Abstract
Objective
Timing of the operation for exchange of right ventricular (RV) to pulmonary artery (PA) conduits is a matter of considerable debate. We aimed to study the course of right ventricular dimension in patients undergoing conduit exchange.
Patients and methods
We retrospectively studied all patients who underwent implantation and or replacement of RV/PA conduits during the time period between 1990 and 2005. Clinical and echocardiographic data were recorded as obtained at follow-up visits.
Results
A total of 229 (144 boys and 85 girls) underwent surgery for implantation and or replacement of RV/PA conduits during the study period. Patients were assigned to three age groups including 37 infants, 125 children aged 1−10 years and 67 patients more than 10 years of age. 185 pulmonary (81%) and 44 aortic homografts (19%) were implanted. Fifty-eight of these 185 patients (25%) required exchange of conduits after a median time of 6.4 (8 months–12 years) (median (range)). The follow-up was 7.55 (0.1–17) years. The survival of the patients after homograft change was 98%. Freedom from failure for aortic and pulmonary homografts at an interval of 10 years for all patients was 38.5% for aortic and 56.2% for pulmonary homografts (P = 0.018; Mann–Whitney). Age at conduit exchange (coefficient: −4.917; P < 0.001) and right ventricular end-diastolic dimension (RVDD) before conduit exchange (coefficient: 8.255; P < 0.001) were related to RVDD as measured by M-mode echocardiography at follow-up (“best subset” regression analysis; R squared = 0.746). RVDD decreased in 48/58 patients, remained unchanged in 8/58 and increased in 2/59 patients at follow-up. An increased RVDD was positively correlated to the duration of artificial ventilation after the operation for conduit exchange (R = 0.56; P < 0.001).
Conclusions
Reoperation for exchange of degenerated conduits should be performed early to prevent the development of irreversible structural myocardial changes and persistence of right ventricular dilatation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Surgical repair employing a valved homograft conduit between the right ventricle (RV) and pulmonary artery (PA) is now considered to be a standard procedure for many complex congenital cardiac anomalies with right ventricular outflow tract obstruction (RVOTO) and/or pulmonary insufficiency. Despite progress in methods for preservation of valved conduits, the progressive degeneration of allogenic and xenogenic conduits are still an unsolved problem requiring repeated changes of conduit during of the patient’s lifetime. It is widely accepted that the ideal conduit has not yet been developed [8]. A distinct subset of patients with degenerated extracardiac conduits develops progressive pulmonary insufficiency and/or stenosis that lead to RV remodeling, causing structural changes of the heart muscle, impairing diastolic, and systolic function.
The detrimental effects of long-standing RV volume overload include diminished exercise performance [17], deterioration in right and left ventricular function [7], and ventricular arrhythmias [21]. However, most of the patients with degenerated extracardiac conduits remain asymptomatic until severe RV dysfunction develops. The problems arising from homografts and xenografts have been addressed in many studies over the past decades [5–8]. When reflecting these data, it is evident that there is no common rule concluding the indicators and optimal timing for conduit replacement. “Conduit failure” and “dysfunction” are not well-defined terms. Criteria for timing of reoperation show a great variation [2, 8]. We aimed to study the influence of right ventricular dimension prior to conduit replacement on right ventricular dimension after conduit replacement and on patient outcome as surrogate parameters of the RV remodeling process.
2 Patients and methods
2.1 Patient population
From 01/1990 to 12/2005, a total of 229 patients with congenital heart disease underwent right ventricular outflow tract (RVOT) reconstruction with cryopreserved homograft conduits at the Department of Cardiac Surgery of the University of Heidelberg Medical Centre. Clinical data were collected from the patients’ medical records. The total patient population (primary implanted homografts and conduit reoperation) consisted of 144 boys and 85 girls. Thirty-seven out of 229 patients were infants, 125/229 were 1–10 years old, and 67/229 were >10 years old. A total of 185 pulmonary (81%) and 44 aortic homografts (19%) were implanted. Clinical and demographic data of the patients are shown in Table 1 and Fig. 1. All patients undergoing primary RV–PA conduit implantation were assigned to a Group A. Fifty-eight (25%) patients required explantation of their original RV–PA homograft and implantation of a new cryopreserved homograft conduit during the period of observation. These 58 implants were assigned to a Group B, which forms the basis for the subsequent analysis. Patient demographics for this group are shown on Table 2. Children undergoing reconstruction of the RVOT with bovine jugular vein conduits were excluded from this study. The time interval from the original repair to the replacement of the conduit was 6.4 years (range: 8 months to 12 years).
Clinical and demographic data for this retrospective study were collected from patients’ records. Length of hospital stay and duration of artificial ventilation were recorded. Echocardiographic data were reviewed and M-mode measurements of right ventricular diameter on a parasternal long axis view were recorded. These measurements were related to data for the right ventricular diameter as obtained in healthy controls of corresponding body surface area [13].
2.2 Indication for re-operation and conduit exchange
During the period of observation, it was the policy of our center to re-operate on patients with RV–PA conduits whenever the following criteria were met:
-
pulmonary regurgitation (grade III as determined by angiography),
-
stenosis of the homograft exceeding peak systolic gradient of >40 mmHg,
-
progressive right ventricular dysfunction associated with pulmonary regurgitation or stenosis of any degree.
2.3 Operative technique
Repeat sternotomy was performed with special care to avoid bleeding caused by adherence of the calcified homograft to the sternum. Central cannulation was preferred whenever possible but femoral artery and vein cannulation was required in selected cases with the aim to establish cardiopulmonary bypass before or during repeat sternotomy. Our preferred technique was to cannulated the aorta and both caval veins with mild to moderate hypothermia without clamping the aorta when the atrial and ventricular septum were intact. Aortic occlusion was used for additional procedures that would require cardioplegic arrest. When perfusion was established the conduit was identified and partially dissected. The obstructed conduit was excised distally including the anastomotic site with the pulmonary arteries and proximally to the right ventricular wall. The origins of the left and right pulmonary arteries was carefully inspected and measured with Hegar dilators. Severe PA stenoses were relieved surgically. Glutaraldehyde-preserved autologous pericardium was used to reconstruct stenosed pulmonary branches when necessary. Alternatively pulmonary branch stenosis was relieved with the new conduit cut into the appropriate shape. The new homograft was anastomosed distally with the PA so that the homograft valve was positioned as close as possible to the distal anastomosis to prevent geometrical changes at the valve level by compression by the sternum. The reconstruction was completed by anastomosing the homograft to the RVOT. For the roof of this anastomosis, we used PTFE patches. After rewarming and discontinuation of cardiopulmonary bypass right ventricular and PA pressures were measured.
2.4 Echocardiographic examinations
Preoperative and postoperative two-dimensional echocardiograms were reviewed. Right ventricular end-diastolic dimension (RVDD) was measured by M-mode echocardiography using the parasternal long axis view at the level of the left ventricular papillary muscles. Values were related to the mean RVDD as observed on controls of corresponding body surface area [13]. RVDD at the last follow-up visit was related to preoperative measurements before conduit implantation.
2.5 Electrocardiographic examinations
The duration of the QRS complex was measured related to the RVDD prior to conduit change.
2.6 Statistics
Data are reported as median (range). Non-parametric testing (Mann–Whitney) was used to test for differences between groups. Linear regression was used to tests the correlation between RVDD and QRS duration and the correlation between RVDD and duration of artificial ventilation. Regression analysis (best subset regression) was used to study the influence of clinical and echocardiographic parameters on RVDD at follow-up. Statistical analysis was performed using the Sigma Stat software version 3.0 (SPSS Inc., USA) Statistical significance was considered at P < 0.05.
3 Results
3.1 Clinical and demographic data
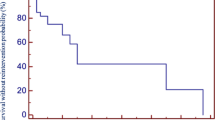
In the whole study group freedom from failure of function for aortic and pulmonary homografts as defined above was 38.5% for aortic and 56.2% for pulmonary homografts as assessed at follow-up of 7.55 years (range 0.1–17 years) after the primary operation (P = 0.018; Mann–Whitney). Younger patients were found to require earlier conduit replacement than older patients (Table 3 and Fig. 2) and aortic homografts showed a higher rate of failure of function in all age groups (Table 3). Predominant pressure overload due to conduit stenosis was the reason for conduit exchange in 22/58 patients whereas 36/58 patients required conduit exchange for significant pulmonary regurgitation. In group B, there was no operative mortality. The survival rate of the patients with changed conduits was 98%. There was one late death.
3.2 Echocardiographic measurements
Postoperative echocardiographic measurements were available in 58 patients at a median follow-up of 7.55 years (range 0.1–17 years). Most patients showed considerable right ventricular dilatation before conduit exchange (Fig. 3). RVDD related to the mean value of normal controls with corresponding body surface area disclosed that the right ventricular diameter before operation was increased up to 3.8 times. At the last follow-up visit after conduit exchange RVDD decreased in 48/58 patients, remained unchanged in 8/58 patients and increased in 2/58 patients (Fig. 4).
Age at operation for conduit exchange (coefficient: −4,917; P < 0.001) and right ventricular diameter before conduit exchange (coefficient: 8.255; P < 0.001) were the single most important factors predicting right ventricular diameter at follow-up (best subset regression analysis; R squared = 0.746).
3.3 Duration of the QRS complex and RVDD
For the whole study population, the correlation between QRS duration and RVDD was not significant (P = 0.30). However, duration of QRS complex was related to RVDD for the subgroup of patients with tetralogy of Fallot requiring conduit exchange for significant pulmonary regurgitation (R = 0.61; P = 0.020; Fig. 5).
3.4 Time of postoperative mechanical ventilation and duration of hospital stay
Prolonged ventilatory support was observed in patients with high RVDD and—on linear regression analysis—the relation of RVDD before conduit exchange was positively correlated with the duration of artificial ventilation after the operation (days of artifical ventilation = −1.687 + (0.0358 * (relative RVDD (in % of expected normal value) before conduit exchange)); R = 0.56; P < 0.001; Fig. 6). Hospital stay was 17 (10−32) days after surgery for conduit replacement. Postoperative complications Postoperative complications occurred in 22 (38%) patients (Table 4). The most prevalent complications were infection (n = 16) and neurological problems such as transient neuropsychatric injury (n = 8) which were more often to be observed in the group of patients with RVDD >25 mm.
4 Discussion
Since the original description of reconstruction of the RVOT with an extracardiac valved conduit by Ross and Somerville [16] the search for “an ideal” conduit to establish subpulmonary ventricle—PA continuity has been a significant challenge for congenital cardiac surgeons. The extracardiac valved conduit has allowed the routine repair of complex anomalies that include tetralogy of Fallot (TOF), truncus arteriosus, complex transposition of the great arteries (TGA) with pulmonary stenosis (PS), pulmonary atresia, double-outlet right ventricle (DORV) with PS, and other forms of complex congenital heart diseases. Data reflecting the long-term follow-up after replacement of the systemic arterial valve in children by using a mechanical prosthesis have been reported [20] but mechanical prostheses rarely are suitable for replacement of the pulmonary valve in children.
The implanted conduit ideally would possess the following characteristics: optimal hemodynamic function, long-term structural durability and functional capacity, low immunological competence, no thrombogenic surfaces, low infectious potential, growth potential and low costs [8]. However such an ideal conduit still does not exist and therefore a progressive structural degeneration process over the years develops in almost all implanted valved conduits [9].
Probably the most important mechanisms for conduit failure in RVOT reconstruction are immunogenicity of the allograft [1], chronic inflammatory process, younger recipient age [3], and small homograft size [11]. Our data confirm that implantation of homografts in infants is associated with a higher rate of reoperation for conduit exchange. Likewise, aortic homografts showed a higher tendency for conduit failure compared with pulmonary homografts [6, 8, 11, 12]. The existence of pathologic pressure—flow relation in abnormal RVOT configuration and persisting pulmonary hypertension in some cases were also reported to be responsible of extracardiac conduit failure [10]. Surgical technical factors like non-anatomic placement and conduit compression [22] play an additional important role in the development of conduit dysfunction and failure.
Right ventricular pressure overload is frequently well tolerated for many years. However, review of experience made in simple tetralogy of Fallot [4] suggests that patients with chronic pulmonary regurgitation, consequent RV dilatation and hypertension have a definite risk of ventricular ectopy and sudden death. Others have shown that right ventricular recovery following pulmonary valve replacement for chronic significant pulmonary regurgitation may be compromised in the adult population with tetralogy of Fallot [19]. Evaluating clinical and echocardiographic findings, Discigil and co-workers [7] reported 7% sudden deaths despite of implantation of competent pulmonary valve and 44% incidence of moderate to severe RV dilatation postoperatively. Many authors therefore have advocated that the timing of inserting a homograft is controversial at present: According to Brown and co-workers [2] conduit stenosis—(P > 40 mmHg, presence of a right ventricular systolic pressure (75% of left ventricular systolic pressure, progressive right ventricular dilatation or tricuspid valve regurgitation or both, associated with right heart failure were the indications of conduit change. In contrast, Forbess and co-workers [8] stated that homograft obstruction was the single indication for replacement. In their study, no homograft was replaced for isolated homograft regurgitation.
According to Stark [18] reoperation was indicated if RV pressure approaches systemic pressure in the presence of obstruction. If RV function was already compromised lower degrees of RV hypertension would warrant reintervention. According to Stark [18] usually the decrease in RV function would suggest that patients were candidates for placement of a competent valve as valved conduit. Our data show that—once considerable right ventricular dilatation due to pulmonary regurgitation is present—the best that can be anticipated is a reduction in RV size but normalization of RV diameter rarely is to be observed. As observed by other groups recently QRS duration correlated with RV size in patients with tetralogy of Fallot and volume overload prior to conduit replacement [10]. In this series, right ventricular dilatation prior to surgery was associated with the need for longer mechanical ventilation postoperatively. This association most likely is explained by right ventricular dysfunction, which will lead to longer convalescence [14]. These findings are supporting the results of Dave and co-workers [5] who showed that the degree of improvement in ventricular dimension and function was directly dependent on the preoperative state of the progressive pathophysiological and pathomorphological alterations of the RV, which should be taken into account when assessing the timing of reoperation.
Limitations: Due to the retrospective study we did not perform a detailed analysis of right ventricular geometry and function as provided by MRI or 3D echocardiography. RVDD as an M-Mode parameter certainly is not the ideal parameter to assess all aspects of right ventricular remodeling. As reflected in a recent review there clearly is a need for serial studies in large numbers of patients assessing right ventricular volume, function and pulmonary regurgitation by MRI to get more information on the ideal timing for pulmonary valve replacement [4]. Moreover, we cannot present data on the functional capacity of these patients as assessed by exercise testing or the ability index [15]. Additional laboratory surrogate parameters such as brain natriuretic peptide (BNP) were not available in this retrospective study.
In summary we demonstrate that right ventricular diameter and age at operation are important factors that influence the capability of the dilated RV to reduce its size after conduit change in patients with congenital heart disease and RV–PA homograft. In addition, the time of mechanical ventilation was significantly longer for the group with a higher degree of preoperative RV dilatation. Reoperation for changing degenerated conduits should be performed early to prevent the development of irreversible structural myocardial changes and persistence of right ventricular dilatation.
References
Baskett RJ, Ross DB, Nanton MA, Murphy DA (1996) Factors in the early failure of cryopreserved homograft pulmonary valves in children: preserved immunogenicity? J Thorac Cardiovasc Surg 112:1170–1178
Brown JW, Ruzmetov M, Rodefeld MD, Vijay P, Turrentine MW (2005) Right ventricular outflow tract reconstruction with an allograft conduit in non-ross patients: risk factors for allograft dysfunction and failure. Ann Thorac Surg 80:655–663; discussion 663–664
Clarke DR, Bishop DA (1995) Ten year experience with pulmonary allografts in children. J Heart Valve Dis 4:384–391
Chaturvedi RR, Redington AN (2007) Pulmonary regurgitation in congenital heart disease. Heart 93:880–889
Dave HH, Buechel ER, Khatami AD, Kadner A, Rousson V, Bauersfeld U, Pretre R (2005) Early insertion of a pulmonary valve for chronic regurgitation helps restoration of ventricular dimensions. Ann Thorac Surg 80:1615–1621
Dearani JA, Danielson GK, Puga FJ, Schaff HV, Warnes CW, Driscoll DJ, Schleck CD, Ilstrup DM (2003) Late follow-up of 1095 patients undergoing operation for complex congenital heart disease utilizing pulmonary ventricle to pulmonary artery conduits. Ann Thorac Surg 75:399–411
Discigil B, Dearani JA, Puga FJ, Schaff HV, Hagler DJ, Warnes CA, Danielson GK (2001) Late pulmonary valve replacement after repair of tetralogy of Fallot. J Thorac Cardiovasc Surg 121:344–351
Forbess JM (2004) Conduit selection for right ventricular outflow tract reconstruction: contemporary options and outcomes. Semin Thorac Cardiovasc Surg Pediatr. Card Surg Annu 7:115–124
Gerestein CG, Takkenberg JJ, Oei FB, Cromme-Dijkhuis AH, Spitaels SE, van Herwerden LA, Steyerberg EW, Bogers AJ (2001) Right ventricular outflow tract reconstruction with an allograft conduit. Ann Thorac Surg 71:911–917
Grothoff M, Spors B, Abdul-Khaliq H, Abd El Rahman M, Alexi-Meskishvili V, Lange P, Felix R, Gutberlet M (2006) Pulmonary regurgitation is a powerful factor influencing QRS duration in patients after surgical repair for tetralogy of Fallot. Clin Res Cardiol 95:643–649
Hawkins JA, Bailey WW, Dillon T, Schwartz DC (1992) Midterm results with cryopreserved allograft valved conduits from the right ventricle to pulmonary artery. J Thorac Cardiovasc Surg 104:910–916
Jashari R, Van Hoeck B, Gaudino M, Daenen W, Van Geldorp T, Kalmar P, Goffin Y (2000) Are pulmonary homografts which were subjected to pulmonary hypertension more appropriate for aortic valve replacement than normal pulmonary homografts? A long-term multicentric echography study. Eur J Cardiothorac Surg 17:140–145
Kampmann C, Wiethoff CM, Wenzel A, Stolz G, Betancor M, Wippermann CF, Huth RG, Habermehl P, Knuf M, Emschermann T, Stopfkuchen H (2000) Normal values of M mode echocardiographic measurements of more than 2000 healthy infants and children in central Europe. Heart 83:667–672
Meliones JN, Kern FH, Schulmann SR, Ungerleider RM, Greeley WJ (1996) Pathophysiological directed approach to congenital heart disease: a perioperative perspective. In: Greeley WJ (ed) Perioperative management of the patient with congenital heart disease. Williams & Wilkins, Baltimore, pp 1–42
Norozi K, Wessel A, Buchhorn R, Alpers V, Arnhold JO, Zooge M, Gayer S (2007) Is the ability index superior to the NYHA classification for assessing heart failure? Comparison of two classification scales in adolescents and adults with operated congenital heart defects. Clin Res Cardiol 96:542–547
Ross DN, Somerville J (1996) Correction of pulmonary atresia with a homograft aortic valve. Lancet 2(7479):1446–1447
Schamberger MS, Hurwitz RA (2000) Course of right and left ventricular function in patients with pulmonary insufficiency after repair of tetralogy of Fallot. Pediatr Cardiol 21:244–248
Stark J (1998) The use of valved conduits in pediatric cardiac surgery. Pediatr Cardiol 19:282–288
Therrien J, Siu SC, McLaughlin PR, Liu PP, Williams WG, Webb GD (2000) Pulmonary valve replacement in adults late after repair of tetralogy of Fallot: are we operating too late? J Am Coll Cardiol 36:1670–1675
Tiete AR, Sachweh JS, Groetzner J, Gulbins H, Muehl EG, Messmer BJ, Daebritz SH (2006) Systemic mechanical heart valve replacement in children under 16 years of age. Clin Res Cardiol 95:281–288
Warner KG, O’Brien PKH, Rhodes J, Kaur A, Robinson DA, Payne DD (2003) Expanding the indications for pulmonary valve replacement after repair of tetralogy of Fallot. Ann Thorac Surg 76:1066–1072
Wells WJ, Arroyo H Jr, Bremner RM, Wood J, Starnes VA (2002) Homograft conduit failure in infants is not due to somatic outgrowth. J Thorac Cardiovasc Surg 124:88–96
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Loukanov, T., Sebening, C., Springer, W. et al. Replacement of valved right ventricular to pulmonary artery conduits: an observational study with focus on right ventricular geometry. Clin Res Cardiol 97, 169–175 (2008). https://doi.org/10.1007/s00392-007-0599-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00392-007-0599-4