Abstract
Background
Females with atrial fibrillation (AF) are at increased risk for ischemic stroke but have been under-represented in AF ablation cohorts. Whether the incidence of TE in women after catheter ablation is higher is unknown. We aimed to analyze the predictive value of thromboembolic scores and other clinical variants for thromboembolism (TE) after AF catheter ablation, separately in women and men.
Methods
TE was combined endpoint of early (within first month) and late (during long-term follow-up) stroke, transient ischemic attack, or systemic embolism. Oral anticoagulation was prescribed for 6 months after catheter ablation and discontinued if CHADS2 was <2 and no AF recurrences were documented.
Results
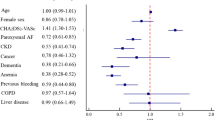
The study population (n = 2,069, 66 % male, 60 ± 10 years; 62 % paroxysmal AF) was followed for a median of 18 months (IQR 12–29). Overall 31 TE (1.5 %) occurred with 16 events within 30 days of ablation and 15 TE during the follow-up. Fourteen females (2.0 %) and 17 males (1.2 %) suffered TE (p = 0.128). On multivariate analysis, higher CHADS2 (HR 1.65, 95 % CI 1.10–2.47, p = 0.015), CHA2DS2-VASc (HR 1.42, 95 % CI 1.03–1.96, p = 0.034), R2CHADS2 (HR 1.76, 95 % CI 1.32–2.35, p < 0.001) scores, and eGFR <60 ml/min/1.73 m2 (HR 3.95, 95 % CI 1.23–12.7, p = 0.021) were significantly associated with TE in men. In females, LV-EF (HR 0.95, 95 % CI 0.91–0.99, p = 0.021) and CHA2DS2-VASc score (HR 1.52, 95 % CI 1.01–2.28, p = 0.044) remained significant predictors for TE.
Conclusion
TE rates after AF catheter ablation are low in both genders. In females, LV-EF and CHA2DS2-VASc score and in males all three scores and renal dysfunction were associated with TE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Catheter ablation of atrial fibrillation (AF) is an effective therapy for the reduction of AF burden, improvement of symptoms, and quality of life [1, 2]. Observational and registry studies have demonstrated low annual rates of thromboembolism (stroke and systemic embolism, TE) in selected populations after AF catheter ablation, ranging between 0.5 [3] and 1.7 % [4], thus reaching event rates that are comparable to patients without AF [4, 5].
Without anticoagulation, women are at higher risk than men for AF-related TE [6–8], but results are not consistent [9]. Vascular complication rates of AF ablation had been recently shown to be also higher in females [10]. Since females are under-represented in AF ablation cohorts, it is unclear whether there are any sex-related differences in TE occurrence in such a specific patient population.
Recently, we demonstrated that all three stroke risk stratification scores—i.e., CHADS2, CHA2DS2-VASc, and R2CHADS2—were associated with late TE risk in anticoagulated AF patients after catheter ablation [11]. Furthermore, the CHA2DS2-VASc score was also predictive for early (i.e., peri-interventional) TE [12].
In current study, we analyzed the predictive value of CHADS2, CHA2DS2-VASc, and R2CHADS2 scores and other clinical variants for TE after AF catheter ablation separately in women and men.
Our objective was to assess sex differences in thromboembolic risk post-ablation and the predictive risk factors (and risk scores) for thromboembolism in females and males.
Methods
Patients and risk scores
The study population comprised 2,069 consecutive patients with symptomatic AF who underwent catheter ablation at the Heart Center Leipzig between January 2007 and December 2011. Stroke risk was assessed using the CHADS2 [congestive heart failure, hypertension, age >75 years, diabetes mellitus, and history of stroke/transient ischemic attack (2 points)] [9] and CHA2DS2-VASc [congestive heart failure, hypertension, age >75 years (2 points), diabetes mellitus, and history of stroke/transient ischemic attack (2 points)—vascular disease (history of myocardial infarction, peripheral artery disease, or vascular plaques), age 65–74 years, and sex category (female)] scores [13, 14]. The R2CHADS2 score incorporated the components of the CHADS2 score and also gave 2 points for renal dysfunction defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 [15]. eGFR was calculated using the Cockroft–Gault equation: (140−age) × weight (kg) × (0.85 if female)/72 × serum creatinine (mg/dl).
The study was performed according to the Declaration of Helsinki and Institutional Guidelines. Patients provided written informed consent.
Catheter ablation and follow-up
Left atrial catheter ablation was performed using a previously described approach [16]. Patients presenting with AF at the beginning of the procedure were electrically cardioverted, and ablation was performed during sinus rhythm (i.e., AF termination with ablation was not attempted). In all patients, circumferential left atrial ablation lines were placed around the antrum of the ipsilateral pulmonary veins (irrigated tip catheter, pre-selected tip temperature of 48 °C, and maximum power of 30–50 W). In patients with persistent AF, additional linear lesions were added at the left atrial roof, the basal posterior wall, and the left atrial isthmus and according to low voltage areas. Ablation of complex fractionated electrograms was not performed. After circumferential line placement, voltage and pace mapping along the ablation line were used to identify and close gaps. The isolation of all pulmonary veins with bidirectional block was verified with a multipolar circular mapping catheter and was defined as the procedural endpoint. After ablation, class I and III antiarrhythmic drugs were not reinitiated, and proton pump inhibitors were added for 4 weeks.
According to the previous guidelines, oral anticoagulation was prescribed for 3–6 months after ablation and depending on the CHADS2 or CHA2DS2-VASc scores thereafter [17]. Because of patient preferences, lack of symptoms, and ECG recordings of sinus rhythm, it was replaced by aspirin in some patients [11].
All patients were followed in the outpatient clinic for at least 12 months after the catheter ablation. During follow-up, serial 7-day Holter ECG recordings were performed immediately and at 3, 6, and 12 months after the procedure. Additional ECGs and Holter ECG recordings were obtained when patients’ symptoms were suggestive of AF.
Outcomes
The primary endpoint of this study was the composite of ischemic stroke, transient ischemic attack (TIA), and/or systemic embolism. Ischemic stroke was a clinical diagnosis that was made on the basis of typical symptoms lasting at least 24 h. Brain imaging, which was available in all patients with peri-interventional stroke and in the vast majority of patients with later TE, was not required but was recommended for the general diagnosis of stroke. A TIA was defined as sudden-onset focal neurological deficit with duration of <24 h. Systemic embolism was defined as TE events that occurred in peripheral organs (e.g., spleen, eye) or extremities.
Statistical analysis
Data are presented as means and standard deviation for normally distributed continuous variables and as proportions for categorical variables. Continuous variables were tested for normal distribution using the Kolmogorov–Smirnov test. The differences between continuous values were assessed using an unpaired two-tailed t test for normally distributed continuous variables, a Mann–Whitney test for skewed variables, and a Chi-square test for nominal variables.
Cox regression analyses were used to identify factors associated with TE events. Multivariable analysis, which included variables with a p value <0.1 found on univariable analysis, was performed to identify independent predictors of TE. In addition, we performed multivariable analyses separately for every stroke risk stratification score with adjustment for renal dysfunction (for the CHADS2 and CHA2DS2-VASc scores) or peripheral artery disease (for the CHADS2 and R2CHADS2 scores) in males. Because impaired left ventricular ejection fraction (LV-EF) could be considered as a part of all scores (as chronic heart failure), we also used separate MV analyses for clinical variables including all scores.
A p value of <0.05 was considered statistically significant. All analyses were performed with SPSS statistical software version 17.
Results
Patient characteristics and thromboembolic events
The clinical characteristics of 696 women (34 %) and 1,373 men (66 %) included into The Leipzig Heart Center AF Ablation Registry are summarized in Table 1. Females were significantly older and had more often paroxysmal AF, hypertension, renal dysfunction, and previous stroke, while males had more coronary artery disease, chronic heart failure, larger LA and LV diameters as well as worse EF. Consequently, all stroke risk stratification scores were higher in women. Patients were followed for median (IQR) 18 months (12–29) (3,078 patient-years).
Overall, TE occurred in 31 patients (1.4 %). Sixteen patients (0.8 %) suffered TE within first 30 days after catheter ablation (peri-interventional TE) and 15 (0.7 %) during follow-up. There were nine ischemic strokes and seven TIAs in patients with peri-interventional TE and five ischemic strokes, eight TIAs, and two systemic embolisms during follow-up. Patients with TE had more often renal dysfunction, previous TE, and consequently higher stroke risk stratification scores than patients without TE (Table 2). There were no significant differences between patients with early and late TE (Table 2).
Sex-related predictors of thromboembolic events
Using three separate multivariate Cox regression (Table 3) analyses, the CHADS2, R2CHADS2, and CHA2DS2-VASc scores were significant predictors of TE in the entire cohort.
In females (n = 696), using the uni- and multivariable Cox regression analyses, LV-EF (HR 0.95, 95 %CI 0.91–0.99, p = 0.021) and CHA2DS2-VASc scores (HR 1.52, 95 %CI 1.01–2.28, p = 0.044) were significant predictors for TE (Table 4). In men, all three stroke risk stratification scores and renal function (MV models 1 and 3 for the CHADS2 and CHA2DS2-VASc scores, respectively) were significant predictors for TE (Table 5).
Discussion
To the best of our knowledge, this is the largest study assessing sex-related incidence and risk factors for thromboembolic complications after AF catheter ablation with a special focus on renal dysfunction and stroke risk stratification scores. The main findings are that both early and late TE after AF catheter ablation are rare. Renal dysfunction and all three stroke risk stratification scores, i.e., CHADS2, CHA2DS2-VASc, R2CHADS2, were associated with TE risk in males, while impaired LV-EF and CHA2DS2-VASc scores were significant independent predictors for TE in females.
Catheter ablation and TE
The first study analyzing peri-interventional events and TE during follow-up associated with AF catheter ablation was presented by Oral et al. [5]. Pooling patients with early and late TE, they did not find any clinical predictors for TE; however, this study was the first which indicated a risk reduction of TE during follow-up, despite being associated with the risk for post-interventional events.
In contrast to Oral et al. [5], we performed our analyses in much larger AF ablation cohort with >2,000 patients with longer follow-up and more thromboembolic events. Despite obvious differences in the likely pathophysiology of early versus late TE (i.e., peri-procedural anticoagulation, procedure characteristics in early TE versus anticoagulation regime in late TE), we found similar incidence of early and late TE without any significant differences among the groups (exception for hypertension). All three stroke stratification scores and renal dysfunction were significant predictors for pooled early and late TE after catheter ablation. Previously, we could not demonstrate that renal dysfunction was a risk factor for late TE occurrence in patients after AF catheter ablation [11]. The current study with selected patient cohort, however, confirms previous suggestions indicating the association between impaired renal function and thromboembolism in AF [18]. Interestingly, the new score R2CHADS2—with renal dysfunction as an additional risk factor—was highly significant as predictor for TE (p < 0.001), while the “classic” CHADS2 and CHA2DS2-VASc scores remained associated with TE after adding renal dysfunction to the multivariable model, too. Of note, renal dysfunction itself was a predictor for TE in the whole population in MV model with CHADS2 score (p = 0.041) but was not significant in the model with CHA2DS2-VASc (p = 0.109). This is partly in accordance with recent study analyzing improvement of predictive ability of the both scores by adding renal dysfunction [19]. A possible explanation for this finding would be the association between renal dysfunction with the stroke risk factors listed within both scores.
Sex-related predictors for TE
Women are often an under-represented cohort in AF ablation populations. Numerous studies and observational data were performed to investigate the role of female sex as a risk factor for AF and with AF-associated co-morbidities [20] as well as thromboembolic complications [6, 8] there are no data about incidence of TE in women after catheter ablation. With current study, we demonstrate that predictors for pooled TE in men and women are different. Because men constitute the large part of ablation cohorts, it was not a surprise to find an association between renal dysfunction and thromboembolic risk scores as we have already demonstrated previously [11]. Despite more renal dysfunction in females, neither impaired eGFR nor R2CHADS2 score was significant predictors for pooled TE in women.
Heart failure has been associated with higher stroke rates [8, 21]. In clinical routine, impaired left ventricular ejection fraction (LV-EF) is often used as an equivalent for the heart failure. However, recently it had been shown that AF patients with heart failure and normal (preserved) LV-EF also have greater TE risk compared with only AF patients [22]. Our finding that impaired EF is significant predictor for TE in females supports previous studies. However, the novel aspect is the importance of EF for risk assessment in women after catheter ablation.
Female sex as a risk factor for TE
Inclusion of female sex has been shown to improve the accuracy of the CHADS2 schema [23]. Later, CHA2DS2-VASc score demonstrated better predictive value compared to CHADS2 score among patients enrolled in the Euro Heart Survey for AF (c-statistic = 0.61 versus 0.56, respectively) [14]. The risk for ischemic stroke in women doubles between the ages of 55 and 65, the menopausal period during which estradiol levels decrease by about 60 % [24]. Interestingly, the effect of hormone replacement therapy (HRT) on the risk for ischemic stroke in females is not consistent. While several studies demonstrated increased TE rates in females with HRT [25], no association between HRT and stroke was found among the ATRIA study population [6]. Although HRT has some known protective effects on the cardiovascular system, it also has significant pro-thrombotic effects including an up-regulation of coagulation factors and down-regulation of anti-coagulant proteins [26]. Furthermore, during menopause occurring, decline in endogenous estrogen receptors contributes to an up-regulated production of inflammatory cytokines, with further link to the hypercoagulable state [27]. Interestingly, N-terminal pro-B-type natriuretic peptide (NT-pro-BNP) levels are higher in females compared to men across age and degree of heart failure [28]. Of note, recently NT-pro-BNP and troponin I were shown to be associated with an increased risk of stroke in AF, independent of CHA2DS2-VASc score [29].
Limitations
This study is limited by its registry design, although we had careful follow-up data in a large consecutive series. The results of this study need to be confirmed by prospective randomized trials. Taken together, although the low event rate may affect statistical power, our findings are consistent and reveal plausible associations. Finally, TE was assumed to be thromboembolic based on clinical history and brain imaging, but other aetiologies may be possible.
In conclusion, the incidence of thromboembolic events after AF catheter ablation is low in men and women. In females, low LV-EF and CHA2DS2-VASc score were independent predictors for TE.
Abbreviations
- AF:
-
Atrial fibrillation
- eGFR:
-
Estimated glomerular filtration rate
- LA:
-
Left atrial
- TE:
-
Thromboembolic events
References
Calkins H, Kuck KH, Cappato R et al (2012) HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace 14:528–606
Camm AJ, Al-Khatib SM, Calkins H et al (2012) A proposal for new clinical concepts in the management of atrial fibrillation. Am Heart J 164(292–302):e1
Hunter RJ, McCready J, Diab I et al (2010) Maintenance of sinus rhythm with an ablation strategy in patients with atrial fibrillation is associated with a lower risk of stroke and death. Heart 98:48–53
Bunch TJ, Crandall BG, Weiss JP et al (2011) Patients treated with catheter ablation for atrial fibrillation have long-term rates of death, stroke, and dementia similar to patients without atrial fibrillation. J Cardiovasc Electrophysiol 22:839–845
Oral H, Chugh A, Ozaydin M et al (2006) Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation 114:759–765
Fang MC, Singer DE, Chang Y et al (2005) Gender differences in the risk of ischemic stroke and peripheral embolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 112:1687–1691
Hart RG, Pearce LA, McBride R, Rothbart RM, Asinger RW (1999) Factors associated with ischemic stroke during aspirin therapy in atrial fibrillation: analysis of 2012 participants in the SPAF I-III clinical trials. The Stroke Prevention in Atrial Fibrillation (SPAF) Investigators. Stroke 30:1223–1229
Wang TJ, Massaro JM, Levy D et al (2003) A risk score for predicting stroke or death in individuals with new-onset atrial fibrillation in the community: the Framingham Heart Study. JAMA 290:1049–1056
Gage BF, Waterman AD, Shannon W, Boechler M, Rich MW, Radford MJ (2001) Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA 285:2864–2870
Zhang XD, Tan HW, Gu J et al (2013) Efficacy and safety of catheter ablation for long-standing persistent atrial fibrillation in women. Pacing Clin Electrophysiol 36:1236–1244
Kornej J, Hindricks G, Kosiuk J et al (2013) Renal dysfunction, stroke risk scores (CHADS2, CHA2DS2-VASc, and R2CHADS2), and the risk of thromboembolic events after catheter ablation of atrial fibrillation: the Leipzig Heart Center AF Ablation Registry. Circ Arrhythm Electrophysiol 6:868–874
Kosiuk J, Kornej J, Bollmann A et al (2014) Early cerebral thromboembolic complications following radiofrequency catheter ablation of atrial fibrillation: incidence, characteristics and risk factors. Heart Rhythm 11:1934–1940
Camm AJ, Kirchhof P, Lip GY et al (2010) Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace 12:1360–1420
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ (2010) Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest 137:263–272
Piccini JP, Stevens SR, Chang Y et al (2013) Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R2CHADS2 index in the ROCKET AF (rivaroxaban once-daily, oral, direct factor Xa inhibition compared with vitamin K antagonism for prevention of stroke and embolism trial in atrial fibrillation) and ATRIA (anticoagulation and risk factors in atrial fibrillation) study cohorts. Circulation 127:224–232
Eitel C, Hindricks G, Sommer P et al (2009) Circumferential pulmonary vein isolation and linear left atrial ablation as a single-catheter technique to achieve bidirectional conduction block: the pace-and-ablate approach. Heart Rhythm 7:157–164
Calkins H, Brugada J, Packer DL et al (2007) HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for personnel, policy, procedures and follow-up. A report of the Heart Rhythm Society (HRS) Task Force on catheter and surgical ablation of atrial fibrillation developed in partnership with the European Heart Rhythm Association (EHRA) and the European Cardiac Arrhythmia Society (ECAS); in collaboration with the American College of Cardiology (ACC), American Heart Association (AHA), and the Society of Thoracic Surgeons (STS). Endorsed and approved by the governing bodies of the American College of Cardiology, the American Heart Association, the European Cardiac Arrhythmia Society, the European Heart Rhythm Association, the Society of Thoracic Surgeons, and the Heart Rhythm Society. Europace 9:335–379
Go AS, Fang MC, Udaltsova N et al (2009) Impact of proteinuria and glomerular filtration rate on risk of thromboembolism in atrial fibrillation: the anticoagulation and risk factors in atrial fibrillation (ATRIA) study. Circulation 119:1363–1369
Roldan V, Marin F, Manzano-Fernandez S et al (2013) Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb Haemost 109:956–960
Meyer S, Brouwers F, Voors A, et al (2014) Sex differences in new-onset heart failure. Clin Res Cardiol (epub ahead of print)
Witt BJ, Gami AS, Ballman KV et al (2007) The incidence of ischemic stroke in chronic heart failure: a meta-analysis. J Card Fail 13:489–496
Jang SJ, Kim MS, Park HJ et al (2013) Impact of heart failure with normal ejection fraction on the occurrence of ischaemic stroke in patients with atrial fibrillation. Heart 99:17–21
Rietbrock S, Heeley E, Plumb JM, Van Staa TP (2008) Chronic atrial fibrillation: Incidence, prevalence, and prediction of stroke using the congestive heart failure, hypertension, age >75, diabetes mellitus, and prior stroke or transient ischemic attack (CHADS2) risk stratification scheme. Am Heart J 156:57–64
Vaccarino V, Badimon L, Corti R et al (2011) Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc Res 90:9–17
Viscoli CM, Brass LM, Kernan WN, Sarrel PM, Suissa S, Horwitz RI (2001) A clinical trial of estrogen-replacement therapy after ischemic stroke. N Engl J Med 345:1243–1249
Lowe GD (2002) Hormone replacement therapy: prothrombotic vs. protective effects. Pathophysiol Haemost Thromb 32:329–332
Novella S, Heras M, Hermenegildo C, Dantas AP (2012) Effects of estrogen on vascular inflammation: a matter of timing. Arterioscler Thromb Vasc Biol 32:2035–2042
Fradley MG, Larson MG, Cheng S et al (2011) Reference limits for N-terminal-pro-B-type natriuretic peptide in healthy individuals (from the Framingham Heart Study). Am J Cardiol 108:1341–1345
Hijazi Z, Oldgren J, Andersson U et al (2012) Cardiac biomarkers are associated with an increased risk of stroke and death in patients with atrial fibrillation: a randomized evaluation of long-term anticoagulation therapy (RE-LY) substudy. Circulation 125:1605–1616
Acknowledgments
Dr. Jelena Kornej was supported by the German Cardiac Society St. Jude Medical Stipend.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
G. Y. H. Lip and A. Bollmann are joint senior authors.
Rights and permissions
About this article
Cite this article
Kornej, J., Kosiuk, J., Hindricks, G. et al. Sex-related predictors for thromboembolic events after catheter ablation of atrial fibrillation: The Leipzig Heart Center AF Ablation Registry. Clin Res Cardiol 104, 603–610 (2015). https://doi.org/10.1007/s00392-015-0823-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-015-0823-6