Abstract
Purpose
Developments in surgical techniques and neoadjuvant treatment have enabled an increasing proportion of patients with rectal cancer to undergo sphincter-sparing resections. The avoidance of a permanent stoma can come at the cost of poor bowel function which can significantly impact patients’ quality of life. The objective of this study was to identify the incidence and risk factors for the development of bowel dysfunction following rectal cancer surgery.
Methods
Patients undergoing anterior resection for rectal cancer between January 2009 and January 2015 were identified from a rectal cancer database at a single centre. All patients who had bowel continuity restored and underwent curative resection were sent a validated low anterior resection syndrome (LARS) questionnaire. Pre-, inter- and postoperative factors were compared between patients with major LARS and those with minor or no LARS using conditional logistic regression.
Results
There was an 80% response rate (n = 68). Thirty-eight patients (56%) had major LARS symptoms. Neoadjuvant radiotherapy, predominantly long-course chemoradiotherapy (LCCRT), was an independent risk factor for development of major LARS symptoms, while restoration of bowel continuity within 6 months was protective.
Conclusions
The use of neoadjuvant radiotherapy (LCCRT) and timing of stoma reversal are risk factors for the development of severe bowel dysfunction. The potential for long-term poor functional results after LCCRT should be discussed with patients and form a part of the decision-making in individual treatment plans. The timing of the ileostomy closure, where safe and feasible, should be performed within 6 months to improve outcome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Over the last decade, the surgical approach to rectal cancer has significantly changed, with greater emphasis on sphincter preservation and a reduction in the rate of abdominoperineal resections [1]. A sphincter-sparing anterior resection is the gold standard for mid to low rectal cancers, with low anastomoses often defunctioned with a loop ileostomy [2]. The National Bowel Cancer Audit’s (NBOCA) formal report in 2015 highlighted that 77% of all patients undergoing an anterior resection had a stoma formed. At 18 months postoperatively, 27% of patients remained with a stoma [3]. As 5-year survival rates have improved for rectal cancer, there is an increasing emphasis on the issues faced by patients following their cancer treatment. A recent report from the Department of Health with the UK National Bowel Cancer Survivorship initiative suggested that almost one in five patients has significant bowel dysfunction following surgery [4].
Anterior resection syndrome was defined in 2012 as “disordered bowel function after rectal resection, leading to a detriment in quality of life” [5]. Symptoms range from bowel fragmentation and emptying difficulties to faecal urgency and incontinence [6, 7]. The term low anterior resection syndrome (LARS) is becoming more commonly used as there is an association with low anastomoses [8]. LARS is reported to be present in as many as 75% of patients following surgery, with 25% having ongoing symptoms for more than 12 months. The incidence of LARS post rectal surgery varies in the literature between 60 to 90%. Several studies have reported that LARS has a significant impact on patients’ quality of life. The aetiology of LARS is thought to be multifactorial, with the potential of sphincter injury during the construction of the anastomosis, alterations in anorectal physiology and the development of a pudendal neuropathy [6]. Further work on functional bowel outcomes after anterior resection has demonstrated that worse outcome is associated with low tumours (i.e. low anterior resections), pelvic sepsis following anastomotic leak and those patients undergoing neoadjuvant and adjuvant radiotherapy [9].
This study aims to identify the incidence and severity of LARS within a tertiary centre’s rectal cancer population, as well as identifying specific risk factors for the development of LARS.
Methods
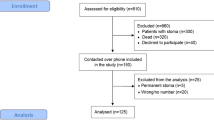
Local research approval was obtained for this study. Patients were identified from a prospectively maintained departmental database (from 2009 to 2014). The inclusion criteria for this study were patients who (i) had undergone anterior resection for rectal neoplasia, (ii) had restored bowel continuity for at least 12 weeks and (iii) had more than 18 years of age. Exclusion criteria included (i) patients who are unable to give informed consent and (ii) patients with stoma.
The LARS score is a simple five-question tool that was first created in 2012 in Denmark and has been validated for the English translation in 2014 [10]. The internationally validated score attempts to allow a uniform assessment of function in patients undergoing low anterior resection. See Appendix.
The LARS questionnaire was posted to each patient and a follow-up telephone was made if they failed to respond. Clinical information regarding TNM staging, surgical intervention and neoadjuvant therapies was obtained from patients’ case notes and clinic letters. Clinical data from the questionnaires were collated and inserted into a LARS database. Patients were grouped into two separate cohorts—those with major LARS scores and those with mild/no LARS symptoms. Categorical outcomes were compared for the major LARS and minor LARS groups using conditional logistic regression. Non-parametric continuous data were compared using the Mann-Whitney test. The LARS database was prospectively updated. Statistical analysis was completed with SPSS (IBM version 20).
Results
Eighty-five patients were identified for this study. Of the 85 questionnaires that were sent out, 80% (n = 68) were completed and returned. The gender split within the cohort was 49 males:19 females. The median age at surgery was 67 years (range 30–88). A LARS score was calculated for each individual. Thirty-eight patients (56%) had major LARS symptoms, 18% had minor symptoms and 26% had no LARS symptoms (Table 1). The time interval between surgery and date of first completion of the LARS questionnaire was calculated; median time was 248 days (range 17–1664 days). Completed questionnaires were subdivided into the following definitive time periods following surgery: <1 year and >4 years. The median LARS score was 35.5 and 27.9, respectively. However, this was not statistically significant (p = 0.19).
Thirty-eight patients (58%) received no chemoradiotherapy. Nineteen patients (29%) underwent neoadjuvant chemoradiotherapy. Eleven patients received adjuvant chemoradiotherapy. Tumour height was determined from preoperative MRI scans. Thirty-one patients had a tumour at 10–15 cm, 32 patients had a tumour at 6–9.9 cm and 5 cases had a tumour at less than 6 cm.
Just over half of the patients (54%) had a defunctioning loop ileostomy created at the time of surgery. Complications were recorded and classified according to Clavien-Dindo criteria; complications of grade 3 or 4 severity required surgical intervention. Twenty-six patients (38%) developed a postoperative complication, of which eight were grade 3 and one was grade 4. Five patients (7%) developed an anastomotic leak within the postoperative period; each patient required surgical intervention (sepsis control and anastomosis takedown with subsequent reversal). Of the five anastomotic leaks, four patients reported major LARS symptoms. Despite a higher incidence of anastomotic leak within the major LARS cohort, this was not statistically significant in the logistic analysis. The time to loop reversal was also recorded. The median time for reversal was 214 days. This ranged from 50 to 1194 days. There was a significant time difference in the number of days until reversal between the major LARS and mild/no LARS cohort (288 days vs 167 days, respectively).
Univariate analysis was performed on individual patient factors to determine if there were any predisposing factors for LARS (Table 2). Of the patient factors, three were deemed to be statistically significant; neoadjuvant radiotherapy, whether an ileostomy was formed after initial procedure and time to ileostomy reversal (within 6 months). Further statistical analysis through a multivariant analysis highlighted that neoadjuvant treatment (radiotherapy) was associated with a nearly 20-fold increased risk of LARS (p < 0.01) and presence of an ileostomy for more than 6 months was associated with a 3.7-fold increased risk of LARS (p = 0.03). For those individuals who were defunctioned, the multivariate analysis suggested that stoma reversal within 6 months was protective against LARS (OR 0.2; p < 0.01).
Discussion
This study highlighted the significant range of functional outcomes following rectal surgery. More than half of the patients (56%) were identified as having major LARS symptoms. Our study highlighted that individuals who undergo chemoradiotherapy were nearly 20 times more likely to develop major LARS symptoms and that delayed closure of ileostomy was associated with 3.7-fold increase risk of major LARS symptoms. It may be suggested that those patients who experience a delay in ileostomy closure of more than 6 months are doing so because of complications and/or adjuvant therapy, all of which have previously been shown to be associated with poor function. We were able to capture high-quality data for a 5-year period and did not demonstrate a significant association for our study, although this may be due to the relatively small number of patients within the cohort. Larger studies are required to further assess the relationship between LARS and anastomotic leaks.
Nine percent (n = 5) of patients had an anastomotic leak, of which four patients had major LARS symptoms. Patients with a collection and associated low-level pelvic sepsis, not defined as a leak, would also contribute to the time delay of stoma reversal. Those patients with pelvic sepsis were considered to have an anastomotic leak, so these patients were captured but did not change the significance. Surgeons would opt for a deferred stoma reversal, in order to ensure that localized sepsis has fully resolved. Rahbari et al. (2010) highlighted that the definition of an anastomotic leak varies significantly within the literature [11].
The timing of the questionnaire completion from surgery did have a wide distribution (median 214 days; range 50 to 1194). It is known that the frequency of bowel dysfunction does improve over time, and ideally, we would have given the questionnaire at the same time points. The study does demonstrate that many patients experience distressing symptoms several years from the original surgery.
Although some of these patients are describing poor function less than 1 year after surgery, the frequency of this is also important to highlight. It has previously been shown that up to 75% of patients can experience significant bowel dysfunction for up to 12 months following surgery. If there is a way of reducing the incidence of bowel dysfunction by improving the time of reversal of ileostomy, then this warrants further investigation as this can be a socially crippling problem for not only the patient but also their family [5].
It is essential that patients are made aware and consented for the risk of developing prolonged bowel dysfunction preoperatively, particularly those undergoing neoadjuvant treatment, to allow them to discuss the options of permanent stoma versus a low anastomosis. Bowel dysfunction following rectal surgery significantly impacts the patient’s social functioning and quality of life [11].
The use of a temporary ileostomy to cover a pelvic anastomosis following low anterior resection is now considered standard practice for most colorectal surgeons in the UK (ACPGBI guidelines) and USA (SAGES guidance) [12, 13]. The use of a temporary ileostomy reduces the mortality associated with an anastomotic leak [14]. Standard timing for reversal is considered to be 3 months; however, there is limited evidence for optimal timings. A French randomized controlled trial looked at the complications associated with closure of ileostomy in selected patients at 7 days versus ‘late’ closure at 2 months, which demonstrated that early closure is feasible [15]. A protocol for an RCT (early closure of temporary ileostomy (EASY) trial) of early (<14 days) reversal of ileostomy was published; however, no records of recruitment or results are currently available [16].
The reality from the NBOCA data is that one in four patients in the UK will remain with a stoma 18 months postoperatively [3]. More precise information is needed on the reasons for this as pelvic sepsis/leak and adjuvant therapy are unlikely to account for all of these patients. In the UK, reversal of ileostomy is a benign procedure without a cancer-driven target and as such may be delayed due to a variety of factors including service demand pressures as well as patient recovery post surgical complications and chemoradiotherapy [17]. There is also the potential for performing the reversal earlier, despite adjuvant therapy. It has been reported that patients can have their stoma successfully reversed while undergoing chemotherapy and some centres elect to reverse the stoma between the second and third chemotherapy cycle [18]. Further research is being conducted to assess the optimal time interval between restoration of gastrointestinal tract continuity and commencing adjuvant chemotherapy. The CoCStom trial aims to compare early reversal (8–10 days post resection) against late reversal (following completion of adjuvant therapy at 26 weeks) [19]. Reported outcomes from the recent EASY (early vs late stoma closure following rectal surgery) trial advocates that early stoma closure (<13 days) is both safe and feasible. The study noted a significant reduction in the mean number of complications during the first year postoperatively in the cohort that underwent early stoma closure (p < 0.0001) [20].
There are limitations within this current study. Despite identifying 85 patients who met the study inclusion criteria, only 80% (n = 68) of the questionnaires were completed and returned. This may have been a consequence of the retrospective data collection that was undertaken. It is acknowledged that one of the main limitations of survey- or questionnaire-based research is ensuring a high response rate [21]. This aspect is difficult to control [21]. An 80% questionnaire return rate is comparable to other studies sending out postal questionnaires to a cohort. The missing 20% could result in overestimation of the frequency of LARS within the cohort. Another limitation of the study is the wide range observed between surgery and questionnaire completion. One of the principal aims of this study was to identify risk factors for the development of LARS. Thus, achieving the largest cohort of patients was most desirable. For this reason, a retrospective analysis of the departmental database enabled identification of all suitable patients between 2009 and 2014. Subsequently, patients who underwent early surgery (2009) would have contributed to the large time range between surgery and questionnaire completion observed. Caution is needed when interpreting the data regarding stoma reversal. Delays in stoma reversal may have been a direct consequence of postoperative morbidities such as an anastomotic leak. However, delays could also represent service and organizational failings within the hospital. Further research is needed to identify the specific reasons for delays in the restoration of gastrointestinal tract continuity.
Pelvic radiotherapy is associated with chronic gastrointestinal symptoms [22]. The symptoms range on a spectrum of severity and can significantly impact on the patients’ quality of life. Evidence has emerged from the current literature that these patients may benefit from input from a gastroenterologist [22]. Andreyev et al. (2013) published an algorithm-based care pathway for pelvic radiotherapy-induced chronic gastrointestinal symptoms [22]. The management of these patients should be multidisciplinary with surgical, gastroenterology, specialist nurse and dietician input.
The presence of a temporary ileostomy is an associated risk factor for bowel dysfunction following anterior resection [6, 23]. This is likely due to a number of factors including potential of sphincter injury during the construction of the anastomosis, alterations in anorectal physiology and the development of a pudendal neuropathy [8, 9]. It may also be postulated that the inactivity of the pelvic floor and sphincter complex for a prolonged period with an ileostomy could contribute to this.
Patients, in particular those undergoing neoadjuvant treatment, should be informed of the risk of postoperative bowel dysfunction and given appropriate strategies for coping with symptoms. Those patients having long-term symptoms should be referred to specialist services for consideration of additional treatments [24]. Patients with treated rectal cancer may have significant morbidity with LARS. Multispecialist input is necessary to ensure that patients who have curative treatment for their rectal cancer have optimal bowel function and quality of life.
Conclusion
This study concludes that risk factors for major LARS symptoms include neoadjuvant chemoradiotherapy and delayed closure of ileostomy. The reasons for delay in closure of ileostomy are multifactorial and may be partly be a proxy for complications. An anastomotic leak was more frequent in the major LARS group, but this did not reach statistical significance.
The data suggests that the timing of reversal of ileostomy should be monitored and, where safe and feasible, prioritized by surgeons and management. Further evidence is needed on the causes of delayed stoma closure.
References
Tilney HS, Heriot AG, Purkayastha S, Antoniou A, Aylin P, Darzi AW, Tekkis PP (2008) A national perspective on the decline of abdominoperineal resection for rectal cancer. Ann Surg 247:77–84. doi:10.1097/SLA.0b013e31816076c3
Morris E, Quirke P, Thomas JD, Fairley L, Cottier B, Forman D (2008) Unacceptable variation in abdominoperineal excision rates for rectal cancer: time to intervene? Gut 57:1690–1697. doi:10.1136/gut.2007.137877
Healthcare Quality Improvement Partnership (2015) National Bowel Cancer Audit Report 2015. Available at http://www.hscic.gov.uk/catalogue/PUB19500/nati-clin-audi-supp-prog-bowe-canc-2015.pdf. Accessed 1 Feb 2016
UK National Cancer Survivorship Initiative and Department of Health report (2013). Available at http://www.ncsi.org.uk/what-we-are-doing/proms/. Accessed 6 Dec 2015
Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL (2012) Anterior resection syndrome. Lancet Oncol 13:403–408. doi:10.1016/S1470-2045(12)70236-X
Ziv Y, Zbar A, Bar-Shavit Y, Igov I (2013) Low anterior resection syndrome (LARS): cause and effect and reconstructive considerations. Tech Coloproctol 17:151–162. doi:10.1007/s10151-012-0909-3
Chen TY-T, Emmertsen KJ, Laurberg S (2013) Bowel dysfunction after rectal cancer treatment: a study comparing the specialist’s versus patient’s perspective. BMJ Open 4(1):e003374. doi:10.1136/bmjopen-2013-003374
Matzel KE, Stadelmaier U, Muehldorfer S, Hohenberger W (1997) Continence after colorectal reconstruction following resection: impact of level of anastomosis. Int J Color Dis 12:82–87
Peeters KC, van de Velde CJ, Leer JW, Martijn H, Junggeburt JM, Kranenbarg EK, Steup WH, Wiggers T, Rutten HJ, Marijnen CA (2005) Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol 23:6199–6206. doi:10.1200/JCO.2005.14.779
Emmertsen KJ, Laurberg S (2012) Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 5:922–928. doi:10.1097/SLA.0b013e31824f1c21
Rahbari NN, Weitz J, Hohenberger W, Heald RJ, Moran B, Ulrich A, Holm T, Wong WD, Tiret E, Moriya Y, Laurberg S, den Dulk M, van de Velde C, Büchler MW (2010) Definition and grading of anastomotic leakage following anterior resection of the rectum: a proposal by the International Study Group of Rectal Cancer. Surgery 147:339–351. doi:10.1016/j.surg.2009.10.012
The Association of Coloproctology of Great Britain & Ireland (2007) Guidelines for the management of colorectal cancer 3rd edition. Available at http://www.acpgbi.org.uk/content/uploads/2007-CC-Management-Guidelines.pdf. Accessed 6 Dec 2015
Society of American Gastrointestinal and Endoscopic Surgeons (2012). Guidelines for laparoscopic resection of curable colon and rectal cancer. Available at http://www.sages.org/publications/guidelines/. Accessed 6 Dec 2015
Montedori A1, Cirocchi R, Farinella E, Sciannameo F, Abraha I (2010) Covering ileo- or colostomy in anterior resection for rectal carcinoma. Cochrane Database Syst Rev 12(5):CD006878. doi:10.1002/14651858.CD006878.pub2
Alves A, Panis Y, Lelong B, Dousset B, Benoist S, Vicaut E (2008) Randomized clinical trial of early versus delayed temporary stoma closure after proctectomy. Br J Surg 95:693–698. doi:10.1002/bjs.6212
Danielsen AK, Correa-Marinez A, Angenete E, Skullmann S, Haglind E, Rosenberg J, SSORG (Scandinavian Outcomes Research Group) (2011) Early closure of temporary ileostomy—the EASY trial: protocol for a randomized controlled trial. BMJ Open 1(1):e000162. doi:10.1136/bmjopen-2011-000162
Waterland P, Goonetilleke K, Naumann DN, Sutcliff M, Soliman F (2015) Defunctioning ileostomy reversal rates and reasons for delayed reversal: does delay impact on complications of ileostomy reversal? A study of 170 defunctioning ileostomies. J Clin Med Res 7:685–689. doi:10.14740/jocmr2150w
Chand M, Nash GF, Talbot RW (2008) Timely closure of loop ileostomy following anterior resection for rectal cancer. Eur J Cancer Care (Engl) 17:611–615. doi:10.1111/j.1365-2354.2008.00972.x
Sandra-Petrescu F, Herrle F, Hinke A et al (2015) CoCStom trial: study protocol for a randomised trial comparing completeness of adjuvant chemotherapy after early versus late diverting stoma closure in low anterior resection for rectal cancer. BMC Cancer 15:923. doi:10.1186/s12885-015-1838-0
Danielsen A, Park J, Jansen JE, Bock D, Skullman S, Wedin A et al (2016) Early closure of a temporary ileostomy in patients with rectal cancer: a multicenter randomized controlled trial. Ann Surg. doi:10.1097/SLA.0000000000001829
Mann C (2003) Observational research methods. Research design II: cohort, cross sectional, and case-control studies. Emerg Med J 20:54–60. doi:10.1136/emj.20.1.54
Andreyev HJ, Benton BE, Lalji A, Norton C, Mohammed K, Gage H, Pennert K, Lindsay JO (2013) Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. Lancet 382:2084–2092. doi:10.1016/S0140-6736(13)61648-7
Emmertsen KJ, Laurberg S (2012) Low anterior resection syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg 255:922–928. doi:10.1097/SLA.0b013e31824f1c21
Visser WS, te Riele WW, Boerma D, van Ramshorst B, van Westreenen HL (2014) Pelvic floor rehabilitation to improve functional outcome after a low anterior resection: a systematic review. Annals of Coloproctology 30:109–114. doi:10.3393/ac.2014.30.3.109
Acknowledgements
The authors thank the LARRIS (Low Anterior Resection and Rectal Irrigation Study) Trial Management Group: Cadogan J, Cornish JA, Davies Z, Edwards K, Hughes D, Keenan S, Lovell-Smith C, McCutchan G, Morris C, O’Neill C, Power K, Torkington J, Turner J and Williams C.
Author contribution
Each author named in this paper significantly contributed to the creation of this manuscript. D Hughes was involved with acquisition of data, analysis and interpretation of data. J Cornish was involved with the design creation of the study, data collection and subsequent analysis of the data. C Morris was involved with study design, data collection and statistical interpretation of gathered data. All three authors have read the final manuscript prior to submission.
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Financial disclosures
This research was supported with a grant from Tenovus (Charity Number 1054015).
Conflict of interest
The authors declare that they have no conflict of interest.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
This study has been presented as an oral presentation by Daniel Hughes at the Welsh Surgical Society Autumn Meeting at the Morriston Hospital, Swansea, Wales, UK. Meeting dates are 26–27th of November 2015. The study has also been presented as a poster presentation at the Association of Coloproctology of Great Britain and Ireland Annual Meeting in Edinburgh, Scotland, UK. Conference dates are 4–6th July 2016.
Appendices
Appendix
On behalf of the LARRIS Trial Management Group
Cadogan J
Cornish JA
Davies Z
Edwards K
Hughes D
Keenan S
Lovell-Smith C
McCutchan G
Morris C
O’Neill C
Power K
Torkington J
Turner J
Williams C
Appendix LARS questionnaire

Rights and permissions
About this article
Cite this article
Hughes, D.L., Cornish, J., Morris, C. et al. Functional outcome following rectal surgery—predisposing factors for low anterior resection syndrome. Int J Colorectal Dis 32, 691–697 (2017). https://doi.org/10.1007/s00384-017-2765-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-017-2765-0