Abstract
Background
Laterally spreading tumors (LSTs) are being increasingly reported nowadays in Japan and the western countries with the application of magnification chromoendoscopy. The aim of this study was to analyze the clinicopathologic features of LSTs and to assess the outcome and safety of endoscopic mucosal resection (EMR) in China.
Patients and methods
One hundred nine patients with LSTs who underwent magnification chromoendoscopy were studied retrospectively. Clinicopathological features of 111 LSTs were analyzed. The efficacy and safety of EMR was assessed in 79 LSTs based on the outcome of follow-up colonoscopy and resection-related complications.
Results
A total of 111 LSTs were diagnosed in 109 patients, including 89 (80%) laterally spreading tumor-granular (LST-G) type and 22 (20%) laterally spreading tumor-non-granular (LST-NG) type. There was significant difference in the dominant pit pattern between LST-G type and LST-NG type (p < 0.001). Type IV pit pattern (62%) was the main crypt pattern in LST-G type; whereas, type IIIL (50%) and type V pit pattern (36%) were predominant crypt patterns in LST-NG type. EMR was performed for 103 lesions. Six of the nine lesions with type VI pit pattern were completely resected by EMR. Eleven (14%) local recurrent lesions were detected in 79 follow-up lesions and were treated successfully during the follow-up.
Conclusions
The type of dominant pit pattern was different between LST-G type and LST-NG type. Many LSTs with a type VI pit pattern can be completely resected by EMR. EMR technique is a safe and efficacious treatment method for LST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term “laterally spreading tumors” (LSTs) has been proposed by Japan in recent years to define a specialized flat neoplasia greater than 10 mm in diameter, extending laterally and circumferentially along the interior luminal wall. These lesions are divided into two subtypes based on endoscopic morphology: laterally spreading tumor-granular (LST-G) type and laterally spreading tumor-non-granular (LST-NG) type. With wider application of magnification chromoendoscopy, the incidence of LSTs reported has been increasing recently. Their clinicopathologic features and the outcome of endoscopic mucosal resection (EMR) have been evaluated in Japan and the western countries in recent years. Some of the clinicopathologic features could be considered as indicators of submucosal invasion, such as tumor size, large nodule, and pit pattern [1, 2], which might influence further management, since it is important to avoid either incomplete treatment or unnecessary surgery.
In previous studies, LSTs with a type V pit pattern were excluded from EMR and were resected by surgery. However, the type V pit pattern was classified as two subtypes: type VI and type VN. Recent studies reported that a type VI pit pattern mainly occurred in lesions with dysplasias or lesions with depth of submucosal invasion less than 1,000 μm [3, 4]. As a special nonpolypoid tumor, LST should be studied in the relationship between the clinicopathological features and treatment methods. Although the clinicopathologic features of LSTs and efficacy of EMR have been reported by Japan and western counties, no data has been published from China. So, we retrospectively analyzed the clinicopathological features of LSTs and evaluated the safety and efficacy of EMR. We found the type of dominant pit pattern was different between LST-G type and LST-NG type. Many LSTs with a VI pit pattern could be completely resected by EMR. In addition, EMR technique was a safe and efficacious treatment method for LST.
Patients and methods
Data was collected retrospectively from the endoscopic center of Gastroenterology Department at the Nanfang Hospital, Guangzhou. We used Dizhongtian computerized patient record system (Shenzhen Dizhongtian Electronic Technique Co., Ltd.) to review all patients with LSTs who underwent total colonoscopy between August 2000 and June 2007. Patients with familial adenomatous polyposis or inflammatory bowel disease were excluded. A total of 111 colorectal LSTs in 109 patients met study inclusion criteria and were resected endoscopically or surgically.
EMR technique
All colonoscopy was performed by an experienced endoscopist using Olympus CF-240Z or Fujinon EC-590ZW magnifying colonoscope. When a lesion was detected by conventional endoscopic examination, its surface was washed with water before spraying 5 mL of 0.4% indigo carmine directly through the biopsy channel of the endoscope. Following chromoscopy, the lesion underwent magnification and was observed carefully to identify the surface pit pattern. Lesions with pit pattern type IIIs, IIIL, IV, and V were regarded as neoplasias according to the classification system proposed by Kudo et al. (Table 1) [5].
After detailed magnification chromoendoscopy, EMR was then performed with en bloc or piecemeal resection following submucosal normal saline injection by using a snare and pure cutting current. Normal saline was injected with a 23-gauge needle (Cook Endoscopy, Winston-Salem, NC, USA). Injection volume of normal saline varied based on the size of the lesion. The lesion was carefully observed during and after submucosal injection to assess for the lift sign. It was regarded as a positive lift sign if the lesion was symmetrically raised by injecting normal saline into the submucosal layer. Conversely, an asymmetrical lift or no lift was considered as negative lift sign. A barbed snare was used for all EMRs with a “cut” mode 30 W Erbe diathermy (Erbe Co., Tuebingen, Germany). Following initial EMR, 0.4% indigo carmine was again sprayed on the lesion so that any residual neoplastic tissue could be identified and further resected. The following types of lesions were excluded from EMR:
-
1.
Presence of an invasive type VN pit pattern,
-
2.
Lesions with non-lifting sign on submucosal normal saline injection, and
-
3.
Lesions where anatomical location precluded endoscopic access for resection.
If lesions belonged to one of the above categories or if there was incomplete resection by EMR, as proven by positive cut margins with carcinoma cells on histopathological examination, surgical operation was carried out.
Histopathology
Resected specimens were retrieved and immediately fixed in a 10% buffered formalin solution prior to examination using hematoxylin and eosin staining. A specialist gastrointestinal pathologist reviewed the specimens. Dysplasia was classified into low grade and high grade according to modified Vienna classification [6]. High-grade neoplasia included high-grade adenoma/dysplasia, carcinoma in situ, suspicious for invasive carcinoma, and intramucosal carcinoma [6]. Carcinoma was defined as neoplastic cellular proliferation extending into submucosal layer, or beyond [6]. The degree of submucosal invasion is classified into three stages, based on the depth of invasion; sm1 (upper 1/3), sm2 (middle 1/3), and sm3 (lower 1/3) [7]. Complete resection was defined histologically if no residual neoplastic tissue was identified at any point on the horizontal or vertical cut margins. Because the lateral margin cannot be evaluated in specimens resected by piecemeal EMR, the cut margins of all lesions treated by piecemeal resection were considered as positive for follow-up purpose.
Complications
The most common complications of EMR include bleeding and perforation. Bleeding was classified into three subtypes:
-
“Procedural” if it occurred during the EMR procedure [8],
-
“Immediate” if it occurred within 24 h of the resection [8], and
-
“Delayed” if it occurred more than 24 h post-procedure [8].
Surveillance colonoscopy
Following “index” EMR, patients underwent surveillance colonoscopy to assess for lesion recurrence. Patients referred for surgical resection were not required to undergo these surveillance procedures. On surveillance colonoscopy, the prior EMR site, which was located based on previous anatomic location description and identified by post-polypectomy scar, was observed carefully using magnification chromoedoscopy to detect minute residual lesions, as evidenced by crypt pattern IIIs, IIIL, IV, and V. The local recurrent lesions were defined as neoplastic tissues at or near the prior EMR site. Endoscopic treatment techniques, such as EMR, hot biopsy, or argon plasma coagulation were applied to resect the recurrent lesion. Complete resection of the initial colonoscopic treatment was defined when no residual neoplastic tissue was found at or near the prior EMR site at the first surveillance colonoscopy.
Statistical analysis
Independent-samples t test and either the chi-square test of independence or Fisher’s exact test were used to compare means and proportions, respectively, between the groups. The relationship between the size of LSTs and resection methods was analyzed by Kruskal–Wallis test. Spearman correlation was used to evaluate the correlation between the size of LSTs and pathology. Differences were considered significant when two-sided p value was less than 0.05. All calculations were performed with the Statistical Package for the Social Sciences (version 13.0).
Results
Clinicopathologic features of LSTs
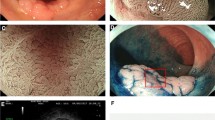
A total of 111 LSTs were detected in 109 patients (Table 2), including 89 LST-G type (Fig. 1) and 22 LST-NG type (Fig. 2). The characteristics of LSTs according to endoscopic morphology are shown in Table 3. The mean size of LST-NG type was smaller than that of LST-G type (p = 0.009). A significant difference in histopathological findings was observed between LST-NG type and LST-G type (p = 0.011). LST-NG type was more often associated with malignancy and mainly located in the right colon as compared to LST-G type which was more commonly diagnosed in the rectum (p < 0.01). There were significant differences between the dominant pit pattern of LST-G type and that of LST-NG type (p < 0.001). A type IV pit pattern was commonly found in LST-G type; whereas, type IIIL and V pit pattern were the predominant crypt patterns in LST-NG type. LST-NG type was resected by en bloc EMR and surgery compared to LST-G type which was mainly removed by piecemeal resection (p = 0.001).
All 111 LSTs were resected either endoscopically or surgically, or both. EMR was performed for 103 lesions, and surgery was performed for 12 lesions including four lesions which underwent previous EMR. Relationships between size of lesions, resection methods, and pathology are shown in Table 4. Resection methods were significantly associated with the size of LSTs (p < 0.001). En bloc EMR was commonly used for lesions in the 10–19 mm range. Forty-one percent of lesions in the 20–29 mm range and 85% of lesions larger than 30 mm were resected by piecemeal EMR. There was no significant difference between the size of lesions treated by surgical resection and EMR. Spearman correlation analysis showed significant correlation between size and pathology of LSTs (r = 0.258, p = 0.006). The malignancy rate of LSTs in the 30–39 mm range was the highest. Carcinoma was found in 16% of these lesions, and 12% were seen infiltrating the muscularis propria.
The characteristic of lesions resected by EMR or surgery
Of 103 LSTs resected by EMR, 83% (85/103) and 17% (18/103) were LST-G type and LST-NG type, respectively. Histopathology revealed 100 lesions to be adenomas (67 low-grade dysplasia (LGD)/33 high-grade dysplasia (HGD)) and three submucosal carcinomas. Characteristics of 12 LSTs resected by surgery are shown in Table 5. Of these 12 LSTs, four lesions that had undergone EMR previously were removed by surgery because their cut margins were positive for malignancy. After surgery, two cases with no residual tumors, one case with intramucosal carcinoma, and another one with carcinoma (sm2) were documented histologically in the surgical specimens. Another eight lesions underwent surgical resection based on the criteria of exclusion from EMR. Of these eight lesions, two lesions with a type VI pit pattern showed non-lifting sign with submucosal injection, five lesions had a type VN pit pattern, and another one located in the sigmoid colon (behind folds) where the procedure of EMR was difficult in accessing the lesion, which was later proved to be tubulovillous adenoma following surgical resection. All five lesions with a type VN pit pattern were carcinomas. The pathologic report of two lesions with a type VI pit pattern was submucosal carcinoma (sm2).
Pit pattern and pathology
All lesions with a type IIIL, IIIs, and IV pit pattern were noninvasive except for one with a type IV pit pattern, which was reported as submucosal carcinoma (sm1). Fourteen lesions showed a type V pit pattern, of which, nine and five were type VI pit and type VN pit. Four high-grade neoplasias, four submucosal carcinomas, and one muscularis propria carcinomas were found in lesions with a type VI pit pattern, and six lesions with positive lift sign were completely resected by endoscopic resection, and no lymphatic involvement was detected in these lesions. All lesions with a type VN pit pattern were carcinomas, and their lift sign were negative.
Complications
Bleeding complications occurred in 11 (11%) patients undergoing EMR (nine procedure, one immediate, and one delayed). All bleeding complications were treated successfully with endoscopic clip or hot biopsy therapy. There was no difference in the frequency of bleeding with respect to LST-G type and LST-NG type (p = 0.34). No perforation or other procedural-related complications were observed.
Post-EMR colonoscopic surveillance
Of 109 patients, 18 patients did not undergo further follow-up colonoscopy because they refused re-examination or could not be contacted due to change in address and telephone numbers. Twelve patients undergoing surgical resection were excluded from the surveillance group. Of the 99 lesions referred for endoscopic resection, 79 lesions in 79 patients underwent first surveillance endoscopy at a mean of 7.8 ± 5.8 months (3 to 26 months). Complete resection was achieved in 97% (31/32) of follow-up lesions resected by en bloc EMR compared to 79% (37/47) of follow-up lesions resected by piecemeal EMR in initial colonoscopy (Figs. 3 and 4). In patients undergoing EMR, local recurrences occurred in 11 patients (14%) in initial surveillance group (Fig. 5; Table 6). Ten of the recurrent lesions were LST-G type that underwent piecemeal resection at initial EMR, and the remaining one was LST-NG type which was removed by en bloc resection. All recurrent lesions underwent further endoscopic therapy by EMR or argon plasma coagulation (APC) and were histologically diagnosed as adenomas (eight LGD/three HGD).
All patients with recurrent lesions underwent subsequent surveillance colonoscopy following the first surveillance, and recurrent lesions were detected in four patients. A total of seven follow-up endoscopic therapies were performed in four cases, four received EMR and APC therapy, two received APC, and another received EMR only. After all patients with recurrent lesions underwent one to five surveillance colonoscopies during a mean follow-up period of 26.6 ± 18 months (13 to 69 months), no residual or recurrent lesions were detected. There were no recurrent lesions detected in patients who had no residual lesions in initial surveillance but underwent subsequent surveillance colonoscopy. Among all follow-up patients, no patients died during the period of surveillance.
Discussion
In the present study, clinicopathologic features of LST-G type significantly differ from that of LST-NG type. LST-NG type was located more often in the right colon and had a smaller size, with higher malignant potential in comparison with LST-G type. These findings were in accordance with that of previous reports [1, 9–12].
There are four pit pattern classes in LSTs: type IIIL, IIIs, IV, and V. Our results showed that the type IV pit pattern was a dominant crypt pattern in LST-G type; whereas, the type IIIL and V pit pattern were found more often in LST-NG type. This result indicated the type of dominant pit pattern was different between LST-G type and LST-NG type, which was in accordance with Kudo pit pattern of LSTs [13]. The previous studies reported that type V pit pattern indicated the presence of submucosal invasion, but the sensitivity was only 50% [14]. The type V pit pattern was classified as two subtypes: type VI and type VN. Recently, Kanao et al. and Onishi et al. analyzed the relation between the type VI pit pattern and history/invasion depth, and they found that a type VI pit pattern mainly occurred in lesions with dysplasias or lesions with depth of submucosal invasion less than 1,000 μm [3, 4]. In our study, submucosal invasion was more frequently encountered in lesions with a type VN pit pattern in comparison with lesions having a type VI pit pattern. Six of the nine lesions with type VI pit pattern were completely resected by EMR, and no lymphatic involvement was detected in these lesions. This result was supported by the report of Kitajima et al. that for nonpedunculated submucosal invasive colorectal carcinoma, rate of lymph node metastasis was 0% if submucosal depth was <1,000 μm [15]. Previous studies also reported lymph node metastasis is observed in 10% of submucosal carcinomas [16] but more frequently present in deeper submucosal invasive carcinoma [17, 18]. So, EMR is considered as an adequate therapy for the lesions with super submucosal invasion. However, almost all lesions with a type VN pit pattern need to be resected surgically because this pit pattern indicates that carcinomas extends into the submucosa or beyond. Previous studies also showed that the reported accuracy of detection of massive submucosal invasion on the basis of the type VN pit pattern is about 97% [19, 20]. In the current study, the pathologic findings of all five lesions with a type VN pit pattern were carcinomas (three muscularis propria carcinomas and two submucosal carcinomas), and the non-lifting sign was positive in all five carcinomas. Although the results in the present study are perfect, possibly due to lesser number of cases with a type V pit pattern, they definitely indicate that many LSTs with a type VI pit pattern can be treated by endoscopic resection as an alternative to surgery; whereas, the type VN pit pattern in LSTs is an indication for surgical resection.
Our study showed LSTs larger than 30 mm are more often resected by piecemeal resection than LSTs smaller than 30 mm. The result was in accordance with the previous study, which reported that the choice of endoscopic resection method depends on the size of lesions [8]. In addition, we found the malignancy rate of LSTs in the 30–39 mm range to be the highest of all lesions. Carcinomas were present in 16% of these lesions, and 12% were muscularis propria carcinoma. Uraoka et al. reported that a larger tumor size (≥20 mm) in LST-NG type and a large nodule (>10 mm) in LST-G type are independent risk criteria for submucosal invasion [1]. Our data showed that five carcinomas occurred in ten LST-NG type larger than 20 mm compared to only one carcinoma occurred in 12 LST-NG type smaller than 20 mm, which was similar with their result, but we could not establish that a large nodule (>10 mm) in LST-G type is significantly associated with submucosal invasion. One of the possible explanations could be the smaller number of carcinomas in this study.
In considering therapeutic strategies, our study clearly showed that as a first-line treatment method for LSTs, EMR is efficacious and safe. Previous studies in Japan and western countries have reported similar findings that most LSTs can be completely resected by EMR, recurrence rate range from 0% to 40% [21–23], and overall cure rate reaches 89–100% after subsequent EMR at 2 years follow-up [9, 24, 25]. In our study, 11 local recurrent lesions occurred in 79 follow-up cases and were completely removed in subsequent surveillance colonoscopy.
At present, the guidelines for follow-up after LSTs resection are not clearly delineated. Many prospective studies reported that the surveillance colonoscopy at 3 to 6 months after initial EMR was most important to decide whether complete resection has been performed [9, 26, 27]. The study of Hurlstone et al. suggested all recurrent lesions were detected within 6 months after initial colonoscopic therapy and 86% of them were endoscopically visible 3 months after resection [24]. The American Cancer Society proposed surveillance recommendations that patients with sessile adenomas removed by piecemeal EMR should be considered for follow-up at short intervals (2 to 6 months) to verify complete removal [28]. In our study, all recurrent lesions were detected at the first surveillance colonoscopy 3 to 26 months after initial EMR. Complete resection criteria were based on both endoscopic and pathologic assessments. After initial colonoscopic therapy, the cure rates for LST undergone en bloc and piecemeal EMR were 97% and 79%, respectively. After one to four surveillance colonoscopies, all recurrent lesions were completely resected. Among recurrent lesions, no carcinomas were found. This result was in accordance with previous reports that carcinoma in recurrent lesions after EMR were also rare [26, 29].
Bleeding and perforation are more common complications of EMR. Based on literature reports, incidence of bleeding complications of EMR range from 1% to 24% [9, 10, 21, 27]. In the current study, bleeding occurred in 11 lesions. Eighty-two percent of bleeding complications were procedure bleeding, while the incidence of both immediate and delayed bleeding was only 9%. There was no significant difference in the incidence of bleeding with respect to LST-G type and LST-NG type. All bleeding complications were controlled by endoscopic clip or hot biopsy therapy. No perforation and other procedure-related complications occurred during this study.
Our study analyzed the clinicopathologic features of LSTs and assessed the efficacy and safety of EMR based on cases obtained using retrospective data. Of all patients undergoing EMR, about 20% patients were lost to follow-up and could not receive surveillance colonoscopy. Among follow-up patients, 25% patients who had no residual lesions at the first surveillance colonoscopy did not consent to subsequent surveillance colonoscopy. These factors may have influenced the result of assessment for efficacy of EMR to some extent.
However, we have utilized the technique of magnification chromoendoscopy in this study to observe residual lesions, which has aided in increasing the accuracy of judging complete resection at first surveillance colonoscopy.
Conclusion
In summary, LSTs are considered nonpolypoid neoplasms with specialized clinicopathologic characteristics, some of which may be regarded as indicators influencing further management. Most LSTs, including many LSTs with a type VI pit pattern, can be completely resected by EMR and even though the majority of LSTs can be resected successfully at initial colonoscopy, surveillance colonoscopy after EMR is essential for curing LSTs completely.
Abbreviations
- LSTs:
-
Laterally spreading tumors
- EMR:
-
Endoscopic mucosal resection
- LST-NG:
-
Laterally spreading tumors (non-granular)
- LST-G:
-
Laterally spreading tumors (granular)
References
Uraoka T, Saito Y, Matsuda T et al (2006) Endoscopic indications for endoscopic mucosal resection of laterally spreading tumours in the colorectum. Gut 55:1592–1597
Hurlstone DP, Cross SS, Adam I et al (2004) Endoscopic morphological anticipation of submucosal invasion in flat and depressed colorectal lesions: clinical implications and subtype analysis of the Kudo type V pit pattern using high magnification-chromoscopic colonoscopy. Colorectal Dis 6(5):369–375
Kanao H, Tanaka S, Oka S et al (2008) Clinical significance of type VI pit pattern subclassification in determining the depth of invasion of colorectal neoplasms. World J Gastroenterol 14(2):211–217
Onishi T, Tamura S, Kuratani Y et al (2008) Evaluation of the depth score of type V pit patterns in crypt orifices of colorectal neoplastic lesions. J Gastroenterol 43:291–297
Kudo S, Rubio CA, Teixeira CR et al (2001) Pit pattern in colorectal neoplasia: endoscopic magnifying view. Endoscopy 33:367–373
Dixon MF (2002) Gastrointestinal epithelial neoplasia: Vienna revisited. Gut 51(1):130–131
Kudo S (1996) Early colorectal cancer: detection of depressed-types of colorectal carcinoma. Igaku-shoin, Tokyo
Ponchon T (2001) Endoscopic mucosal resection. J Clin Gastroenterol 32:6–10
Tanaka S, Haruma K, Oka S et al (2001) Clinicopathologic features and endoscopic treatment of superficially spreading colorectal neoplasms larger than 20 mm. Gastrointest Endosc 54:62–66
Hurlstone DP, Sanders DS, Cross SS et al (2004) Colonoscopic resection of lateral spreading tumours: a prospective analysis of endoscopic mucosal resection. Gut 53:1334–1339
Yoshikane H, Hidano H, Sakakibara A et al (1999) Endoscopic resection of laterally spreading tumours of the large intestine using a distal attachment. Endoscopy 31:426–430
Saito Y, Fujii T, Kondo H et al (2001) Endoscopic treatment for laterally spreading tumors in the colon. Endoscopy 33:682–686
Kudo S (2000) Endoscopic treatment of neoplasms in colon and rectum. Igaku-shoin, Tokyo
Hurlstone DP, Cross SS, Adam I et al (2004) Efficacy of high magnification chromoscopic colonoscopy for the diagnosis of neoplasia in flat and depressed lesions of the colorectum: a prospective analysis. Gut 53:284–290
Kitajima K, Fujimori T, Fujii S et al (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39:534–543
Tamura S, Yokoyama Y, Ookawauchi K et al (2003) Evaluation of the type V pit pattern in the lesions of colonic Tis and T1 cancer. Dig Endosc 15:185–189
Tanaka S, Haruma K, Teixeira CR et al (1995) Endoscopic treatment of submucosal invasive colorectal carcinoma with special reference to risk factors for lymph node metastasis. J Gastroenterol 30:710–717
Kitajima K, Fujimori T, Fujii S et al (2004) Correlations between lymph node metastasis and depth of submucosal invasion in submucosal invasive colorectal carcinoma: a Japanese collaborative study. J Gastroenterol 39:534–543
Kawano H, Tsuruta O, Ikeda H et al (1998) Diagnosis of the level of depth in superficial depressed-type colorectal tumors in terms of stereomicroscopic pit patterns. Int J Oncol 12:769–775
Oka S, Tanaka S, Kaneko I et al (2005) Diagnosis of the invasion depth using magnifying videocolonoscopy in early colorectal carcinoma. Early Colorectal Cancer 9:161–168
Kanamori T, Itoh M, Yokoyama Y et al (1996) Injection-incision: assisted snare resection of large sessile colorectal polyps. Gastrointest Endosc 43:189–195
Iishi H, Tatsuta M, Iseki K et al (2000) Endoscopic piecemeal resection with submucosal saline injection of large sessile colorectal polyps. Gastrointest Endosc 51:697–700
Brooker JC, Saunders BP, Shah SG et al (2002) Treatment with argon plasma coagulation reduces recurrence after piecemeal resection of large sessile colonic polyps: a randomized trial and recommendations. Gastrointest Endosc 55:371–375
Hurlstone DP, Sanders DS, Thomson M et al (2006) “Salvage” endoscopic mucosal resection in the colon using a retroflexion gastroscope dissection technique: a prospective analysis. Endoscopy 38(9):902–906
Higaki S, Hashimoto S, Harada K et al (2003) Long-term follow-up of large flat colorectal tumors resected endoscopically. Endoscopy 35(10):845–849
Bories E, Pesenti C, Monges G et al (2006) Endoscopic mucosal resection for advanced sessile adenoma and early-stage colorectal carcinoma. Endoscopy 38(3):231–235
Kaltenbach T, Friedland S, Maheshwari A et al (2007) Short- and long-term outcomes of standardized EMR of nonpolypoid (flat and depressed) colorectal lesions ≥1 cm. Gastrointest Endosc 65:857–865
Winawer SJ, Zauber AG, Fletcher RH, US Multi-Society Task Force on Colorectal Cancer et al (2006) Guidelines for colonoscopy surveillance after polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer and the American Cancer Society. Gastroenterology 130:1872–1885
Jameel JK, Pillinger SH, Moncur P et al (2006) Endoscopic mucosal resection (EMR) in the management of large colo-rectal polyps. Colorectal Dis 8:497–500
Tanaka S, Kaltenbach T, Chayama K, Soetikno R (2006) High-magnification colonoscopy (with videos). Gastrointest Endosc 64:604–613
Acknowledgement
We express our special thanks to Prof. Hu Jin for the statistical analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, Y., Liu, S., Gong, W. et al. Clinicopathologic features and endoscopic mucosal resection of laterally spreading tumors: experience from China. Int J Colorectal Dis 24, 1441–1450 (2009). https://doi.org/10.1007/s00384-009-0749-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-009-0749-4