Abstract
Background
Novel cardioprotective strategies are required to improve clinical outcomes in high risk patients undergoing coronary artery bypass graft (CABG) ± valve surgery. Remote ischemic preconditioning (RIC), in which brief episodes of non-lethal ischemia and reperfusion are applied to the arm or leg, has been demonstrated to reduce perioperative myocardial injury following CABG ± valve surgery. Whether RIC can improve clinical outcomes in this setting is unknown and is investigated in the effect of remote ischemic preconditioning on clinical outcomes (ERICCA) trial in patients undergoing CABG surgery. (ClinicalTrials.gov Identifier: NCT01247545).
Methods
The ERICCA trial is a multicentre randomized double-blinded controlled clinical trial which will recruit 1,610 high-risk patients (Additive Euroscore ≥ 5) undergoing CABG ± valve surgery using blood cardioplegia via 27 tertiary centres over 2 years. The primary combined endpoint will be cardiovascular death, non-fatal myocardial infarction, coronary revascularization and stroke at 1 year. Secondary endpoints will include peri-operative myocardial and acute kidney injury, intensive care unit and hospital stay, inotrope score, left ventricular ejection fraction, changes of quality of life and exercise tolerance. Patients will be randomized to receive after induction of anesthesia either RIC (4 cycles of 5 min inflation to 200 mmHg and 5 min deflation of a blood pressure cuff placed on the upper arm) or sham RIC (4 cycles of simulated inflations and deflations of the blood pressure cuff).
Implications
The findings from the ERICCA trial have the potential to demonstrate that RIC, a simple, non-invasive and virtually cost-free intervention, can improve clinical outcomes in higher-risk patients undergoing CABG ± valve surgery.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Coronary heart disease (CHD) is the leading cause of death and disability worldwide and accounts for 3.8 million of men and 3.4 million of women deaths every year. CABG surgery remains the procedure of choice for coronary artery revascularization in patients with multi-vessel coronary artery disease. Currently, high-risk CHD patients are being operated on due to the aging population, the increasing prevalence of co-morbidities such as diabetes, hypertension, valve disease and the presence of more complex disease [1]. Surgery in these patients is associated with an elevated overall operative risk of 5–6% compared to 1% in lower-risk patients [2], as well as an increased risk of peri-procedural myocardial injury (as measured by serum CK-MB, Troponin T or I) [3], acute kidney injury (AKI) [4] and stroke [5], the presence of which are associated with worse clinical outcomes [6–16]. Myocardial injury during CABG surgery may be attributed to different mechanisms, including most importantly acute myocardial ischemia–reperfusion injury (IRI), but also inflammatory response to the extraneous substances in the cardiopulmonary bypass circuit, left ventricular over-distension, coronary atheroembolism [17], increased cardiac workload during the intraoperative period, and direct myocardial injury due to retraction and handling of the heart [18].
According to the technique used, myocardial IRI can be the result of intermittent cross-clamping, intermittent or continuous administration of cardioplegic solution, cross-clamp fibrillation or a combination of these methods and may manifest as myocardial stunning [19], the so called no-reflow phenomenon [20], reperfusion arrhythmias [21] and lethal reperfusion injury [22], the latter of which would be of most concern. A variety of factors are believed to contribute to lethal myocardial IRI and include oxidative stress [23], pH changes [24] calcium overload [22], the acute inflammatory response [25] and important metabolic changes [26], many of which impact on the opening of the mitochondrial permeability transition pore (mPTP), a critical determinant of cell death in the setting of acute IRI [27].
Whilst cardioprotective strategies have been overall extremely encouraging in experimental studies, the translation from bench to bedside has not always resulted in positive outcomes and this could be due to the obvious differences between the species involved, the size and age of animals used and the absence of co-morbidities and concomitant treatments in the experimental models [28, 29].
Both pharmacological and non-pharmacological interventions have been investigated to enhance the innate process of cardioprotection and therefore to limit or prevent acute myocardial IRI. Amongst the non-pharmacological strategies, ischemic preconditioning (IPC), perconditioning (IPerC) and postconditioning (IPost) have been extensively investigated in both the laboratory and clinical settings. In IPC, the heart is subject to brief episodes of non-lethal ischemia and reperfusion prior to the sustained episode of lethal ischemia and reperfusion; in IPerC and IPost the protective stimulus is applied after the onset of myocardial ischemia or at the time of myocardial reperfusion, respectively [30]. However, both IPerC and IPost require an invasive intervention applied directly to the myocardium in order to achieve cardioprotection and may therefore be impractical or even harmful, particularly in the setting of an acute myocardial infarction (AMI). In this perspective, the phenomenon of remote ischemic conditioning (RIC) appears extremely encouraging: it describes the phenomenon in which brief episodes of non-lethal ischemia and reperfusion to an organ (i.e. kidney, liver or small intestine) or tissue (i.e. skeletal muscle), protect the heart against a sustained episode of lethal IRI [31, 32]. Therefore, RIC does not imply a direct intervention on the heart to achieve cardioprotection.
The concept of RIC was first introduced in 1993 by Przyklenk et al. [31], who demonstrated that IPC protects canine myocardium both in the territory exposed to brief coronary occlusion and in a vascular bed distant or remote or “virgin”. Following this, pioneering studies by MacAllister et al. [33] demonstrated that RIC could be reproduced by non-invasively applying brief episodes of ischemia and reperfusion to the forearm using a standard blood pressure cuff. Since then, a number of proof-of-concept clinical studies have demonstrated that RIC comprising brief episodes of non-lethal ischemia and reperfusion to the arm or leg, non-invasively applied by inflating a blood pressure to supra-systolic pressures placed on the arm or leg, can protect the heart during CABG surgery from peri-operative myocardial injury as evidenced by reduced serum troponin T [34–37]. A similar RIC stimulus has been reported to be protective in a number of different clinical settings including elective surgical repair of abdominal aortic aneurysm (AAA) [38, 39], elective cervical decompression surgery [40]; elective PCI [41] and in ST-segment elevation myocardial infarction patients undergoing primary percutaneous intervention (PCI) [42].
Currently, whether this non-invasive virtually cost-free intervention can improve clinical outcomes in higher-risk patients undergoing CABG ± valve surgery is unknown and is the objective of the proposed effect of remote ischemic preconditioning on clinical outcomes (ERICCA) trial in patients undergoing coronary artery bypass graft (CABG) surgery. (ClinicalTrials.gov Identifier: NCT01247545).
Methods
Study objectives
The primary objective of this study is to determine whether RIC improves 1 year clinical outcomes (cardiovascular death, non-fatal myocardial infarction, revascularization and stroke) in higher-risk adult patients undergoing CABG ± valve surgery. The secondary research objectives are to determine whether RIC improves the above clinical outcomes at 30 days post-surgery; has an effect on all-cause death; reduces peri-operative myocardial injury and preserves LV systolic function; reduces AKI; improves patient morbidity [intensive care unit (ICU) stay and hospital stay], lessens inotrope requirements and improves exercise tolerance and quality of life.
Study design
The study has received Ethical Committee approval. The ERICCA trial is a controlled randomized multi-centre double blind trial. It investigates the effect of RIC on 1 year clinical outcomes in 1,610 high-risk (Euroscore ≥ 5) patients undergoing CABG ± valve surgery recruited via 27 tertiary cardiac centres in the UK.
Study population
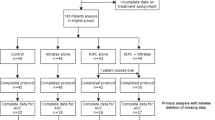
Patient inclusion criteria are as follows: the patient is ≥18 years old, scheduled for CABG ± valve surgery with blood cardioplegia, and has an additive Euroscore equal or above 5 (Fig. 1). Patient exclusion criteria include the following: history of cardiogenic shock or cardiac arrest during the current admission; pregnancy; significant peripheral arterial disease affecting the upper limbs; significant hepatic impairment (Bilirubin > 20 mmol/L, INR > 2.0); significant pulmonary disease (FEV1 < 40% predicted); renal failure with a GFR <30 mL/min/1.73 m2; concomitant treatment with glibenclamide or nicorandil (as these medications may interfere with the cardioprotection elicited by RIC). All patients will freely give their informed consent to participate in the study and may decide to withdraw from the study at any time. The study will conform to the spirit and the letter of the declaration of Helsinki, and in accordance with the UCL Good Clinical Practice Guidelines.
Intervention
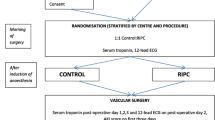
The intervention being assessed is RIC, which will be performed after the induction of anesthesia but prior to surgery and will occur within 1 hour of the institution of cardiac bypass. Those patients randomized to receive RIC will have a standard blood pressure cuff placed on the upper arm, inflated to 200 mmHg for 5 min and then deflated for 5 min, a cycle which will be performed four times in total. For patients with systolic blood pressures >185 mmHg, the cuff will be inflated to at least 15 mmHg above the patient’s systolic blood pressure. The sham RIC protocol is described as follows: the air valve on the blood pressure cuff is first opened such that the cuff is not inflated on squeezing the attached bulb. The bulb will then be squeezed for a duration of 15 s to give the impression that the cuff is being inflated. After 5 min, the air valve will be closed to give the impression that the cuff is being deflated. After 5 min, the air valve will be opened again and the bulb squeezed as before: the above cycle will be repeated four times in total. This is to ensure the rigorous blindness of the anesthetic and surgical teams as well as non-medical theatre staff. These interventions will be undertaken after the induction of anesthesia and will not prolong the anesthetic time or delay the onset of surgery.
We have decided to use four cycles of 5 min of cuff inflation and deflation as we wish to maximize the RIC stimulus to overcome potential resistance of diabetic heart and the presence of other factors interfering with cardioprotection such as volatile anesthetics including isoflurane. Moreover, the application of four cycles of 5 min inflation/deflation was reported to be beneficial in patients with STEMI undergoing primary PCI [42].
Randomization and allocation
On the morning of surgery, patients will be randomized to one of two groups, either RIC or control. Randomization will be coordinated centrally by the Clinical Trials Unit based at the London School of Hygiene and Tropical Medicine via a secure web-site and will be stratified by center using random permuted blocks. This will only be accessed by the research nurse responsible for performing either the RIC or sham RIC protocol. The same research nurse will be the only person in each centre aware of the treatment allocation for the patient and he/she will not be involved with the data collection other than those relating to the actual randomization procedure. Treatment allocations will only be known by one research nurse at each centre. The patient, cardiac surgeons and anesthetists, the research nurses collecting the data, and the assessor of clinical outcomes will all be blinded to the treatment allocation.
Study endpoints
Primary clinical endpoint
Major adverse cardiac events (cardiovascular death, non-fatal myocardial infarction, repeat revascularization) and cerebrovascular events (stroke) calculated at 12 months post-surgery.
Cardiovascular death will be defined as death due to a known cardiovascular cause or where the cause of death is unknown, i.e. where no other cause of death has been identified from the medical history or an autopsy.
Repeat revascularization will be defined as any PCI or repeat-CABG ± valve surgery within the first year post-surgery.
Myocardial infarction will include both peri-operative myocardial infarction and myocardial infarction following cardiac surgery. Peri-operative myocardial infarction (type 5 myocardial infarction) [43] will be indicated by biomarker (high-sensitive Troponin T) values more than five times the 99th percentile of the normal reference range during the first 72 h following CABG ± valve surgery, when associated with the appearance of new pathological Q-waves or new left bundle branch block (LBBB), or angiographically documented new graft or native coronary artery occlusion, or imaging evidence of new loss of viable myocardium. Post-surgical myocardial infarction will be defined by: (1) a rise and/or fall of Troponin T compared to baseline with at least one value above the 99th percentile of the upper reference limit together with evidence of myocardial ischemia with at least one of the following: symptoms of ischemia, ECG changes indicative of new ischemia (new ST-T changes of new LBBB), development of Q waves in the ECG, imaging evidence of new loss of viable myocardium or new regional wall motion abnormality; (2) sudden unexpected cardiac death involving cardiac arrest often with symptoms suggestive of myocardial ischemia and accompanied by presumably new ST-elevation or new LBBB and/or fresh thrombus on coronary angiography and/or at autopsy, but death occurring before blood samples could be obtained or at time before the appearance of cardiac troponin T in the blood.
Stroke will be defined as a focal, central neurological deficit lasting >72 h which results in irreversible brain damage or body impairment.
Secondary clinical end-points
Clinical outcome at 30 days, including CV death, non-fatal myocardial infarction, revascularization and stroke.
All-cause death
Peri-operative high-sensitive Troponin-T
This will be assessed by measuring serum high-sensitive Troponin-T pre-operatively and at 6, 12, 24, 48, 72 h post coming off cardiac bypass. Following elective CABG ± valve surgery, several studies have demonstrated that peri-operative myocardial injury, indicated by the release of the cardiac enzymes CK-MB, Troponin-T and Troponin-I is associated with worse clinical outcomes following surgery [6–16].
Length of ICU/hospital stay and inotrope score
The length of ICU and hospital stay and the inotrope score are factors which can be influenced by the outcome of surgery and which have an important impact on health-care resources. The inotrope score provides an objective measurement of the requirement of inotropes in the immediate post-operative period. Data on inotrope requirement will be collected daily from the medical drug chart on the ICU. This will be adapted from a study by Ko et al. [44] and will be calculated at 0 (time when coming off bypass), 24, 48 and 72 h after the surgery using the formula below. The inotrope score for the particular time point is calculated as follows: at time 0, the inotrope score will be calculated from the dose of the individual inotropes used at the time of coming off bypass. For 24-, 48- and 72-h time-points, the inotrope score will be calculated from the maximum dose of the individual inotropes used in the previous 24-h period.
Inotrope score =
-
1.
Dopamine + Dobutamine +
-
2.
+ [(Adrenaline + Noradrenaline + Isoproterenol + Isoproterenol) × 100]
-
3.
+ [Enoximone (or Milrinone) × 15]
All dosages will be in μg/kg/min.
The dosage of Levosimendan will be documented when given.
Remote ischemic preconditioning may impact on these outcome measures by reducing myocardial ischemic injury and preserving left ventricular (LV) systolic function.
Peri-operative AKI
This will be measured by (1) the AKI Score, calculated over the 3-day peri-operative period (Table 1) with serum creatinine measured daily for 3 days (and at 6 weeks and 1-year post-surgery) and urine volumes monitored daily; (2) Neutrophil gelatinase-associated lipocalin (NGAL), a new early marker for AKI, with levels rising rapidly after renal injury [45, 46], measured pre-operatively, 6, 12 and 24 h (post-coming off cardiac bypass).
The six minute walk test (6MWT)
This will be performed at baseline, 6 weeks (in the outpatient clinic follow-up appointment), and at 1-year (in the research outpatient clinic follow-up appointment) post-CABG ± valve surgery. Patients will be instructed to walk as far as possible along a straight, flat hospital corridor in 6 min. The 6MWT will be used to evaluate the effect of RIC on the functional status of patients undergoing CABG ± valve surgery [47]. Shortly after CABG ± valve surgery, functional capacity is significantly reduced, but it rapidly improves after cardiac rehabilitation. This improvement has been found to be independent of age, sex, co-morbidities and baseline functional capacity [47].
Quality of life
The EuroQol EQ-5D Health-Related Quality of Life (HRQOL) questionnaire (http://www.euroqol.org) will be used to assess patients’ quality of life post-CABG ± valve surgery [48], at baseline, at 6 weeks (in the surgical outpatient clinic follow-up appointment), at 3 months (by post/e-mail), at 6 months (by post/e-mail), at 9 months (by post/e-mail) and at 1-year post surgery (in the research outpatient clinic follow-up appointment). Non-responders will be telephoned.
Left ventricular ejection fraction
A subgroup of 140 patients at two recruitment centres will have a transthoracic echocardiogram performed by bi-planar Simpson’s technique and 3D echo techniques, in order to assess left ventricular ejection fraction (LVEF) at baseline and at 1-year post surgery (in the research outpatient clinic follow-up appointment).
Statistical considerations
A detailed statistical analysis plan will be produced prior to unblinding of any data.
Sample size calculation
There will be two arms to the trial: RIC and control. We plan to recruit 1,610 patients through 27 tertiary centres. In the SYNTAX study the MACCE (death, myocardial infarction, revascularization and stroke) rate was 12.4% of patients at 12 months following CABG surgery [49]. However, the patients recruited into the SYNTAX study were generally lower-risk than those to be recruited in ERICCA with a mean EuroSCORE of 3.8, whereas the patients we intend to recruit in our study are higher-risk with EuroSCORE ≥ 5. In another study comprising higher-risk patient defined by them all having left main stem coronary lesions, the MACCE rate (which included some additional neurological criteria) at 1 year was estimated to be 25% [50]. Therefore, for our higher-risk CABG ± valve surgery patients we have estimated an MACCE rate of 20% at 1 year, which means that to detect a 27% relative reduction in this primary endpoint in the RIC-treated group (from 20.0 to 14.6%), with a power of 80% and a significance level of 5%, a sample size of 770 patients will be required for each trial arm (1,540 in total). This was chosen to represent a clinically significant effect, which is less than the reductions in myocardial injury observed in the previously mentioned proof-of-concept clinical studies (i.e. 40–50% reductions in serum cardiac enzymes). To allow for up to 5% dropouts, we plan to recruit 1,610 patients in total (805 patients each arm).
With regards to the ECHO subgroup analysis involving 140 patients in two of the centres, in a previous study [51] IPost was reported to improve LVEF by 7% (absolute increase) from 49 to 56% at 1 year in ST-elevation myocardial infarction patients. In order to detect a smaller mean difference of 5% with a common SD of 10.5%, the substudy requires 70 patients in each group (140 in total) using 80% power and a 5% significance level.
Statistical analysis
The primary analysis will be a comparison of the 1-year MACCE rate between the RIC and control arms of the trial. Survival analyses techniques will be used for MACCE and other clinical endpoints. Hazard ratios and confidence intervals will be calculated using Cox proportional hazards modeling and Kaplan–Meier curves produced. The assumptions underlying the Cox model will be assessed. In addition, risk differences at 1 year together with 95% confidence intervals will be calculated. Differences in means (continuous variables) together with 95% confidence intervals will be calculated using linear regression models and analysis of covariance techniques where appropriate. Analysis will be by intention to treat on using all available data. We plan to undertake a limited number of subgroup analyses: these will include age, baseline EuroSCORE, LVEF, diabetic status, aortic cross-clamp time, cardiac bypass time and method of cardioplegia (anterograde vs. retrograde blood cardioplegia). All subjects randomized to the study will be analyzed on an intention to treat basis. Data will be validated and the data analysis will take appropriate account of missing values.
Study monitoring
A Trial Steering Committee (TSC) will be responsible for drafting the final report and submission for publication and will meet every 6 months. A Trial Management Group (TMG) will meet weekly during the planning stages of the study and less frequently when the study is actually recruiting. An independent clinical events committee will be convened comprising an independent cardiologist, cardiac surgeon and neurologist. A Data Monitoring and Ethics Committee (DMEC) will meet at the start of the trial to establish a DMEC charter, soon after recruitment has started and then at least annually to determine if there are any unforeseen effects of RIC. This will be the only group, along with the statistician producing the reports for the DMEC, who will see interim analyses by treatment.
Discussion
In the ERICCA trial, we will investigate whether RIC, a non-invasive virtually cost free cardioprotective strategy can improve clinical outcomes at 1 year in higher-risk patients undergoing CABG ± valve surgery. The risk profile of patients undergoing CABG surgery continues to change with factors such as (a) the aging population (the proportion of patients over 75 years old has increased by more than 4.5-fold over the last decade with the 5-year mortality in this age group being 35%); (b) the increasing prevalence of diabetes (the proportion of diabetic patients has risen from 15 to 22%, with the operative mortality in this patient group being 2.6%) resulting in an increase in the number of higher-risk patients (defined as an additive EuroSCORE ≥ 5) being operated upon and a corresponding increase in overall operative risk to 5–6% [1, 2]. These higher-risk patients are at a greater risk of peri-operative complications, which are associated with a worse clinical outcome, such as peri-procedural myocardial injury [3], inotropic support post-surgery, significant AKI (up to 34% of patients) [4] and stroke (1–3%) [5]. Peri-operative myocardial injury, as measured by serum CK-MB, Troponin-T or Troponin-I during surgery has been associated with worse clinical outcomes post-surgery [6–16].
The discovery that the RIC stimulus could be reproduced by applying brief episodes of ischemia and reperfusion to the upper or lower limb [52, 53] has facilitated its recent translation from animal studies into the clinical arena. MacAllister et al. [30, 54] were the first to demonstrate the concept of RIC in human volunteers by inflating a blood pressure cuff around the upper arm to 200 mmHg for 5 min and deflating the cuff for 5 min (to induce ischemia and reperfusion, respectively), a cycle which was repeated two more times, with subsequent attenuation of ischemia-induced endothelial dysfunction in the contralateral arm. Cheung et al. [36] applied RIC in children undergoing cardiac surgery using four-5 min cycles of lower limb ischemia and reperfusion, with reduction of myocardial injury, airway resistance and inotrope score. We then demonstrated [34, 35] that three-5 min cycles of upper limb ischemia and reperfusion reduced myocardial injury in adult patients undergoing elective CABG ± valve surgery.
More recently, RIC using lower limb ischemia/reperfusion has also been reported to induce cardiac, renal and neurological protection in elective surgery for AAA [38, 39] and cervical decompression [40]. Hoole et al. [41] have reported that RIC using brief ischemia and reperfusion of the arm reduced the peri-procedural myocardial injury associated with elective PCI for stable CHD. Botker et al. [42] have recently demonstrated that RIC using four-5 minute cuff inflations/deflations administered in ambulance reduced myocardial infarct size in ST-elevation myocardial infarction patients undergoing primary PCI. In patients undergoing valve surgery alone, RIC comprising three 4-min cycles of cuff inflation and deflation on the lower limb applied at the time of aortic bypass was found to reduce peri-operative myocardial injury although RIC with the same stimulus applied prior to surgery was reported to be ineffective [55]. In patients undergoing off-pump CABG surgery, RIC only resulted in a non-significant 26% reduction in peri-operative myocardial injury as measured by cTnI [56].
Interestingly, not all the studies investigating the effects of RIC in CHD have been positive: Iliodromitis et al. [57] showed more myocardial injury in low-risk patients undergoing elective PCI receiving RIC, although a non-standard RIC protocol was applied, comprising bilateral arm cuff inflation and deflation, and the study may have been underpowered. Moreover, the largest clinical study of RIC in CABG surgery to be published (162 patients randomized to RIC or control) [58] failed to demonstrate any benefits with RIC (three-5-min cycles of inflation and deflation of a blood pressure cuff placed on the upper arm) in terms of peri-operative myocardial injury (cTnT release), ECG changes, cardiac index, inotrope requirements, renal impairment and lung injury. The reason for this negative study is not clear but may be attributable to patient selection (patients with unstable angina were included- these patients may have been inadvertently preconditioned), the RIC stimulus (which was applied in a ‘blinded’ fashion with the position of the cuff on the arm hidden from view) or concomitant medication (the presence of inhalational anesthetics and intravenous glycerine trinitrate may have interfered with the cardioprotective effect of RIC). In a recently published small study comprising 54 patients undergoing elective CABG under a strict anesthetic regime (the volatile anesthetic isoflurane was given for maintenance of anesthesia until institution of cardio-pulmonary bypass and the intravenous anesthetic propofol was used for induction and following cardio-pulmonary bypass initiation until the end of surgery), Karuppasamy et al. [59] again demonstrated no significant difference in myocardial injury and inflammatory response between RIC and placebo subjects59, although it is possible that the same anesthetics choice and/or their timing might have interfered with RIC [60].
Importantly, in our proposed ERICCA trial, we also intend to include patients undergoing valve surgery in addition to CABG surgery in order to determine whether this higher-risk surgical group may benefit in terms of improved clinical outcomes with RIC. Furthermore, in the previously cited proof-of-concept clinical trials [34, 35] valve surgery patients were included and were demonstrated to benefit from RIC and recent studies specifically investigating ischemic postconditioning have reported benefit in patients undergoing aortic valve surgery [55].
In summary, the ERICCA trial is a large multicentre randomized double blinded clinical trial, which will investigate whether RIC can improve clinical outcomes at 1 year in higher-risk patients undergoing CABG ± valve surgery. The findings from this trial have the potential to change clinic practice with the introduction of a non-invasive virtually cost-free cardioprotective strategy for improving clinical outcomes in patients with IHD.
References
Biancari F, Kangasniemi OP, Mahar MA, Rasinaho E, Satomaa A, Tiozzo V, Niemela M, Lepojarvi M (2009) Changing risk of patients undergoing coronary artery bypass surgery. Interact Cardiovasc Thorac Surg 8:40–44
Kathiresan S, Servoss SJ, Newell JB, Trani D, MacGillivray TE, Lewandrowski K, Lee-Lewandrowski E, Januzzi JL Jr (2004) Cardiac troponin T elevation after coronary artery bypass grafting is associated with increased one-year mortality. Am J Cardiol 94:879–881
Brener SJ, Lytle BW, Schneider JP, Ellis SG, Topol EJ (2002) Association between CK-MB elevation after percutaneous or surgical revascularization and three-year mortality. J Am Coll Cardiol 40:1961–1967
Karkouti K, Wijeysundera DN, Yau TM, Callum JL, Cheng DC, Crowther M et al (2009) Acute kidney injury after cardiac surgery: focus on modifiable risk factors. Circulation 119:495–502
Whitaker DC, Stygall J, Newman SP (2002) Neuroprotection during cardiac surgery: strategies to reduce cognitive decline. Perfusion 17(Suppl):69–75
Kathiresan S, Servoss SJ, Newell JB, Trani D, MacGillivray TE, Lewandrowski K et al (2004) Cardiac troponin T elevation after coronary artery bypass grafting is associated with increased one-year mortality. Am J Cardiol 94:879–881
Lehrke S, Steen H, Sievers HH, Peters H, Opitz A, Muller-Bardorff M et al (2004) Cardiac troponin T for prediction of short- and long-term morbidity and mortality after elective open heart surgery. Clin Chem 50:1560–1567
Mohammed AA, Agnihotri AK, van Kimmenade RR, Martinez-Rumayor A, Green SM, Quiroz R et al (2009) Prospective, comprehensive assessment of cardiac troponin T testing after coronary artery bypass graft surgery. Circulation 120:843–850
Fellahi JL, Gue X, Richomme X, Monier E, Guillou L, Riou B (2003) Short- and long-term prognostic value of postoperative cardiac troponin I concentration in patients undergoing coronary artery bypass grafting. Anesthesiology 99:270–274
Croal BL, Hillis GS, Gibson PH, Fazal MT, El Shafei H, Gibson G et al (2006) Relationship between postoperative cardiac troponin I levels and outcome of cardiac surgery. Circulation 114:1468–1475
Muehlschlegel JD, Perry TE, Liu KY, Nascimben L, Fox AA, Collard CD et al (2009) Troponin is superior to electrocardiogram and creatinine kinase MB for predicting clinically significant myocardial injury after coronary artery bypass grafting. Eur Heart J 30:1574–1583
Mangano CM, Diamondstone LS, Ramsay JG, Aggarwal A, Herskowitz A, Mangano DT (1998) Renal dysfunction after myocardial revascularization: risk factors, adverse outcomes, and hospital resource utilization. The Multicenter Study of Perioperative Ischemia Research Group. Ann Intern Med 128:194–203
Suen WS, Mok CK, Chiu SW, Cheung KL, Lee WT, Cheung D et al (1998) Risk factors for development of acute renal failure (ARF) requiring dialysis in patients undergoing cardiac surgery. Angiology 49:789–800
Rosner MH, Okusa MD (2006) Acute kidney injury associated with cardiac surgery. Clin J Am Soc Nephrol 1:19–32
Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J (1998) Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 104:343–348
Lassnigg A, Schmidlin D, Mouhieddine M, Bachmann LM, Druml W, Bauer P et al (2004) Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 15:1597–1605
Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, Erbel R (2009) Coronary microembolization: from bedside to bench and back to bedside. Circulation 120(18):1822–1836
Wheatley DJ (2003) Protecting the damaged heart during coronary surgery. Heart 89:367–368
Braunwald E, Kloner RA (1982) The stunned myocardium: prolonged, postischaemic ventricular dysfunction. Circulation 66(6):1146–1149
Ito H (2006) No-reflow phenomenon and prognosis in patients with acute myocardial infarction. Nat Clin Pract Cardiovasc Med 3(9):499–506
Manning AS, Hearse DJ (1984) Reperfusion induced arrhythmias: mechanisms and prevention. J Mol Cell Cardiol 16(6):497–518
Piper HM, Garcia-Dorado D, Ovize M (1998) A fresh look at reperfusion injury. Cardiovasc Res 38:291–300
Hearse DJ, Humphrey SM, Chain EB (1973) Abrupt reoxygenation of the anoxic potassium arrested perfused rat heart: a study of myocardial enzyme release. J Mol Cell Cardiol 5(4):395–407
Lemasters JJ, Bond JM, Chacon E et al (1996) The pH paradox in ischaemia-reperfusion injury to cardiac myocytes. EXS 76:99–114
Vinten-Johansen J (2004) Involvement of neutrophils in the pathogenesis of lethal myocardial reperfusion injury. Cardiovasc Res 61(3):481–497
Jonassen AK, Sack MN, Mjos OD, Yellon DM (2001) Myocardial protection by insulin at reperfusion requires early administration and is mediated via Akt and p70s6 kinase cell-survival signaling. Circ Res 89(12):1191–1198
Heusch G, Boengler K, Schulz R (2010) Inhibition of mitochondrial permeability transition pore opening: the Holy Grail of cardioprotection. Basic Res Cardiol 105(2):151–154
Hausenloy DJ, Baxter G, Bell R, Bøtker HE, Davidson SM, Downey J, Heusch G, Kitakaze M, Lecour S, Mentzer R, Mocanu MM, Ovize M, Schulz R, Shannon R, Walker M, Walkinshaw G, Yellon DM (2010) Translating novel strategies for cardioprotection: the Hatter Workshop Recommendations. Basic Res Cardiol 105(6):677–686
Schwartz Longacre L, Kloner RA, Arai AE, Baines CP, Bolli R, Braunwald E, Downey J, Gibbons RJ, Gottlieb RA, Heusch G, Jennings RB, Lefer DJ, Mentzer RM, Murphy E, Ovize M, Ping P, Przyklenk K, Sack MN, Vander Heide RS, Vinten-Johansen J, Yellon DM, National Heart, Lung, and Blood Institute, National Institutes of Health (2011) New horizons in cardioprotection: recommendations from the 2010 National Heart, Lung, and Blood Institute Workshop. Circulation 124(10):1172–1179
Zhao ZQ, Corvera JS, Halkos ME et al (2003) Inhibition of myocardial injury by ischaemic postconditioning during reperfusion: comparison with ischaemic preconditioning. Am J Physiol Heart Circ Physiol 285(2):H579–H588
Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P (1993) Regional ischemic ‘preconditioning’ protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 87:893–899
Hausenloy DJ, Yellon DM (2008) Remote ischaemic preconditioning: underlying mechanisms and clinical application. Cardiovasc Res 79:377–386
Kharbanda RK, Mortensen UM, White PA, Kristiansen SB, Schmidt MR, Hoschtitzky JA et al (2002) Transient limb ischemia induces remote ischemic preconditioning in vivo. Circulation 106:2881–2883
Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E et al (2007) Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 370:575–579
Venugopal V, Hausenloy DJ, Ludman A, Di Salvo CM, Kolvekar S, Yap J et al (2009) Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold blood cardioplegia: a randomised controlled trial. Heart 95:1567–1571
Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J et al (2006) Randomized controlled trial of the effects of remote ischemic preconditioning on children undergoing cardiac surgery: first clinical application in humans. J Am Coll Cardiol 47:2277–2282
Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, Jakob H, Heusch G (2010) Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Res Cardiol 105(5):657–664
Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM et al (2007) Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 116:I98–I105
Walsh SR, Boyle JR, Tang TY, Sadat U, Cooper DG, Lapsley M et al (2009) Remote ischemic preconditioning for renal and cardiac protection during endovascular aneurysm repair: a randomized controlled trial. J Endovasc Ther 16:680–689
Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ et al (2010) Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol 22(2):157
Hoole SP, Heck PM, Sharples L, Khan SN, Duehmke R, Densem CG et al (2009) Cardiac remote ischemic preconditioning in coronary stenting (CRISP Stent) study: a prospective, randomized control trial. Circulation 119:820–827
Botker HE, Kharbanda RK, Schmidt MR, Bottcher M, Kaltoft AK, Terkelsen CJ, Munk K, Anderson NH, Hansen TM, Trautner S, Lassen JF, Christiansen EH, Krusell LR, Kristensen SD, Thuesen L, Nielsen SS, Rehling M, Sorensen HF, Redington AN, Nielsen TT (2010) Prehospital remote ischaemic conditioning increases myocardial salvage in acute myocardial infarction. Lancet 375(9716):727–734
Thygesen K, Alpert JS, White HD, Jaffe AS, Apple FS, Galvani M et al (2007) Universal definition of myocardial infarction. Circulation 116:2634–2653
Ko WJ, Lin CY, Chen RJ, Wang SS, Lin FY, Chen YS (2002) Extracorporeal membrane oxygenation support for adult postcardiotomy cardiogenic shock. Ann Thorac Surg 73:538–545
Haase M, Bellomo R, Devarajan P, Ma Q, Bennett MR, Mockel M et al (2009) Novel biomarkers early predict the severity of acute kidney injury after cardiac surgery in adults. Ann Thorac Surg 88:124–130
Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R et al (2008) Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol 3:665–673
Fiorina C, Vizzardi E, Lorusso R, Maggio M, De Cicco G, Nodari S et al (2007) The 6-min walking test early after cardiac surgery. Reference values and the effects of rehabilitation programme. Eur J Cardiothorac Surg 32:724–729
Dunning J, Waller JR, Smith B, Pitts S, Kendall SW, Khan K (2008) Coronary artery bypass grafting is associated with excellent long-term survival and quality of life: a prospective cohort study. Ann Thorac Surg 85:1988–1993
Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ et al (2009) Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease. N Engl J Med 360:961–972
Lee MS, Kapoor N, Jamal F, Czer L, Aragon J, Forrester J et al (2006) Comparison of coronary artery bypass surgery with percutaneous coronary intervention with drug-eluting stents for unprotected left main coronary artery disease. J Am Coll Cardiol 47:864–870
Thibault H, Piot C, Staat P, Bontemps L, Sportouch C, Rioufol G et al (2008) Long-term benefit of postconditioning. Circulation 117:1037–1044
Birnbaum Y, Hale SL, Kloner RA (1997) Ischemic preconditioning at a distance: reduction of myocardial infarct size by partial reduction of blood supply combined with rapid stimulation of the gastrocnemius muscle in the rabbit. Circulation 96:1641–1646
Ren C, Gao X, Steinberg GK, Zhao H (2008) Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience 151:1099–1103
Loukogeorgakis SP, Panagiotidou AT, Broadhead MW, Donald A, Deanfield JE, MacAllister RJ (2005) Remote ischemic preconditioning provides early and late protection against endothelial ischemia-reperfusion injury in humans: role of the autonomic nervous system. J Am Coll Cardiol 46:450–456
Li L, Luo W, Huang L, Zhang W et al (2010) Remote perconditioning reduces myocardial injury in adult valve replacement: a randomized controlled trial. J Surg Res 164(1):e21–e26
Hong DM, Mint JJ, Kim JH et al (2010) The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off-pump coronary artery bypass graft surgery. Anaesth Intensive Care 38(5):924–929
Iliodromitis EK, Kyrzopoulos S, Paraskevaidis IA et al (2006) Increased C reactive protein and cardiac enzyme levels after coronary stent implantation. Is there protection by remote ischaemic preconditioning? Heart 92:1821–1826
Rahman IA, Mascaro JG, Steeds et al (2010) Remote ischaemic preconditioning in human coronary artery bypass surgery: from promise to disappointment? Circulation 122(11 Suppl):S53–S59
Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, John L, Shah AM, Marber MS, Kunst G (2011) Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury, inflammation? Basic Res Cardiol 106(4):511–519
Peters J (2011) Remote ischaemic preconditioning of the heart: remote questions, remote importance, or remote preconditions? Basic Res Cardiol 106(4):507–509
Acknowledgments
We are extremely grateful to the National Institute of Health Research, the Medical Research Council and the British Heart Foundation who have kindly agreed to fund the ERICCA trial with an Efficacy and Mechanism Evaluation research grant: Reference number 09/100/05. The Efficacy and Mechanism Evaluation programme is funded by the MRC and NIHR and managed by the NIHR Evaluation, Trials and Studies Coordinating Centre (NETSCC). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the MRC, NHS, NIHR or the Department of Health.
Author information
Authors and Affiliations
Consortia
Corresponding author
Additional information
D. J. Hausenloy and L. Candilio are joint first authors.
Research collaborators
Research collaborators
Mr. Geoff Berg, Golden Jubilee Hospital, Mr. Moninder Bhabra, Wolverhampton Hospital, New Cross Hospital, Mr. Chris Blauth, St Thomas’ Hospital, Mr. Norman Briffa, Northern General Hospital, Prof. John Dark, The Freeman Hospital, Mr. Jatin Desai, Kings College London Hospital, Mr. Steven Griffin, Castle Hill Hospital, Marjan Jahangiri, St George’s Hospital, Mr. David Jenkins, Papworth Hospital, Prof. Daniel Keenan, Manchester Royal Infirmary, Mr. Shyam Kolvekar, Heart Hospital, Mr. Dheeraj Mehta, Cardiff University Hosptial, Prof. John Pepper, Royal Brompton Hospital, Mr. Renzo Pessotto, Edinburgh Royal Infirmary, Mr. Mario Petrou, John Radcliffe Hospital, Mr. Prakash Punjabi, Hammersmith Hospital, Mr. David Richens, Trent Cardiac Centre, Mr. Andrew Ritchie, Essex Cardiothoracic Centre, Mr. André Simon, Harefield Hospital, Prof. Tom Spyt, Glenfield Hospital, Mr. Augustine Tang, Blackpool Victoria Hospital, Mr. Uday Trivedi, Royal Sussex County Hospital, Mr. Jonathan Unsworth-White, Derriford Hospital, Mr. Rakesh Uppal, London Chest Hospital and St Barts’ Hospital, Prof. Nizar Yonan, Wythenshawe Hospital, Mr. Aprim Youhana, Morriston Hospital.
Rights and permissions
About this article
Cite this article
Hausenloy, D.J., Candilio, L., Laing, C. et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi-centre randomized double-blinded controlled clinical trial. Clin Res Cardiol 101, 339–348 (2012). https://doi.org/10.1007/s00392-011-0397-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-011-0397-x