Abstract
Purpose
Although the multimodal cancer treatment techniques have greatly improved over the years, irradiation-induced late gastrointestinal toxicity remains a great concern as it may highly affect the quality of life of a patient. The aim of this study was to define the prevalence of late gastrointestinal toxicities.
Methods
Electronic databases of Cochrane Library, Embase, Web of Science, CENTRAL and PubMed were searched until September 2019. We used the following keywords: radiotherapy, radiation therapy, irradiation, rectal cancer, gastrointestinal toxicity, adverse effects, late effects, pelvic radiation and pelvic radiation disease.
Results
Nine studies were included into this review out of 4785 that were preidentified as potentially relevant. Overall prevalence of severe (Grade 3 or higher) late irradiation-induced gastrointestinal toxicities was up to 19%. Most frequent toxicities of any grade were reported to be diarrhoea (up to 35%), faecal incontinence (22%), incontinence to gas (71%), rectal bleeding (9%), rectal pain (13%) and obstruction (7.4%). Preoperative treatment approaches and more advance radiotherapy techniques such as intensity-modulated and image-guided radiotherapy (IMRT) and volumetric modulated arc therapy (VMAT) turn out to result in lower late gastrointestinal toxicity rates.
Conclusion
After great improvements in rectal cancer treatment, late gastrointestinal toxicity after radiotherapy is experienced less frequent and less severe; however, it remains a great concern associated with worse quality of life.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colorectal cancer remains one of the most frequently diagnosed cancers and the second leading cause of death for both men and women worldwide [1]. Rectal cancer is treated with a combined modality therapy that includes surgery, radiation therapy and chemotherapy. Although radiotherapy has improved the rectal cancer treatment approach over time and led to a reduction of the risk for local recurrences, the therapeutic benefit of radiation therapy itself is balanced against potential damage to noncancerous tissue [2]. Many studies focus on early-onset toxicity since most commonly acute symptoms which may include nausea, diarrhoea, vomiting, enteritis, proctitis and dehydration appear 1–3 weeks after the start of radiotherapy and reach a peak at fifth week of treatment; therefore, it is less complicated to record results, and no long follow-up is required as for the evaluation of late outcomes of which little knowledge is published yet.
The aim of this article was to review published studies on late adverse effects caused by radiotherapy for rectal cancer treatment, focusing on gastrointestinal toxicity. The intention is to define the prevalence and severity of late irradiation-induced gastrointestinal symptoms and compare different treatment techniques.
Materials and methods
We performed this systematic review according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement [3].
Literature search and inclusion criteria
Two authors searched independently electronic databases of Cochrane Library, Embase, Web of Science, CENTRAL and PubMed until September 2019. Search string for Medline and Embase were used: radiotherapy and/or radiation therapy and/or irradiation and/or rectal cancer and/or gastrointestinal toxicity and/or adverse effects and/or late effects and/or pelvic radiation and/or pelvic radiation disease.
Studies on both preoperative and postoperative external beam radiotherapy were included, while articles on brachytherapy were excluded.
This review focuses on studies published in the English language between January, 1995, and September, 2019.
The inclusion criteria were as follows: (1) original studies; (2) studies that analysed late radiotherapy toxicity to gastrointestinal tract and (3) patients who underwent treatment only for rectal cancer;
Exclusion criteria were as follows: (1) studies where other pelvic cancers were included, (2) unable to separate other cancers or toxicity to other organs and (3) articles or abstracts written in non-English.
The disagreement was solved by consensus or a third reviewer. Data from included studies was extracted into a datasheet and pretested to prove its suitability. In addition, references and abstracts were searched.
Definition of late gastrointestinal toxicity
Radiotherapy causes early onset gastrointestinal symptoms that may remain for long years as late adverse effects of the treatment. The cut-off to separate the timing of acute and late toxicity in overviewed publications was considered to be 3 months counting from the start of treatment. In accordance with literature, the most common standards used to evaluate toxicity caused by radiotherapy were the national cancer institute Common Terminology Criteria for Adverse Events (CTCAE) [4] classification, the Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC) [5] radiation morbidity scoring scheme and scoring system of late effects of radiations on normal tissues (LENT SOMA scale) [6].
The updated version (CTCAE v4.0) contains lower GI adverse events such as diarrhoea, fistula, haemorrhage, necrosis, obstruction, perforation, stricture/stenosis and ulcer. Also, the site of the symptom is noted as ‘anal’ or ‘rectal’. Toxicity in CTCAE is graded as mild (Grade 1), moderate (Grade 2), severe (Grade 3), life-threatening (Grade 4) or death (Grade 5). The Radiation Therapy Oncology Group and the European Organization for Research and Treatment of Cancer (RTOG/EORTC) radiation late morbidity scoring scheme evaluates toxicity that occurs 90 days after the start of the treatment. The RTOG/EORTC criteria grade severity by symptoms from none (Grade 0) to severe (Grade 4). LENT SOMA scale is used for irradiation-induced toxicity assessment by evaluating the severity and frequency of the symptoms (from Grade 1 to Grade 4) subjectively, objectively, analytically by the means of medical examination and grading the management used for these adverse effects. Toxicity of Grade 3 and higher are regarded as high.
We applied ten review criteria that had been previously developed by Gill and Feinstein [7] and refined by Moons et al. [8].
Results
Search results
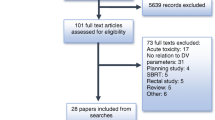
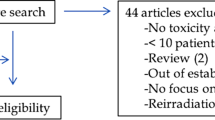
There were 4785 potentially relevant studies identified according to the predefined search strategy. A total of 3978 studies remained because of elimination of 807 duplicates. After reading abstracts of the articles that remained, 3892 were excluded since they were not fulfilling the criteria and not eligible for the topic; therefore, 86 studies remained. After review, 77 articles were excluded because of different reasons: 30 articles were unavailable, 18 studies appeared to be irrelevant for the topic, 15 studies were published in other language than English and 14 studies were impossible to separate data in pelvic cancer. Therefore, nine studies were suitable for the included criteria (Fig. 1).
Incidence of late gastrointestinal toxicity
Overviewed studies report that up to 19% of all rectal cancer patients after the radiotherapy treatment suffer from late gastrointestinal toxicity symptoms of Grade 3 or higher that clearly worsens the quality of life (Table 1). Most frequently mentioned late adverse symptoms include diarrhoea (up to 35%), faecal incontinence (22%), incontinence to gas (71%), rectal bleeding (9%), rectal pain (13%) and obstruction (7.4%). Although small bowel obstruction is not the most frequently met side effect, it is considered to be most complicated as it requires closer observation and interventional procedures [9, 10].
Side effects of radiotherapy are best illustrated by the studies that compare groups of irradiated patients with non-irradiated. Norwegian study comparing male patients with rectal cancer treated either with pre- or postoperative (chemo)radiotherapy and control group that underwent surgery alone found a statistically significant difference in late gastrointestinal toxicity experienced by the two groups of patients without stoma. Bowel movements were statistically more frequent in irradiated group compared with non-irradiated patients (19% and 3%, respectively, of Grade ≥ 3 and 54% and 20%, respectively, of Grade 2). Also, loose stools were more frequently reported in irradiated group with 36% compared with non-irradiated with 16%. The prevalence of other toxicity symptoms that included mucus in the stools (12% and 5%), rectal pain (13% and 6%), blood in the stools (9% and 4%), anal stricture (10% and 6%) and incontinence for solid stools (15% and 5%) and gas (71% and 58%) was higher but not statistically different in compared groups. Also, no significant difference was found in toxicity comparing preoperative radiotherapy with postoperative treatment as well as comparing patients who underwent chemoradiotherapy with those treated without chemotherapy. Late adverse gastrointestinal effects induced by radiation strongly correlate with lower quality of life scores where social life is mostly affected [11].
A study form Sweden and Norway presented no statistically significant differences between the chemoradiotherapy and radiotherapy alone, although higher prevalence of toxicity was noted in chemoradiotherapy group regarding bowel obstruction (28% compared with 15%, p = 0.27) and rectal or abdominal pain (50% compared with 30%, p = 0.10). The proportion of patients with stoma in compared groups did not differ significantly. Incontinence for patients without stoma was slightly higher in chemoradiation group but not statistically significant: for liquid stools 58% in CRT group and 38% in RT group, for gas 75% and 56% respectively [12]. Later, the study on the follow-up of the patients reported a higher mean scale score of diarrhoea in radiotherapy group (25) compared with chemoradiotherapy group (20), and that has a statistically significant difference compared with the normal population (p < 0.05). However, no significant difference was revealed for mean scale scores for constipation (12 vs 16, p = 0.30), appetite loss (2 vs 6, p = 0.28) and nausea and vomiting (2 vs 4, p = 0.27) comparing radiotherapy and chemoradiotherapy groups and also compared with the normal population [13].
A prospective study on comparison of preoperative chemoradiotherapy and postoperative chemoradiotherapy for rectal cancer validates a standard approach for today and reveals a statistically significant lower incidence of high-grade late toxicity in the group which underwent chemoradiation before the surgery (p = 0.002) inflicting the later treatment approach as superior. The total severe late toxicity reported in preoperative CRT group was 7% in comparison with 13% in postoperative group. In the postoperative CRT group, 3% of patients had a bowel obstruction that needed a surgical intervention, 6% developed anastomotic stenosis and 2% developed fistulas, whereas in the preoperative group, 1% of patients developed obstruction, 1% developed stenosis and 1% developed fistula [10].
Korean retrospective study compared younger and elderly rectal cancer patients treated with neoadjuvant chemoradiation where the cut-off between the observed groups was 70 years old. The elderly patients experienced more high-grade late gastrointestinal radiation-induced side effects (2.6% in younger arm vs 4.5% in elderly arm) that included rectal bleeding, fistula and pelvic abscess; however, the study found no significant difference between the groups [14]. High tolerability of radiation treatment of elderly patients is supported by studies by Tougeron et al. [15] and Choi et al. [16].
Study on intensity-modulated and image-guided radiotherapy with and without a simultaneous integrated boost, revealed high-grade (Grade ≥ 3) late gastrointestinal toxicity in 9% of all rectal cancer patients, where 57 patients out of 108 received radiotherapy with a boost on a tumour. One patient in the no-boost group experienced Grade 5 diarrhoea, while Grade 5 fistula between small bowel and bladder was observed in a patient who received treatment with a boost. The study reported diarrhoea of Grade ≥ 1 in 52% patients in no-boost group and 46% in boost group (p = 0.49) and small bowel obstruction of Grade ≥ 1 in 5% and 16% of patients, respectively (p = 0.06); however, no statistically significant difference between the groups was found [17].
The comparison of more advanced radiotherapy treatment techniques, a volumetric modulated arc therapy (VMAT) and conventional 3D conformal radiotherapy (3DCRT) reported VMAT to causes less gastrointestinal late toxicity. Proctitis of Grade 3 and higher was reported for 3% of patients in VMAT group vs 8% in 3DCRT group, while any grade of enteritis was 0% and 6%, respectively. Rates of the 2-year period of time free from radiation-induced toxicity were 81% and 91% for 3DCRT and VMAT patients, respectively [18].
Evaluation according to the criteria
The evaluation of methodological and conceptual quality or rigour according to the criteria of Gill and Feinstein [7, 8] (Table 2) revealed that none of the studies defined quality of life while evaluating; two (22%) of the nine studies state the measured domains in evaluating quality of life (criterion 2). In the single study (11%), a specific reason for the choice of instrument to measure QOL (criterion 3) was given. In one (11%) of the studies, results were aggregated from multiple items and domains into a single composite score illustrating global quality of life (criterion 4). Evaluation of the studies showed that criteria 5–10 were not fulfilled; none of the studies distinguished overall and health-related quality of life and provided an option for the participants to select additional items that are important to them.
Based on the wide heterogeneity of included studies, the meta-analysis could not be performed.
Discussion
Our systematic review showed that radiotherapy leads to severe late gastrointestinal toxicity. Most frequently diarrhoea, rectal pain, bleeding and incontinence affect the life of rectal patients after the treatment. Reduced ability to defer defecation and incontinence to loose stools induced by irradiation worsen the quality of life the most by impairing social functions.
Measures such as antidiarrheal medication, sanitary pads and a particular diet may improve quality of life by preventing faecal incontinence. Surgical interventions such as sphincter-sparing surgery that preserves rectal function and stoma formation would also be an option to prevent patients from faecal incontinence and, therefore, could improve the social life and whole quality of life. Previously, stoma has related a negative effect for the quality of life; however, a reviewed study showed no difference in any function scale and quality of life scores between patients with stoma and without [11].
Total radiation dose exposed to irradiated normal tissue correlates with the severity of adverse gastrointestinal effects. VMAT and IMRT allow better target volume coverage and organ at risk sparing, compared with other techniques, specifically 3DCRT, leading to less damage to irradiated normal tissue, and therefore could cause less severe toxicity, yet VMAT-induced late toxicity is insufficiently studied [18]. IMRT and IGRT with a SIB boost approach to rectal cancer allow to boost dose to target and minimize the irradiation volume for normal tissue, precisely small bowel and bladder that are most sensitive to radiation and are dose-limiting factors. Despite the advance and promising concept of radiotherapy approach, Engels et al. conclude that this treatment should not be chosen routinely for rectal cancer patients as it does not lower late toxicity rates [17]. Also, a proper patient selection and imaging for the planning of the radiotherapy should not be underestimated as it lowers the possibility to overtreat and helps to minimize the irradiation volume to surrounding tissue or avoid unnecessary irradiation [9, 11, 19].
Preoperative radiotherapy has become a standard approach for rectal cancer treatment as it shrinks the tumour in volume and decreases its seeding in surrounding tissues, and tumour oxygenation is better during the preoperative irradiation compared with the postoperative approach. This leads to better surgical results, overall lower toxicities and high tolerability for rectal cancer patients regardless of their age [14, 20].
The treatment including chemotherapy in addition to radiotherapy is supposed to lead to greater rates and enhanced gastrointestinal toxicities, although Brændengen et al. suggest that late bowel toxicity should not be directly assigned to chemotherapy as it can be partly related to better survival rates of patients with advanced rectal tumours [13].
Our systematic review has obvious limitations: some studies of retrospective nature, limited numbers of followed-up patients and a high proportion of them lost during years, low statistical power and potential biases. Moreover, prolonged observation, uniform and adequate evaluation of late toxicities are required to make reliable conclusions.
Conclusion
Radiotherapy as a mean of rectal cancer treatment decreases the local recurrence rate without increasing the survival; however, it causes a lot of adverse effects that remain long years after the irradiation. Development of radiotherapy techniques and profound pretreatment imaging and planning are expected to cause less gastrointestinal side effects; however, more improvements and investigations have to be carried out in the future in order to treat rectal cancer patients more efficiently, with the intention to preserve gastrointestinal functions and maintain a high quality of life.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A (2018) Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 68(6):394–424
Birgisson H, Påhlman L, Gunnarsson U, Glimelius B (2007) Late adverse effects of radiation therapy for rectal cancer – a systematic overview. Acta Oncol 46(4):504–516
Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62(10):1006–1012
National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services (2009) Common terminology criteria for adverse events (CTCAE) Version 4.0. NIH publication # 09-7473. Published May 29, 2009; Revised Version 4.03 June 14, 2010. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. Accessed 16 Nov 2019
Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31(5):1341–1346
Lent soma scales for all anatomic sites. Int J Radiat Oncol. 1995;31(5):1049–1091
Gill TM, Feinstein AR (1994) A critical appraisal of the quality of quality-of-life measurements. JAMA 272(8):619–626
Moons P, Van Deyk K, Budts W, De Geest S (2004) Caliber of quality-of-life assessments in congenital heart disease: a plea for more conceptual and methodological rigor. Arch Pediatr Adolesc Med 158(11):1062–1069
Ciabattoni A, Cavallaro A, Potenza AE, Colli R, Maurizi F, Miccichè F, Valentini V (2003) Preoperative concomitant radiochemotherapy with a 5-fluorouracil plus folinic acid bolus in the combined treatment of locally advanced extraperitoneal rectal cancer: a long-term analysis on 27 patients. Tumori J 89(2):157–163
Akgun E, Ozkok S, Tekin M, Yoldas T, Caliskan C, Kose T, Karabulut B, Sezak M, Elmas N, Ozutemiz O (2017) The effects of chemoradiotherapy on recurrence and survival in locally advanced rectal cancers with curative total mesorectal excision: a prospective, nonrandomized study. World J Surg Oncol 15:205
Bruheim K, Guren MG, Skovlund E, Hjermstad MJ, Dahl O, Frykholm G, Carlsen E, Tveit KM (2010) Late side effects and quality of life after radiotherapy for rectal cancer. Int J Radiat Oncol 76(4):1005–1011
Brændengen M, Tveit KM, Bruheim K, Cvancarova M, Berglund Å, Glimelius B (2011) Late patient-reported toxicity after preoperative radiotherapy or chemoradiotherapy in nonresectable rectal cancer: results from a randomized phase III study. Int J Radiat Oncol Biol Phys 81(4):1017–1024
Brændengen M, Tveit KM, Hjermstad MJ, Johansson H, Berglund Å, Brandberg Y, Glimelius B (2012) Health-related quality of life (HRQoL) after multimodal treatment for primarily non-resectable rectal cancer. Long-term results from a phase III study. Eur J Cancer 48(6):813–819
Sung S-Y, Jang H, Kim S, Jeong JU, Jeong S, Song JH, Chung MJ, Cho HM, Kim HJ, Kim JG, Lee IK, Lee JH (2019) Oncologic outcome and morbidity in the elderly rectal cancer patients after preoperative chemoradiotherapy and total mesorectal excision: a multi-institutional and case-matched control study. Ann Surg 269(1):108–113
Tougeron D, Roullet B, Paillot B, Hamidou H, Tourani JM, Bensadoun RJ, Michel P, Silvain C (2012) Safety and outcome of chemoradiotherapy in elderly patients with rectal cancer: results from two French tertiary centres. Dig Liver Dis 44(4):350–354
Choi Y, Kim JH, Kim J-W, Kim JW, Lee KW, Oh HK, Kim DW, Kang SB, Song C, Kim JS (2016) Preoperative chemoradiotherapy for elderly patients with locally advanced rectal cancer-a real-world outcome study. Jpn J Clin Oncol 46(12):1108–1117
Engels B, Platteaux N, Van den Begin R, Gevaert T, Sermeus A, Storme G, Verellen D, De Ridder M (2014) Preoperative intensity-modulated and image-guided radiotherapy with a simultaneous integrated boost in locally advanced rectal cancer: report on late toxicity and outcome. Radiother Oncol 110(1):155–159
Dröge LH, Weber HE, Guhlich M, Leu M, Conradi LC, Gaedcke J, Hennies S, Herrmann MK, Rave-Fränk M, Wolff HA (2015) Reduced toxicity in the treatment of locally advanced rectal cancer: a comparison of volumetric modulated arc therapy and 3D conformal radiotherapy. BMC Cancer 15(1):750
Lee SF, Chiang CL, Lee FAS, Wong YW, Poon CM, Wong FCS, Tung SY (2018) Outcome of neoadjuvant chemoradiation in MRI staged locally advanced rectal cancer: retrospective analysis of 123 Chinese patients. J Formos Med Assoc 117(9):825–832
Tseng M, Zheng H, Ng I, Leong YH, Leong CN, Yong WP, Cheong WK, Tey JCS (2018) Outcomes of neoadjuvant chemoradiotherapy followed by total mesorectal excision surgery for locally advanced rectal cancer: a single-institution experience. Singap Med J 59(6):305–310
Author information
Authors and Affiliations
Contributions
ES, AS, AB and AD contributed to this work, satisfying the following four criteria of the guidelines of the International Committee of Medical Journal Editors (ICMJE): substantial contributions to the conception or design of the work or the acquisition, analysis or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
What does this paper add to the literature?
Our systematic review shows that radiotherapy in rectal cancer treatment has a long-term negative effect on bowel function. Up to 30% of patients experience late postradiotherapy effect: diarrhoea, faecal incontinence (22%), incontinence to gas, rectal bleeding, rectal pain and obstruction.
Rights and permissions
About this article
Cite this article
Sipaviciute, A., Sileika, E., Burneckis, A. et al. Late gastrointestinal toxicity after radiotherapy for rectal cancer: a systematic review. Int J Colorectal Dis 35, 977–983 (2020). https://doi.org/10.1007/s00384-020-03595-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-020-03595-x