Abstract
Purpose
In recent years, colorectal cancer surgery has benefitted from new techniques such as laparoscopy and robotic surgery. However, many treatment disparities exist among different centers for patients affected by the same kind of tumors.
Methods
Forty-five (41%) open (OCO) vs. 30 (28%) laparoscopic (LCO) vs. 34 (31%) robotic-assisted (RCO) colectomies and 34 (40%) open (ORR) vs. 52 (60%) robotic (ROR) rectal resections performed during a 15-month period, in elective setting, were compared. Patients presenting contraindications for minimally invasive procedures were excluded from the study, so that all the enrolled patients were suitable for either of the surgical procedures.
Results
Overall morbidity rates were similar among groups. Perioperative mortality was nil. No significant differences were noted as for total number of lymph nodes harvested between arms. Mean time (days) to first bowel movement to gas was 3.3 vs. 2.3 vs. 2.6 for OCO, LCO, and RCO, respectively (p < 0.001), and 3.3 vs. 2.0 for ORR and ROR, respectively (p = 0.003). Among several European Organization in Research and Treatment of Cancer QLQ-C30 functional scales considered only physical functioning was significantly better at 30 days for RCO vs. OCO (96.3 ± 10 RCO vs. 85.5 ± 12.6 OCO; p = 0.015). Robotic surgery was much more expensive in comparison to open as well as laparoscopic procedures.
Conclusions
Laparoscopic and robotic surgeries for colorectal cancer present both the same advantages in comparison to open procedures in terms of faster recovery. However, our data do not seem to support the routine use of RCO as a cost-effective procedure.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Surgical care today for colorectal cancer patients can benefit from new technologies such as laparoscopic and robotic surgeries, and more recently by evidence-based enhanced recovery programs like fast-track surgery (FTS) [1–3]. However, clear indications for their use are lacking, in particular for locally advanced cancers. Quality-of-life (QoL) indicators have been evaluated for these patients in recent years [4, 5], as well as more traditional outcomes such as perioperative mortality, complications, long-term survival rates, and all these have been taken into consideration to assess the overall appropriateness of the treatment delivered. Moreover, new concepts especially regarding colonic cancer surgery have recently been addressed focusing on the oncologic quality of surgery [6]. This is reflected by the quality of the specimen both for colon and rectal cancers, the status of the circumferential resection margin (CRM) in rectal cancer, the distance of the tumor to the vascular tie, rectal or colonic inadvertent perforation and the number of lymph nodes retrieved [6, 7]).
The aim of the study was to provide a prospective registration of data concerning colorectal cancer resections using three different approaches (open vs. laparoscopic vs. robotic), where all the patients enrolled were suitable for any one of the procedures, and to define the cost-effectiveness, the oncologic quality of surgery and QoL following each treatment modality.
Patients and methods
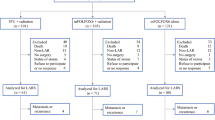
This is an observational cohort prospective study. Data from 195 consecutive and unselected patients affected by colorectal cancer who underwent elective radical resection during a 15-month period were prospectively collected. Study tasks comprised systematic registration of data regarding the surgical procedures and the postoperative period. This study was approved by our institutional review board, and written informed consent from each patient was obtained. Exclusion criteria were: cancer with intestinal obstruction or perforation, local tumors that were resectable via transanal access, adjacent organ invasion requiring en bloc multiorgan resections, distant metastasis, and previous open abdominal surgery for cancer. The choice from among three different surgical approaches for colon cancer (open, laparoscopic, and robotic) and two for rectal cancer (open and robotic) was based on the skill and experience of the referring physician. Patients presenting a contraindication to a minimally invasive approach were excluded from the study.
Preoperative staging was performed using a thoraco-abdominal computed tomography scan, abdominal magnetic resonance imaging, and endoscopic ultrasound as single modalities or in combination depending on surgeon preference. All patients underwent at least one form of preoperative imaging for staging purposes. Patients whose tumor was localized within 12 cm of the anal verge, as defined by Nelson et al. [8], were treated according to institutional policy with elective total mesorectal excision (TME). Rectal cancer patients with locally advanced tumors (T3, T4, or N positive) defined by preoperative staging investigations, received conventional radiotherapy for a total of 50.4 Gy and concomitant chemotherapy.
Preoperative antibiotics (cephoxitin, 2 g) and antithrombotic prophylaxis (low molecular weight heparin) were administered before surgery. Mechanical bowel preparation (MBP) with polyethylene glycol was administered the day before surgery according to surgeon preference. Some patients enrolled in this study were also randomized to receive MBP or not in a trial started at our Institute in October 2007 (NCT00940030).
Surgical technique
Rectal resections were performed in cases of tumors located within 12 cm above the anal verge (by rigid sigmoidoscopy) and colectomies for all the remaining cases.
Tumor resections were performed en bloc after the complete mobilization of the right colon and ligation of the ileocolic and, where present, the right colic vessels at the origin from the superior mesenteric vein and artery, respectively. In cancers of the transverse colon, middle colic vessels were usually ligated after mobilization of hepatic and splenic flexure. For cancers involving the splenic flexures and proximal descending colon, the root of the inferior mesenteric artery was usually preserved, with a central tie of the left ascending colonic artery. In cancer of the middle down to the sigmoid colon, left colectomy was performed with ligation of the root of the inferior mesenteric artery (Fig. 1) and the vein below the pancreas (Fig. 2). Proximal colonic division was performed depending upon the site of tumor, with the distal transection line always at the upper third of the rectum. Anastomoses were established by stapling devices, usually performed in an end-to-end fashion for left and rectal resections and were termino-lateral for right colon resections. In low rectal cases, we performed anastomoses according to Knight–Griffen’s technique [9]. Staplers were routinely used. Standard resections were defined as tumor resections including standard lymph nodes dissections restricted to the tumor-bearing bowel section [10]. Anterior and abdominoperineal resections of rectum were invariably performed employing the TME technique for both arms. Robotic surgery was performed employing the Da Vinci System® in all cases. Open procedures were performed by seven surgeons (BA, RB, AC, PB, EB, FL, and SP), laparoscopic by two surgeons (PB and CC) and robotic by three surgeons (RB, PB, and FL), each one having performed at least 30 colorectal resection/technique (study arm) at the time of first patient’s enrolment in this study. In Fig. 3, the moving average curves show no significant changes in operating time for all 86 robotic colorectal resections, indicating that the first plateau was reached before the study was started. Indication for minimally invasive approach for colon and rectal cancer patients was based on the referring physician’s surgical experience and preference.
In laparoscopic colectomies (LCO), intracorporeal anastomoses were usually fashioned [11]. Full robotic left colon and rectal resections were always performed with robotic mobilization of the splenic flexure and intracorporeal anastomosis in all cases [12]. The distal rectum was stapled laparoscopically, and the specimen extracted through a 7-cm transversal incision in the lower abdominal quadrant—usually enlarging a robotic trocar access—when a low anterior resection was performed. In case of intersphyncteric rectal resection, no suture line was applied to the rectum and the specimen was retrieved through the intersphyncteric perineal access, followed by sectioning the colon at the desired level and performing a hand-sewn double-layered colo-anal anastomosis at the dentate line. In robotic right colectomies, the anastomosis was extracorporeal.
Clinicopathological data
We prospectively registered type and duration of surgery, postoperative hospital stay, intraoperative bleeding (mL), perioperative blood transfusions, overall complication rate, anastomotic dehiscence, wound infection, intraabdominal abscesses, postoperative day of first bowel movement to gas and stool and postoperative day of resumption of solid diet. For this, we defined the surgical site infections (SSI) as follows [13]:
-
1.
Anastomotic dehiscence: any anastomotic leak verified at surgery, at contrast radiography or evidence of faecal discharge from drain
-
2.
Wound infection: superficial infection needing surgical drainage of the wound associated with positive bacterial culture
-
3.
Deep abscess: deep infection verified by radiography or surgery needing surgical and/or antimicrobial therapy
Pneumonia was defined as abnormal chest radiographwith fever (>38°C) and a white blood cell count exceeding 12,000 cells/μl with a positive culture of sputum or at bronchoalveolar lavage. We assumed overall morbidity to include grades I–IV surgical complications as defined by Dindo et al. [14].
All patients were closely followed postoperatively with two independent observations per day. Solid diet was administered to patients within 12 h from the first bowel movement to gas. Patients were discharged according to the enhanced recovery after surgery (ERAS) [15] discharge criteria (1, adequate analgesia with oral medication; 2, tolerating oral intake and passage of flatus; 3, adequate and safe mobility).
We registered the total number of lymph nodes harvested and calculated the lymph node ratio as the number of positive lymph nodes divided by the total number of lymph nodes within one sample. We reported the length of the specimen (mm) and the tumor distance to the closest margin (mm). In rectal cancer, we evaluated the CRM positivity rate.
QoL data
Quality of life was measured using the European Organization for Research and Treatment of Cancer (EORTC) QLQ C-30, developed by the EORTC study group [16, 17]. This represents a frequently used (internationally), validated, 30-question cancer-specific health-related questionnaire. In this study, we analyzed the global QoL score and all functional (physical, emotional, role, social, and cognitive) and symptom (fatigue, pain, insomnia, nausea, and vomiting) scales as well as financial and global health status. Each item has four answer categories: 1 = not at all, 2 = a little, 3 = rather a lot and 4 = very much. Scores were transformed into a scale from 0 to 100 according to the manual on which a higher global QoL score and higher functional scores corresponded with better QoL. Patients were given the questionnaire at discharge, and were asked to fill it in 30 days after surgery.
Administrative (cost) data
We considered the following cost items for each surgical procedure:
-
1.
Diagnostic (preoperative blood analysis, chest radiogram, ECG, and anesthesiology evaluation)
-
2.
Pathologic (specimen pathology evaluation)
-
3.
Drugs and materials
-
4.
Disposable instruments (e.g., staplers, trocars, energy cautery devices, etc.)
-
5.
Robot depreciation costs
-
6.
Hospital stay costs
-
7.
Indirect costs
-
8.
Personnel costs
Da Vinci® depreciation costs were calculated assuming that the robot system is routinely employed in general surgery, urology, gynecology, thoracic surgery, and head and neck surgery. In our Institute, about 15 robotic procedure are performed per week overall.
Statistical analysis
For the scope of the study, we performed subgroup analysis for colon resections establishing three surgical arms according to surgical approach: open colectomies (OCO), laparoscopic colectomies (LCO), and robotic colectomies (RCO) and two arms for rectal resections: open rectal resections (ORR) and robotic rectal resections (ROR).
Preoperative (age, gender, ASA class, and neoadjuvant therapy, BMI, previous laparotomies) and pathological (pT3–4 tumors, pN+ tumors) characteristics, clinical results (duration of surgery, hospital stay, intraoperative bleeding, post-operative day of first bowel movement to gas and oral alimentation, overall morbidity, wound infections, anastomotic leaks, and abdominal abscess) and QoL scores were summarized according to surgery and intervention using either mean, median, and standard deviation or counts and percentages for continuous and categorical variables, respectively.
Normality assumption was checked using the Kolmogorov–Smirnov test. Between-group comparisons were conducted using the Kruskal–Wallis or the Wilcoxon two-sample two-sided test for non-normal continuous data or chi-square and Fisher’s exact test as appropriate.
The proportion of patients exceeding a given QoL score for the Physical functioning domain and for Pain was plotted against the score according to surgery. A time series for the duration of surgery was constructed and plotted against the sequential case index and tested for randomness (e.g., no trend for duration of surgery) using a Wald–Wolfovitz test [18]. Costs were calculated as weighted averages.
Results
Clincopathological findings
From February 2009 to April 2010, 365 patients underwent colorectal resection for primary cancer at the European Institute of Oncology, Milan. Among these, 152 were excluded from the present study mainly due to one or more contraindications to laparoscopic or robotic surgery such as previous major abdominal surgery and/or tumor infiltrating at least one adjacent organ and 18 patients were also excluded because of the presence of synchronous liver and/or lung metastases. Accordingly, the body of the analysis comprised a total of 195 patients (113 males and 82 females; median age, 63 years; range, 26–82 years) who underwent colectomy (109 cases) and rectal resection (86 cases). Among the 109 colectomies, we performed 45 OCO (41%), 30 LCO (28%), and 34 RCO (31%). Among the 86 rectal resections, there were 34 ORR (40%) and 52 ROR (60%). Patient characteristics according to arm are reported for colectomies and rectal resections in Tables 1 and 2, where there are no statistically significant differences among groups at baseline. Duration of surgery was significantly longer for laparoscopic and robotic procedures in comparison to open procedures both for colectomies and rectal resections (Tables 3 and 4). Conversion to open surgery rates were 2/30 (7%) for LCO, 2/34(6%) for RCO, and 2/52 (4%) for ROR. No significant differences were noted considering intraoperative bleeding (Tables 3 and 4) between groups, although ORR was associated with higher but still not significant intraoperative bleeding in comparison to ROR. There were two cases of peritonitis due to an inadvertent perforation of the small bowel, one following a laparoscopic right colectomy and the other after a robotic low anterior resection of rectum. In both cases, patients were reoperated on postoperative day 1. No other intraoperative complications that were recognized during surgery occurred. There were five of 34 (15%) and four of 52 (8%) abdominoperineal resections in ORR vs. ROR (p = 0.349) and 17/29 (59%) vs. 35/48 (73%) diverting stomas, respectively (p = 0.134). Minimally invasive procedures showed a better outcome in terms of both time to first bowel movement to gas (days) and time to resumption of solid diet (days) both for colectomy and rectal resection arms (Tables 3 and 4).
No significant differences regarding overall morbidity rates (grades I–IV according to Dindo et al. [14] were noted among the different arms (Tables 3 and 4). Perioperative mortality (within 30 days from surgery) was nil. The same result was obtained considering SSI complications separately where anastomotic dehiscence rates registered were two of 45 (4%) OCO vs. one of 30 (3%) LCO vs. one of 34 (3%) RCO (p = 0.869) and three of 34 ORR (9%) vs. six of 52 ROR (12%) (p = 0.311). A significant difference in wound infections was registered in favor of ROR vs. ORR (1/52, 2% vs. 5/34, 15%, respectively, p = 0.034).
Postoperative hospital stay was significantly shorter for LCO vs. OCO (mean, 5.3 days vs. 7.4 days, respectively; p < 0.001) and for ROR vs. ORR (7.9 vs. 8.7, respectively; p = 0.004) (Tables 3 and 4).
At pathology evaluation, there were no differences in the distribution of locally advanced cases pT3–4 and/or N-positive tumors between arms (Tables 5 and 6). In N1/2 (stage III) patients, there was a significantly lower lymph node ratio for RCO, in comparison with OCO (0.25 OCO vs. 0.07 RCO; p = 0.014. The length of bowel resected was significantly longer following either laparoscopic or robotic procedures in comparison to open procedures both for colectomies and rectal resections (see Tables 5 and 6).
QoL data
Of the 195 EORTC QLQ-C30 questionnaires administered, 131 (67%; 71 for colon and 60 for rectal subgroups) were returned after a median of 34 days (range, 28–42 days). We evaluated all functional and symptom scales. No significant differences were registered between arms, except for physical functioning which was better for robotic procedures in comparison to open resections overall (RCO, 96.3 ± 10.0 vs. OCO, 85.5 ± 12.6; p = 0.015). The inferior non-significant difference between arms was noted for RCO, 90.9 ± 15.2 vs. OCO, 80.4 ± 23.6 as for role functioning scale (p = 0.067). Physical functioning was significantly better after RCO vs. OCO (93.6 ± 8.1 vs. 88.2 ± 11.3; p = 0.02) (Fig. 4a) as well as after ROR vs. ORR (89.9 ± 9.4 vs. 83.6 ± 10.2) (p = 0.03) (Fig. 4b). For patients who underwent rectal resection the impact of a stoma was not significant for each functional and symptom scale. In particular, we registered the scores of 85.0 ± 16.7 vs. 84.3 ± 11.2 (p = 0.41) for physical and 67.1 ± 9.2 vs. 62.2 ± 14.3 (p = 0.48) for social functioning and a global health status of 84.5 ± 12.5 vs. 87.2 ± 15.3 (p = 0.95) in patients with or without a stoma, respectively.
a QoL scores for patients undergoing who underwent colectomy resection (p = 0.02 in favor of robotic resection for physical functioning). Values calculated on 71 questionnaires returned out of 109 (65.1%); b QoL scores for patients undergoing who underwent rectal resection (p = 0.03 in favor of robotic resection for physical functioning). Values calculated on 60 questionnaires returned out of 86 (69.8%)
Costs issues
Considering hospital costs, robotic surgery was more expensive in comparison to open or laparoscopic procedures, mainly due to the Da Vinci depreciation costs (Tables 7 and 8). RCO was 2059 € more expensive than LCO and 2139 € than OCO. ROR was associated with an increase in costs of 1356 € in comparison to ORR.
Discussion
We found minimally invasive colorectal surgery to be associated with a faster recovery in terms of bowel function and resumption of solid diet than traditional open surgery. Following this, we noted a shorter hospital stay for laparoscopic and robotic procedures vs. open surgery in comparison to open procedures, using ERAS criteria for hospital discharge. No significant difference was noted regarding postoperative complications, as well as for intraoperative bleeding or the need of blood transfusions, although a significant shorter duration of surgery was registered for OCO and rectal resections. Regarding QoL, a significantly better physical functioning was found following robotic procedures among groups as evaluated by the EORTC QLQ-C30 questionnaire. Nonetheless, we reported increased costs for robotic surgery. No substantial short-term advantages were noted for robotic vs. laparoscopic colon resections.
COST Study Group, MRC CLASICC, COLOR, and ALCCaS randomized trials [4, 5, 19, 20] advocated the superiority of laparoscopic colon surgery in comparison to open surgery when considering short-term outcomes. After those trials were initiated, the introduction of several perioperative treatment protocols (FTS and ERAS criteria) pointed out the need for health care providers to establish which treatment was to be considered appropriate for each patient. Although many of the abovementioned papers compared open vs. laparoscopic colon resection, no literature exist comparing open vs. laparoscopic vs. RCO.
In our study, patients were not treated within a formally addressed FTS protocol: indeed it is debatable whether better recovery following minimally invasive procedures would be confirmed if all our patients were treated assuming the same FTS elements. On this aspect, the blinded trial by Basse et al. [21] was unable to demonstrate any difference between laparoscopic and open colorectal surgery within a multimodal rehabilitation protocol, as regards length of hospital stay or functional recovery. Another trial [22], which was not blinded, suggested a superiority of laparoscopy (hospital stay significantly shorter, 5.2 vs. 7.4 days and better performance score in day 2), but one cannot exclude a placebo effect due to the absence of blinding. These conflicting results have also been reported in non randomized studies. However, several questions remain to be answered regarding the cost-effectiveness of fast-track laparoscopy compared with fast-track laparotomy. The ongoing LAFA trial [23] could answer these questions.
Some authors have recently advocated the robotic option as a possible way to facilitate the adoption of minimally invasive rectal surgery [24] with no detrimental effects on oncologic outcomes [25]. In this respect, the oncologic quality of surgery was not affected by the laparoscopic or robotic approach both for colon and rectal resections. Moreover, lymph node ratio in stage III patients was more favorable after RCO. Most notably, our series of minimally invasive-treated patients is composed of a high proportion of T3–4 cases both in colectomies and rectal resections.
On this topic, two comparative, non-randomized studies have recently been published [26, 27]. One of these was by our group, which compare robotic and laparoscopic TME for rectal cancer, where no substantial differences in short-term outcomes were reported between robotic and laparoscopic rectal resections. However, the authors of both papers concluded that for rectal resections with TME the technical advantages of the robotic surgical system made it easier to adopt as an alternative approach to open surgery, in comparison to laparoscopy.
This is the first study considering QoL issues in a population of patients who underwent robotic colorectal resection. We found a better physical functioning for robotic procedures after 1 month in comparison to open procedures and no difference between the laparoscopic and robotic approach. This positive effect of robotic surgery on physical functioning was confirmed both for colectomies and rectal resections separately.
This study presents several weak points. First, this is not a randomized study, so any new possible advantage for laparoscopic or robotic procedures demonstrated should be carefully considered (e.g., QoL results, lymph node ratio). Second, we did not administer the EORTC QLQ-C30 to patients before surgery, and QoL results could be affected by the fact that our study is not blind. Indeed, this issue is quite complex to manage, where heterogeneity of the colorectal cancer patients population, especially related to whether or not preoperative chemo-radiation was administered, stage of disease and patients’ expectations for different type of surgical technologies used, can cause confounding information at baseline. Finally, we did not provide a laparoscopic rectal resection arm, due to the policy of our institution. Our reasons take into account the strategic value of this choice with the commencement of robotics as the leading procedure for minimally invasive surgery at that time and the need to avoid a too long learning curve, resulting from a small number of robotic-treated rectal cancers.
We found that RCO was more expensive than LCO, and this is consistent with recently published papers [28, 29]. This difference was quite high in particular for robotic in comparison with LCO (2059 €), despite there being no difference in complications, advantages in a faster recovery and QoL after 1 month. However, we believe that this finding deserves careful consideration. Although our analysis was quite accurate for hospital costs, assuming direct and indirect costs, we did not take into account the possible advantages in terms of society costs for minimally invasive procedures, as a consequence of a faster recovery. Moreover, we reported a significantly higher incidence of wound infections for ORR in comparison to ROR, where this kind of surgical complication has been demonstrated to correlate with increased costs [30].
Abbreviations
- FTS:
-
Fast-track surgery
- QoL:
-
Quality of life
- CRM:
-
Circumferential resection margin
- CT:
-
Computed tomography
- TME:
-
Total mesorectal excision
- MBP:
-
Mechanical bowel preparation
- IMV:
-
Inferior mesenteric vein
- IMA:
-
Inferior mesenteric artery
- SSI:
-
Surgical site infection
- ERAS:
-
Enhanced recovery after surgery
- EORTC:
-
European Organization in Research and Treatment of Cancer
- OCO:
-
Open colectomies
- LCO:
-
Laparoscopic colectomies
- RCO:
-
Robotic colectomies
- ORR:
-
Open rectal resections
- ROR:
-
Robotic rectal resection
- BMI:
-
Body mass index
- LTME:
-
Laparoscopic TME
References
Phillips EH, Franklin M, Carroll BJ et al (1992) Laparoscopic colectomy. Ann Surg 216(6):703–707
Spinoglio G, Summa M, Priora F et al (2008) Robotic colorectal surgery: first 50 cases experience. Dis Colon Rectum 51(11):1627–1632
Kehlet H (2008) Fast-track colorectal surgery. Lancet 371(9615):791–793
Weeks JC, Nelson H, Gelber S et al (2002) Clinical Outcome of Surgical Therapy (COST) Study Group. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA 278:321–328
Guillou PJ, Quirke P, Thorpe H et al (2005) Short term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 365:1718–1726
Hohenberger W, Weber K, Matzel K et al (2009) Standardized surgery for colonic cancer: complete mesocolic excision and central ligation—technical notes and outcome. Colorectal Dis 11:354–365
West NP, Hohenberger W, Weber K et al (2009) Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 28:272–278
Nelson H, Petrelli N, Carlin A et al (2001) Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 93:583–596
Griffen FD, Knight CD, Whitaker JM Sr et al (1990) The double stapling technique for low anterior resection. Results, modifications and observations. Ann Surg 211:745–752
Chiappa A, Biffi R, Bertani E et al (2006) Surgical outcomes after total mesorectal excision for rectal cancer. J Surg Oncol 94(3):182–193
Bianchi PP, Ceriani C, Rottoli M et al (2007) Laparoscopic lymphatic mapping and sentinel lymph node detection in colon cancer: technical aspects and preliminary results. Surg Endosc 21(9):1567–1571
Luca F, Cenciarelli S, Valvo M et al (2009) Full robotic left colon and rectal cancer resection: technique and early outcome. Ann Surg Oncol 16(5):1274–1278
Horan TC, Gaynes RP, Martone WJ et al (1992) CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol 13(10):606–608
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients as results of a survey. Ann Surg 240(2):205–213
Wind J, Polle SW, Fung Kon Jin PH et al (2006) Systematic review of enhanced recovery programmes in colonic surgery. Br J Surg 93:800–809
Aaronson NK, Ahmedzai S, Bergman B et al (1993) The European Organisation for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 85:365–376
Fayers PM, Aaronson NK, Bjordal K et al (2001) The EORTC QLQ-C30 Scoring Manual, 3rd edn. European Organisation for Research and Treatment of Cancer, Brussels
Wald A, Wolfowitz J (1940) On a test whether two samples are from the same population. Ann Math Stat 11:147–162
Veldkamp R, Kuhry E, Hop WC et al (2005) Colon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 6:477–484
Hewett PJ, Allardyce RA, Bagshaw PF et al (2008) Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer. The ALCCaS Trial. Ann Surg 248:728–738
Basse L, Jakobsen DH, Bardram L et al (2005) Functional recovery after open versus laparoscopic colonic resection: a randomized, blinded study. Ann Surg 241:416–423
King PM, Blazeby JM, Ewings P et al (2006) Randomized clinical trial comparing laparoscopic and open surgery for colorectal cancer within an enhanced recovery programme. Br J Surg 93:300–308
Wind J, Hofland J, Preckel B et al (2006) Perioperative strategy in colonic surgery; laparoscopy and/or fast track multimodal management versus standard care (LAFA trial). BMC Surg 6:16
Hellan M, Anderson C, Ellenhorn JDI et al (2007) Short-term outcomes after robotic-assisted total mesorectal excision for rectal cancer. Ann Surg Oncol 14(11):3168–3173
Baek JH, McKenzie S, Garcia-Aguilar J et al (2010) Oncologic outcomes of robotic-assisted total mesorectal excision for the treatment of rectal cancer. Ann Surg 251(5):882–886
Park JS, Choi GS, Lim KH et al (2010) Robotic-assisted versus laparoscopic surgery for low rectal cancer: case-matched analysis of short-term outcomes. Ann Surg Oncol 17(12):3195–3202
Bianchi PP, Ceriani C, Locatelli A et al (2010) Robotic versus laparoscopic total mesorectal excision for rectal cancer: a comparative analysis of oncological safety and short-term outcomes. Surg Endosc 24(11):2888–2894
deSouza AL, Prasad LM, Park JJ et al (2010) Robotic assistance in right hemicolectomy: is there a role? Dis Colon Rectum 53(7):1000–1006
Maeso S, Reza M, Mayol JA et al (2010) Efficacy of the Da Vinci surgical system in abdominal surgery compared with that of laparoscopy: a systematic review and meta-analysis. Ann Surg 252(2):254–262
Smith RL, Bohl JK, McElearney ST et al (2004) Wound infection after elective colorectal resection. Ann Surg 239(5):599–605
Acknowledgments
The authors thank William Russell-Edu for help with English and Liliana Tadini, Marina Mancini (research nurses), Laura Ogliari, Marisa Lamberti, (scrub nurses), Alfonso Lorusso (administrative staff) for their contribution to this study.
Conflict of interest
All the Authors declare no potential conflict of interests connected with this paper.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bertani, E., Chiappa, A., Biffi, R. et al. Assessing appropriateness for elective colorectal cancer surgery: clinical, oncological, and quality-of-life short-term outcomes employing different treatment approaches. Int J Colorectal Dis 26, 1317–1327 (2011). https://doi.org/10.1007/s00384-011-1270-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-011-1270-0