnancy. Preconceptionally, she had been exposed to rituximab and cyclophosphamide.
This patient was the only one in our case series developing thrombosis during pregnancy. No further patients with pregnancy complications were reported that could be attributed to thromboembolic events. Postpartum VTE was not reported in any woman during a median follow-up of 17 weeks after birth.
Outcome of retrospectively reported cases (n = 2)
Two pregnancies were reported retrospectively. Treatment with 10 mg rivaroxaban daily had been started in a 37-year-old mother with tetralogy of Fallot after a first episode of atrial fibrillation 8 days before conception and was stopped by the patient 1 week after conception. Pregnancy was complicated by recurrent atrial fibrillation necessitating electric cardioversion at gestational weeks 17 and 20, respectively. Only after the second episode in week 17, anticoagulation was started again with 60 mg enoxaparin twice daily. Intrauterine growth retardation was diagnosed in week 31 and a girl was delivered by Caesarean section at week 33 + 6 days. Neonatal birth weight was 1830 g (35th percentile). After mild hypoglycaemia and episodes of apnoea-bradycardia the infant developed normally.
In another 37-year-old woman rivaroxaban was prescribed 4 months following her first pregnancy complicated by DVT at gestational week 26. Still being on 10 mg rivaroxaban once daily, she got pregnant again. At week 10 a missed abortion was diagnosed necessitating uterine evacuation which was followed by unusually severe bleeding. The embryo was described as having abnormal (“crumpled”) limbs. No further details are available for this case.
Discussion
Our case series presents the first data on pregnancies exposed to rivaroxaban including detailed documentation of exposure related to gestational age. Rivaroxaban is an oral factor Xa inhibitor currently approved for several indications [36]. Whereas indications such as VTE prophylaxis in elective hip and knee replacement surgery, stroke prevention in non-valvular atrial fibrillation, and prevention of atherothrombotic events after ACS are more prevalent in older patients, the most frequent indication for use of rivaroxaban or other NOACs in women of childbearing age is treatment and prophylaxis of recurrent VTE [36]. Accordingly, we have noticed a significant increase in rivaroxaban exposures in pregnant women after the approval for the treatment of acute DVT and PE, and for the prevention of recurrent DVT and PE in Germany in November 2012 (Fig. 2). While case reports or smaller case series on the selective and parenteral factor Xa inhibitor fondaparinux totalling at least 65 pregnancies have been published [12, 25, 30], there is only one case on a pregnant patient treated with rivaroxaban published so far [26].
Compared to established anticoagulants, practical experience with rivaroxaban is still limited and as with any new drug, responsible use of NOACs within the label of approval is an important issue [42]. Whereas a recent study did not observe important off-label use of rivaroxaban in Germany [23], our case series demonstrates that inappropriate dosing and off-label treatment (Fig. 2) occur even during pregnancy although the European Medicines Agency (EMA) has labelled rivaroxaban as contraindicated during pregnancy due to its potential reproductive toxicity and the intrinsic risk of bleeding (product information Xarelto® [13]). According to this product information, embryo-foetal toxicity (post-implantation loss, retarded/progressed ossification, hepatic multiple light-coloured spots) and an increased incidence of common malformations as well as placental changes were observed at clinically relevant plasma concentrations in animal studies with rats and rabbits.
In contrast, the US Food and Drug Administration (FDA) stated that there is no increased malformation risk in rats or rabbits but a decrease in viable rat foetuses after exposure to 41 times the human dose based on surface area. Increased resorption rates and decreased number of live foetuses and decreased foetal body weight were observed when pregnant rabbits were given oral doses that are about four times the human exposure of unbound drug, based on AUC comparisons at the human dose of 20 mg/day [22]. However, animal experiments did not provide evidence of teratogenicity, i.e., an increase of structural malformations [15]. Consequently, the FDA has assigned rivaroxaban to pregnancy category C [22] indicating that animal reproduction studies have shown an adverse effect on the foetus and, although no adequate and well-controlled studies in humans are available, potential benefits may warrant use of the drug in pregnant women despite potential risks [16].
In the more restrictive European approval of rivaroxaban, it is recommended that women of childbearing potential should avoid becoming pregnant during treatment with the drug and should, therefore, practise reliable contraception [13].
In the current case series, we observed only one major malformation documented in the prospective cohort. The mother suffered from severe systemic lupus erythematosus and recurrent VTE and had several co-medications, some of which are insufficiently studied during pregnancy (e.g., aliskiren, ezetimibe) or definitely fetotoxic (e.g., ramipril). Her obstetric history with a previous foetus affected by tetralogy of Fallot (without rivaroxaban exposure) suggests other risk factors or a genetic contribution to the cardiac malformation in both foetuses. Interestingly, Elsaigh et al. [12] reported a foetus with tetralogy of Fallot and Dandy–Walker syndrome after maternal exposure to the parenteral selective factor Xa inhibitor fondaparinux (started in week 7 of pregnancy), but we would consider this as a coincidence, as tetralogy of Fallot is the most frequent cyanotic cardiac defect [3]. Our retrospective case report of an aborted embryo with crumpled limbs might still represent a normal phenotype at that early developmental stage with a body size of 1.5 cm.
The rate of spontaneous abortions of 16 % lies within the expected range in the general pregnant population [5, 8].
Finally, it is noteworthy that one woman receiving rivaroxaban until week 26 delivered a healthy infant. The mother reported two previous spontaneous abortions while being on phenprocoumon, in weeks 22 and 11, respectively.
There was one pregnancy with mild preeclampsia, but no preterm birth reported in the prospective cohort and only one infant was born preterm among the two retrospective case reports (Table 2).
An increased risk for bleeding complications during pregnancy and around delivery has to be considered in women treated with any anticoagulant including NOACs. While a reduction of intracerebral bleeding events during the treatment with NOACs as compared with VKA is considered as an important advantage of these drugs [34, 43] they may expose specific patient groups to a higher risk for gastrointestinal bleeding events [34]. Moreover, bleeding complications have been observed more frequently in women than in men [2]. In late pregnancy, similar to VKA, rivaroxaban might cause cerebral bleeding in the foetus. Except for one case with an evacuation of a missed abortion we did not observe (maternal) bleeding complications in our case series. However, rivaroxaban exposure was limited to the first trimester in most of the cases. All women of the current case series discontinued rivaroxaban after discovering their pregnancy. A delay of initiation of conversion to LMWH as observed in some of our pregnancies should be avoided in high risk pregnancies, since the switching from rivaroxaban to LMWH therapy can be easily conducted in clinical practice [42] (Fig. 4).
Proposed procedure after inadvertent treatment with rivaroxaban during pregnancy. Switch from rivaroxaban to LMWH should be decided upon individual risk assessment. The first dose of the replacement anticoagulant (e.g. LMWH) can be given at the time the next dose of rivaroxaban would have been due. LMP, last menstrual period. Asterisk indicates individual decision about re-initiation of rivaroxaban after lactation period
In summary, our observations do not provide evidence of a substantial embryotoxic risk for rivaroxaban during early pregnancy. Therefore, our results may be used to reassure those women, who were inadvertently exposed to rivaroxaban in early pregnancy. These women should be carefully counselled to prevent overestimation of embryotoxic risks. Apart from reconsidering the need of continuous anticoagulation a detailed ultrasound should be recommended to confirm normal foetal development (Fig. 4).
The available data are still insufficient to document safety and to recommend the use of rivaroxaban during pregnancy. Before prescribing rivaroxaban to women of childbearing age they should be advised to use effective birth control. According to the European labelling of rivaroxaban (EMA) as contraindicated during pregnancy, rivaroxaban should be discontinued in unplanned pregnancies. More observational studies on rivaroxaban and other NOACs during pregnancy are required because it appears likely that the number of pregnant women exposed to these drugs will increase in the future.
References
Ahrens I, Bode C (2014) Direct oral anticoagulants in acute coronary syndrome. Semin Hematol 51:147–151. doi:10.1053/j.seminhematol.2014.03.004
Alotaibi GS, Almodaimegh H, McMurtry MS, Wu C (2013) Do women bleed more than men when prescribed novel oral anticoagulants for venous thromboembolism? A sex-based meta-analysis. Thromb Res 132:185–189. doi:10.1016/j.thromres.2013.07.017
Apitz C, Webb GD, Redington AN (2009) Tetralogy of Fallot. Lancet 374:1462–1471. doi:10.1016/S0140-6736(09)60657-7
Bates SM, Greer IA, Middeldorp S, Veenstra DL, Prabulos AM, Vandvik PO (2012) VTE, thrombophilia, antithrombotic therapy, and pregnancy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 141:e691S–e736S. doi:10.1378/chest.11-2300
Borrell A, Stergiotou I (2013) Miscarriage in contemporary maternal-fetal medicine: targeting clinical dilemmas. Ultrasound Obstet Gynecol 42:491–497. doi:10.1002/uog.12442
Caldeira D, Costa J, Ferreira JJ, Lip GY, Pinto FJ (2015) Non-vitamin K antagonist oral anticoagulants in the cardioversion of patients with atrial fibrillation: systematic review and meta-analysis. Clin Res Cardiol 104:582–590. doi:10.1007/s00392-015-0821-8
Camm JA, Lip GYH, De Caterina R, Savelieva I, Atar D, Hohnloser SH, Hindricks G, Kirchhof P, Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck-Brentano C, Hasdai D, Hoes A, Knuuti J, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, Vardas P, Al-Attar N, Alfieri O, Angelini A, Blomstrom Lundqvist C, Colonna P, De Sutter J, Ernst S, Goette A, Gorenek B, Hatala R, Heidbüchel H, Heldal M, Kristensen SD, Kolh P, Le Heuzey JY, Mavrakis H, Mont L, Filardi PP, Ponikowski P, Prendergast B, Rutten FH, Schotten U, Van Gelder IC, Verheugt FWA (2012) 2012 focused update of the ESC Guidelines for the management of atrial fibrillation. Eur Heart J 33:2719–2747. doi:10.1093/eurheartj/ehs253
Carrington B, Sacks G, Regan L (2005) Recurrent miscarriage: pathophysiology and outcome. Curr Opin Obstet Gynecol 17:591–597
Conti E, Zezza L, Ralli E, Comito C, Sada L, Passerini J, Caserta D, Rubattu S, Autore C, Moscarini M, Volpe M (2014) Pulmonary embolism in pregnancy. J Thromb Thrombolysis 37:251–270. doi:10.1007/s11239-013-0941-9
Cutts BA, DasGupta D, Hunt BJ (2013) New directions in the diagnosis and treatment of pulmonary embolism in pregnancy. Am J Obstet Gynecol 208:102–108. doi:10.1016/j.ajog.2012.06.035
Desai NR, Krumme AA, Schneeweiss S, Shrank WH, Brill G, Pezalla EJ, Spettell CM, Brennan TA, Matlin OS, Avorn J, Choudhry NK (2014) Patterns of initiation of oral anticoagulants in patients with atrial fibrillation—quality and cost implications. Am J Med 127:1075–1082. doi:10.1016/j.amjmed.2014.05.013
Elsaigh E, Thachil J, Nash MJ, Tower C, Hay CR, Bullough S, Byrd L (2014) The use of fondaparinux in pregnancy. Br J Haematol 168:762–764. doi:10.1111/bjh.13147
EMA EPAR-Product information Rivaroxaban (2015) http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000944/WC500057108.pdf. Accessed 27 May 2015
Ewen S, Rettig-Ewen V, Mahfoud F, Bohm M, Laufs U (2014) Drug adherence in patients taking oral anticoagulation therapy. Clin Res Cardiol 103:173–182. doi:10.1007/s00392-013-0616-8
FDA Rivaroxaban pharmacology review (2015) http://www.accessdata.fda.gov/drugsatfda_docs/nda/2011/022406Orig1s000PharmR.pdf. Accessed 27 May 2015
Federal Register Content and Format of Labeling for Human Prescription Drug and Biological Products; Requirements for Pregnancy and Lactation Labeling (2015) https://www.federalregister.gov/articles/2008/05/29/E8-11806/content-and-format-of-labeling-for-human-prescription-drug-and-biological-productsrequirements. Accessed 27 May 2015
Ferner M, Wachtlin D, Konrad T, Deuster O, Meinertz T, von Bardeleben S, Munzel T, Seibert-Grafe M, Breithardt G, Rostock T (2015) Rationale and design of the RE-LATED AF-AFNET 7 trial: REsolution of Left atrial-Appendage Thrombus-Effects of Dabigatran in patients with Atrial Fibrillation. Clin Res Cardiol. doi:10.1007/s00392-015-0883-7
Fischer D, Gardiwal A, Haentjes J, Klein G, Meyer GP, Drexler H, Hausmann D, Schaefer A (2012) Sustained risk of recurrent thromboembolic events in patients with patent foramen ovale and paradoxical embolism: long-term follow-up over more than 15 years. Clin Res Cardiol 101:297–303. doi:10.1007/s00392-011-0392-2
Greer IA (2012) Thrombosis in pregnancy: updates in diagnosis and management. Hematol Am Soc Hematol Educ Program 2012:203–207. doi:10.1182/asheducation-2012.1.203
Haghikia A, Podewski E, Berliner D, Sonnenschein K, Fischer D, Angermann CE, Bohm M, Rontgen P, Bauersachs J, Hilfiker-Kleiner D (2015) Rationale and design of a randomized, controlled multicentre clinical trial to evaluate the effect of bromocriptine on left ventricular function in women with peripartum cardiomyopathy. Clin Res Cardiol. doi:10.1007/s00392-015-0869-5
Husted S, de Caterina R, Andreotti F, Arnesen H, Bachmann F, Huber K, Jespersen J, Kristensen SD, Lip GY, Morais J, Rasmussen LH, Siegbahn A, Storey RF, Weitz JI (2014) Non-vitamin K antagonist oral anticoagulants (NOACs): no longer new or novel. Thromb Haemost 111:781–782. doi:10.1160/TH14-03-0228
Janssen Pharmaceuticals, Inc (2011) Prescribing Information Xarelto. http://www.xareltohcp.com/sites/default/files/pdf/xarelto_0.pdf. Accessed 27 May 2015
Jobski K, Enders D, Amann U, Suzart K, Wallander MA, Schink T, Garbe E (2014) Use of rivaroxaban in Germany: a database drug utilization study of a drug started in hospital. Eur J Clin Pharmacol 70:975–981. doi:10.1007/s00228-014-1697-7
Kaemmerer M, Vigl M, Seifert-Klauss V, Nagdyman N, Bauer U, Schneider KT, Kaemmerer H (2012) Counseling reproductive health issues in women with congenital heart disease. Clin Res Cardiol 101:901–907. doi:10.1007/s00392-012-0474-9
Knol HM, Schultinge L, Erwich JJ, Meijer K (2010) Fondaparinux as an alternative anticoagulant therapy during pregnancy. J Thromb Haemost 8:1876–1879. doi:10.1111/j.1538-7836.2010.03926.x
Königsbrugge O, Langer M, Hayde M, Ay C, Pabinger I (2014) Oral anticoagulation with rivaroxaban during pregnancy: a case report. Thromb Haemost 112:1323–1324. doi:10.1160/TH14-04-0393
Kornej J, Kosiuk J, Hindricks G, Arya A, Sommer P, Rolf S, Husser D, Lip GY, Bollmann A (2015) Sex-related predictors for thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Clin Res Cardiol 104:603–610. doi:10.1007/s00392-015-0823-6
Meister R, Schaefer C (2008) Statistical methods for estimating the probability of spontaneous abortion in observational studies—analyzing pregnancies exposed to coumarin derivatives. Reprod Toxicol 26:31–35. doi:10.1016/j.reprotox.2008.06.006
Merks JH, van Karnebeek CD, Caron HN, Hennekam RC (2003) Phenotypic abnormalities: terminology and classification. Am J Med Genet A 123A:211–230. doi:10.1002/ajmg.a.20249
Nagler M, Haslauer M, Wuillemin WA (2012) Fondaparinux—data on efficacy and safety in special situations. Thromb Res 129:407–417. doi:10.1016/j.thromres.2011.10.037
Nielsen PB, Lane DA, Rasmussen LH, Lip GY, Larsen TB (2015) Renal function and non-vitamin K oral anticoagulants in comparison with warfarin on safety and efficacy outcomes in atrial fibrillation patients: a systemic review and meta-regression analysis. Clin Res Cardiol 104:418–429. doi:10.1007/s00392-014-0797-9
Rasmussen SA, Olney RS, Holmes LB, Lin AE, Keppler-Noreuil KM, Moore CA (2003) Guidelines for case classification for the National Birth Defects Prevention Study. Birth Defects Res A Clin Mol Teratol 67:193–201. doi:10.1002/bdra.10012
Regitz-Zagrosek V, Blomstrom LC, Borghi C, Cifkova R, Ferreira R, Foidart JM, Gibbs JS, Gohlke-Baerwolf C, Gorenek B, Iung B, Kirby M, Maas AH, Morais J, Nihoyannopoulos P, Pieper PG, Presbitero P, Roos-Hesselink JW, Schaufelberger M, Seeland U, Torracca L (2011) ESC Guidelines on the management of cardiovascular diseases during pregnancy: the Task Force on the Management of Cardiovascular Diseases during Pregnancy of the European Society of Cardiology (ESC). Eur Heart J 32:3147–3197. doi:10.1093/eurheartj/ehr218
Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM (2014) Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 383:955–962. doi:10.1016/S0140-6736(13)62343-0
Sabouret P, Depret-Bixio L, Cotte FE, Marie P, Bedira N, Blin P (2014) Sex differences in stroke prevention in atrial fibrillation in French primary care. Results of the AFIGP (Atrial Fibrillation In General Practice) database. Clin Res Cardiol 103:887–893. doi:10.1007/s00392-014-0726-y
Sarich TC, Peters G, Berkowitz SD, Misselwitz F, Nessel CC, Burton P, Cook-Bruns N, Lensing AW, Haskell L, Perzborn E, Kubitza D, Moore KT, Jalota S, Weber J, Pan G, Sun X, Westermeier T, Nadel A, Oppenheimer L, DiBattiste PM (2013) Rivaroxaban: a novel oral anticoagulant for the prevention and treatment of several thrombosis-mediated conditions. Ann N Y Acad Sci 1291:42–55. doi:10.1111/nyas.12136
Schindewolf M, Gobst C, Kroll H, Recke A, Louwen F, Wolter M, Kaufmann R, Boehncke WH, Lindhoff-Last E, Ludwig RJ (2013) High incidence of heparin-induced allergic delayed-type hypersensitivity reactions in pregnancy. J Allergy Clin Immunol 132:131–139. doi:10.1016/j.jaci.2013.02.047
Schultinge L, Knol HM, Kluin-Nelemans HC, Erwich JJ, Meijer K (2013) Incidence of hypersensitivity skin reactions in patients on full-dose low-molecular-weight heparins during pregnancy. Neth J Med 71:518–522
Sedaghat A, Nickenig G, Hammerstingl C (2014) Left atrial appendage closure in a patient with atrial fibrillation after mechanical mitral valve replacement and cardio-embolic stroke despite effective oral anticoagulant therapy: a case report. Clin Res Cardiol 103:587–589. doi:10.1007/s00392-014-0704-4
Sliwa K, Ojji D, Bachelier K, Bohm M, Damasceno A, Stewart S (2014) Hypertension and hypertensive heart disease in African women. Clin Res Cardiol 103:515–523. doi:10.1007/s00392-014-0660-z
Steiner T, Bohm M, Dichgans M, Diener HC, Ell C, Endres M, Epple C, Grond M, Laufs U, Nickenig G, Riess H, Rother J, Schellinger PD, Spannagl M, Veltkamp R (2013) Recommendations for the emergency management of complications associated with the new direct oral anticoagulants (DOACs), apixaban, dabigatran and rivaroxaban. Clin Res Cardiol 102:399–412. doi:10.1007/s00392-013-0560-7
Turpie AG, Kreutz R, Llau J, Norrving B, Haas S (2012) Management consensus guidance for the use of rivaroxaban—an oral, direct factor Xa inhibitor. Thromb Haemost 108:876–886. doi:10.1160/TH12-03-0209
van Es N, Coppens M, Schulman S, Middeldorp S, Buller HR (2014) Direct oral anticoagulants compared with vitamin K antagonists for acute venous thromboembolism: evidence from phase 3 trials. Blood 124:1968–1975. doi:10.1182/blood-2014-04-571232
Wacker-Gussmann A, Thriemer M, Yigitbasi M, Berger F, Nagdyman N (2013) Women with congenital heart disease: long-term outcomes after pregnancy. Clin Res Cardiol 102:215–222. doi:10.1007/s00392-012-0522-5
Wells PS, Forgie MA, Rodger MA (2014) Treatment of venous thromboembolism. JAMA 311:717–728. doi:10.1001/jama.2014.65
Acknowledgments
Documentation and evaluation of pregnancy outcomes under medication are performed on behalf of the German Federal Institute for Drugs and Medical Devices (BfArM). The authors would like to thank all participating physicians and patients who provided detailed information and contributed to the study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
MH, EB, KM, CS declare no conflict of interest. RK has been a consultant or participated in advisory boards for Bayer HealthCare, Berlin-Chemie Menarini, Bristol-Myers-Squibb, Daiichi Sankyo and Servier.
Additional information
C. Schaefer and R. Kreutz contributed equally to this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hoeltzenbein, M., Beck, E., Meixner, K. et al. Pregnancy outcome after exposure to the novel oral anticoagulant rivaroxaban in women at suspected risk for thromboembolic events: a case series from the German Embryotox Pharmacovigilance Centre. Clin Res Cardiol 105, 117–126 (2016). https://doi.org/10.1007/s00392-015-0893-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0893-5