Abstract
Background and aims
The objective of this study was to investigate the effect of Boswellia serrata extract (BSE) on symptoms, quality of life, and histology in patients with collagenous colitis.
Materials and methods
Patients with chronic diarrhea and histologically proven collagenous colitis were randomized to receive either oral BSE 400 mg three times daily for 6 weeks or placebo. Complete colonoscopy and histology were performed before and after treatment. Clinical symptoms and quality of life were assessed by standardized questionnaires and SF-36. The primary endpoint was the percentage of patients with clinical remission after 6 weeks (stool frequency ≤3 soft /solid stools per day on average during the last week). Patients of the placebo group with persistent diarrhea received open-label BSE therapy for a further 6 weeks.
Results
Thirty-one patients were randomized; 26 patients were available for per-protocol-analysis. After 6 weeks, the proportion of patients in clinical remission was higher in the BSE group than in the placebo group (per protocol 63.6%; 95%CI, 30.8–89.1 vs 26.7%, 95%CI, 7.7–55.1; p = 0.04; intention-to-treat 43.8% vs 26.7%, p = 0.25). Compared to placebo, BSE treatment had no effect on histology and quality of life. Five patients discontinued BSE treatment prematurely. Discontinuation was due to adverse events (n = 1), unwillingness to continue (n = 3), or loss to follow-up for unknown reasons (n = 1). Seven patients received open-label BSE therapy, five of whom achieved complete remission.
Conclusions
Our study suggests that BSE might be clinically effective in patients with collagenous colitis. Larger trials are clearly necessary to establish the clinical efficacy of BSE.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Collagenous colitis (CC) is a form of microscopic colitis with an incidence of 0.6 to 5.2/100,000 person years and a prevalence of 10 to 15.7/100,000 in Europe [1–3]. The disease is clinically characterized by chronic watery diarrhea and few or no endoscopic abnormalities [4, 5]. The diagnosis of CC relies on histopathologic examination of biopsy specimens from the colorectal mucosa, with a typical feature being diffuse thickening of the subepithelial collagen layer (≥10 μm) beneath the basement membrane and a nonspecific chronic inflammatory infiltrate of the lamina propria [6–10]. The etiology of CC is unknown. Several hypotheses have been suggested. Autoimmunity may play an important role arising from a poorly regulated epithelial immune response to luminal antigens [11–14]. There is also an increasing evidence for specific drugs to cause or worsen collagenous colitis [15].
The treatment of CC has been purely empirical in the past and remains a challenge. The first randomized placebo-controlled trial suggested that treatment with bismuth subsalicylate has a positive effect on clinical symptoms and histopathology in patients with collagenous and lymphocytic colitis. However, because the number of patients in that study was rather small (n = 9), these data may need confirmation by larger trials [16]. Recently, budesonide capsules have been shown to be effective for induction of remission in collagenous colitis in three placebo-controlled trials [17–19]. The majority of patients treated with budesonide capsules experience a rapid induction of clinical remission, a significant improvement of quality of life and histology [20, 21]. However, a considerable clinical relapse rate after cessation of treatment has been described [17, 22]. Thus, alternative treatment modalities for acute and long-term treatment of CC are needed.
Due to their anti-inflammatory properties, Boswellia serrata extract (BSE) has been used in various inflammatory disorders, such as bronchial asthma, chronic polyarthritis, and inflammatory bowel diseases [23–28].
This led us to hypothesize that BSE might also be effective in collagenous colitis. The aim of this study was therefore to evaluate the clinical and histologic effects of oral BSE in patients with CC in an appropriate study design.
Materials and methods
Study design and recruitment of patients
The clinical trial was conducted in a randomized, placebo-controlled, double-blind fashion performed at several centers in Germany.
Patients, aged between 18 and 80 years were eligible for the study if they had at least five liquid or soft stools per day on average per week, a complete colonoscopy performed within the last 4 weeks before randomization, and a histologically confirmed diagnosis of collagenous colitis.
Exclusion criteria included treatment with budesonide, salicylates, steroids, prokinetics, antibiotics, ketoconazole, or non-steroidal anti-inflammatory drugs within 4 weeks before randomization, other endoscopically or histologically verified causes for diarrhea, infectious diarrhea, pregnancy or lactation, previous colonic surgery, and known intolerance to BSE.
The study protocol and consent form were approved by the Ethics Committee of the University Hospital Dresden in accordance with the revised Declaration of Helsinki. Written informed consent was obtained from all patients before inclusion in the trial.
Randomization and therapy
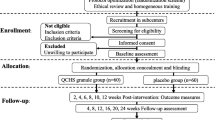
Eligible patients were randomized by groups of four patients according to a central computer-generated randomization list to receive either BSE 400 mg or placebo given three times daily with meals for 6 weeks (Fig. 1). Physicians, patients, and pathologist were blinded to the treatment group. Study medication was provided in identical-looking white boxes labeled with consecutive numbers corresponding to the randomization list. In addition, the placebo containers were prepared from the inside to mimic the typical scent of incense to prevent unblinding by the typical odor of BSE.
For each box of study medication, a sealed white envelope was available to be opened only in case of emergency for unblinding.
Concomitant use of loperamide was allowed for the first 3 weeks but was not allowed for the last 3 weeks of the study. Patients were allowed to use butylscopolamine in case of abdominal pain.
Patients who did not respond to treatment after 6 weeks were individually unblinded. If they were in the active treatment group, they were judged as treatment failure. If they were in the placebo group, crossover therapy with open-labeled BSE 400 mg, given orally three times daily was offered.
Study medication
The preparation used in this study contained 400 mg of BSE per capsule (80% Boswellia acid). High performance liquid chromatography (HPLC) analysis of individual Boswellia acids revealed the following results: 21.2 mg 11-keto-β-boswellia acid, 27.3 mg α-boswellia acid, 50.9 mg β-boswellia acid, 11.3 mg acetyl-11-keto-β-boswellia acid, 9.8 mg acetyl-α-boswellia acid, and 28.7 mg acetyl-β-boswellia acid. The study medication was prepared and delivered by a local pharmacist in Western Germany (O.E.) associated with a hospital for naturopathy.
Clinical symptoms, safety, and compliance
Stool frequency and consistency, intake of study medication, adverse events, and any intake of allowed concomitant medication were assessed by standardized questionnaire. Spontaneous reports of adverse events were documented at the time of onset. The documentation sheets were collected at the end of the treatment period.
Compliance was assessed by pill count. Patients who took less than 80% of the prescribed pills were considered to be non-compliant and excluded from the per-protocol analysis, but were included in the intention-to-treat analysis.
The primary endpoint of the study was the percentage of patients with clinical remission after 6 weeks. Histology and quality of life served as secondary endpoints of the study. Clinical remission was defined as stool frequency equal to or less than three soft or solid stools per day on average during the last week of treatment.
Quality of life
Quality of life was assessed by using SF-36 at baseline and after 6 weeks of treatment. The SF-36 consists of four domains of physical health (physical functioning, role limitation-physical, bodily pain, general health) and four domains of mental health (role limitation-emotional, vitality, mental health, social functioning).
Endoscopy and histology
A complete colonoscopy including inspection of the terminal ileum was performed at baseline and after 6 weeks, i.e., at the end of therapy. At each colonoscopy, at least two biopsy specimens each from the ileum, ascending colon, transverse colon, descending colon, sigmoid colon, and rectum were taken for histological examination. Biopsy specimens were fixed in 10% formalin and embedded in paraffin. Hematoxylin and eosin stain and van Gieson stain were performed. On well-oriented sections, in which at least three adjacent crypts were cut in the vertical plane, the following three parameters were assessed: thickness of the collagen band (μm), inflammation of the lamina propria (semiquantitative score 0–3), and degeneration of surface epithelium (present/absent). The diagnosis of CC was made when the subepithelial collagen layer on at least one well-oriented section of mucosa exceeded 10 μm.
For each individual patient, the values of each parameter at the various biopsy sites were pooled and the mean was calculated.
Statistical analysis
For statistical analysis, the Banard’s exact test, Fisher’s exact test, and the Mann–Whitney test were used when appropriate. Less than 0.05 p values were considered to indicate statistical significance. The sample size of n = 23 per group was required that a Fisher’s exact test with a 0.05 two-sided significance level will have 80% power to detect the difference in the response rate between the active group of 60% and the placebo group of 20%.
Statistical analyses were performed utilizing the software package SPSS 13.0 for windows and StatXact-4.
Results
Study population
Between October 2002 and April 2005, a total of 31 patients (16 BSE group) were enrolled. In May 2005, the study was prematurely stopped due to insufficient recruitment. The baseline characteristics of the two groups were similar (Table 1). Eight patients (25.8%) of the study population reported concomitant autoimmune-like disorders such as diabetes, thyroid disease, or fibromyalgia. A total of seven patients (22.5%; 4 patients BSE group; 3 patients of placebo group) received concomitant medications that are reportedly associated with microscopic colitis, such as aspirin, NSAIDs, and lisinopril. Patients continued their concomitant medications during the entire study.
A total of four patients discontinued treatment prematurely either due to adverse events (one BSE group) or due to unwillingness to continue (three BSE group, 2 weeks after starting treatment with persistent diarrheal symptoms). One patient of the BSE group was lost to follow-up for unknown reason. Thus, a total of 26 patients (83.8%) were available for per protocol analysis.
Efficacy of treatment
After 6 weeks, the rate of clinical remission was significantly higher in the BSE group than in the placebo group (per protocol 63.6%; 95%CI, 30.79–89.07 vs 26.7%; 95%CI, 7.7–55.1) p = 0.04); intention-to-treat, 43.8% vs 26.7%, p = 0.25).
In the per-protocol analysis, the median stool frequency per day was reduced from 6.5 (range, 4–15) to 3 (range, 1–7) in the BSE group and from 5 (range, 4–10) to 4 (range, 1–9) in the placebo group after 6 weeks of treatment (Fig. 2). Change of stool consistency after treatment is depicted in Fig. 3. After 3 weeks of treatment, clinical remission rates were not significantly different between the two groups (p = 0.18). None of the patients used any antidiarrheals or butylscopolamine during the entire study period.
Seven patients of the placebo group with persistent diarrhea after 6 weeks of treatment agreed and received an open-label BSE therapy for further 6 weeks. Of those, five patients (71,4%) achieved clinical remission at the end of cross-over BSE treatment.
Effect on histopathology
The thickness of the collagen layer was patchy distributed through the entire the colon ranged between 0 to 15 μm. The diagnosis of CC was made when the subepithelial collagen layer on at least one well-oriented section of mucosa exceeded 10 μm.
At baseline, the mean of the collagen band thickness was 10.1 μm in the BSE group and 8.8 μm in the placebo group. The corresponding inflammatory scores were 2.3 and 1.9, respectively. There was no significant difference between both groups at baseline. After 6 weeks of treatment, there was only a small reduction of collagen band thickness and inflammation score without statistical significance compared to baseline or between the groups.
Effect on quality of life
Complete SF-36 assessments at baseline and after 6 weeks were available in 26 patients. At baseline, the mean scores of all eight domains in the entire study population were markedly lower in patients with collagenous colitis compared to normal controls. After 6 weeks of treatment, there were no statistical significant changes of quality of life in the two groups compared to baseline and between groups.
Adverse events
BSE therapy was well tolerated, and no serious adverse events were reported. Two patients of the BSE group did experience some side effects. One of them withdrew after 3 weeks of treatment because of dizziness, hypoglycemia (not judged as a severe adverse event by the local investigator and protocol committee), and lack of appetite and recovered to normal after cessation of treatment. The second patient completed the 6-week treatment with persistent diarrhea and newly diagnosed bacterial enteritis. In the placebo group, one patient experienced adverse events (eczema and Coxsackie virus infection) but still completed the treatment.
Discussion
At present, this is the second largest randomized, placebo-controlled trial investigating medical intervention in collagenous colitis. Although our study failed to show a statistically significant difference between Boswellia serrata extract and placebo, the per-protocol analysis indicates that Boswellia serrata extract may be clinically effective and safe in the treatment of collagenous colitis.
The clinical course of collagenous colitis is variable with spontaneous improvement or exacerbation of symptoms. Thus, therapeutic trials of patients with collagenous colitis require a placebo-controlled, randomized study design to eliminate the effect of spontaneous improvement or other sources of bias. Recently, budesonide capsules have been proven effective for induction of remission in collagenous colitis in three placebo-controlled trials [17–19]. A recent Cochrane review of these trials has calculated a number needed to treat of two patients [29]. The same Cochrane review included preliminary data from the present study [30] and concluded that there was a trend towards clinical improvement in patients receiving BSE treatment compared to placebo and that there may have been a lack of power given the small numbers of patients in each group [29].
Several clinical studies suggest an effect of Boswellia serrata extract in patients with Crohn’s disease, ulcerative colitis, and nonspecific colitis [26–28]. In a randomized, double-blind, parallel group study, Gerhardt et al. [26] have shown that Boswellia serrata extract was not inferior to mesalazine in maintenance therapy of patients with Crohn disease. In another study, Boswellia serrata extract was effective in patients with ulcerative colitis [27].
Thus, the rationale of our study was to investigate Boswellia serrata extract in patients with collagenous colitis in a randomized, placebo-controlled trial. Unfortunately, the study had to be stopped prematurely due to insufficient recruitment. We speculate that the meanwhile widely distributed acceptance of budesonide as first-line treatment of collagenous colitis in Germany has contributed to a declining willingness of patients to participate in this trial. Despite the low patient number, the per-protocol analysis suggests a clinical effect of BSE with a therapeutic gain of 37% over placebo. The clinical benefit was further supported by the observation that five out of seven patients responded to crossover BSE.
The study was powered under the assumption of a placebo response rate of 20%, but the placebo response rate actually observed was far greater (26,7%) compared to other placebo controlled trials [17–19]. Possible explanations for the high placebo response may be the spontaneous course of disease or aggravation of the placebo effect by the odor of incense of the placebo container. Thus, the high placebo response combined with the low patient number may have obscured statistical separation between BSE and placebo regarding the secondary study endpoints.
Quality of life, assessed by SF-36, was markedly reduced in the present population with collagenous colitis when compared to normal controls, which confirms previous data of our study group using GILQI [20]. However, in contrast to our previous study, we did not observe a significant change in quality of life comparing both groups after therapy, which may be due the low patient number or due to the short treatment period.
Treatment with Boswellia serrata extract was well tolerated and safe. Only two patients reported side effects. One of them discontinued treatment prematurely because of self-reported side-effects. Therefore, Boswellia serrata extract might be considered as a useful therapeutic option for patients with collagenous colitis.
The mode of action of BSE has been investigated in several experimental studies showing that Boswellia acids inhibit production of 5-lipooxygenase and synthesis of leucotrienes [32–35]. Furthermore, Boswellia acids are catalytic inhibitors of human topoisomerase I and IIα [36]. In contrast to our previous clinical trial [18], we did not find a significant influence on the histopathology in the present study as would be expected based upon the anti-inflammatory effect of BSE. A possible explanation could be again that the treatment period of 6 weeks may have been too short to improve histolology. The possible slow mode of action was further supported by the observation that most of the patients experienced clinical benefit only after 6 weeks of BSE treatment. In contrast, budesonide treatment leads to rapid induction of clinical remission [21].
In conclusion, our study suggests that oral Boswellia serrata extract might be a clinically effective and safe treatment modality for patients with collagenous colitis. However, we strongly recommend to further investigate BSE in larger clinical randomized trials before definite conclusions can be drawn.
References
Fernandez-Banares F, Salas A, Forne M, Esteve M, Espinos J, Viver JM (1999) Incidence of collagenous and lymphocytic colitis: a 5-year population-based study. Am J Gastroenterol 94:418–423
Bohr J, Tysk C, Eriksson S, Jarnerot G (1995) Collagenous colitis in Orebro, Sweden, an epidemiological study 1984–1993. Gut 37:394–397
Agnarsdottir M, Gunnlaugsson O, Orvar KB, Cariglia N, Birgisson S, Bjornsson S, Thorgeirsson T, Jonasson JG (2002) Collagenous and lymphocytic colitis in Iceland. Dig Dis Sci 47:1122–1128
Tremaine WJ (2000) Collagenous colitis and lymphocytic colitis. J Clin Gastroenterol 30:245–249
Pardi DS (2004) Microscopic colitis. An update. Inflamm Bowel Dis 10:860–870
Bohr J, Olesen M, Tysk C, Järnerot G (2000) Collagenous and lymphocytic colitis: a clinical and histopathological review. Can J Gastroenterol 14:943–947
Veress B, Lofberg R, Bergman L (1995) Microscopic colitis syndrome. Gut 36:880–886
Lazenby AJ, Yardley JH, Giardiello FM, Jessurun J, Bayless TM (1989) Lymphocytic (microscopic) colitis: a comparative histopathologic study with particular reference to collagenous colitis. Hum Pathol 20:18–28
Stolte M, Ritter M, Borchard F, Koch-Scherrer G (1990) Collagenous gastroduodenitis on collagenous colitis. Endoscopy 22:186–187
Carpenter HA, Tremaine WJ, Batts KP, Czaja AJ (1992) Sequential histologic evaluations in collagenous colitis. Correlations with disease behavior and sampling strategy. Dig Dis Sci 37:1903–1909
Armes J, Gee DC, Macrae FA, Schroeder W, Bhathal PS (1992) Collagenous colitis: jejunal and colorectal pathology. J Clin Pathol 45:784–787
Bohr J, Tysk C, Yang P, Danielsson D, Jarnerot G (1996) Autoantibodies and immunoglobulins in collagenous colitis. Gut 39:73–76
Jarnerot G, Tysk C, Bohr J et al (1995) Collagenous colitis and fecal stream diversion. Gastroenterology 109:449–455
Duerr RH, Targan SR, Landers CJ et al (1991) Anti-neutrophil cytoplasmic antibodies in ulcerative colitis: comparison with other colitides/diarrheal illnesses. Gastroenterology 100:1590–1596
Beaugerie L, Pardi DS (2005) Review article: drug-induced microscopic colitis-proposal for a scoring system and review of the literature. Aliment Pharmacol Ther 22:277–284
Fine K, Ogunji F, Lee E, Lafon G, Tanzi M (1999) Randomized, double-blind placebo-controlled trial of bismuth subsalicylate for microscopic colitis (abstr.). Gastroenterology 166:A880
Baert F, Schmit A, D’Haens G, Dedeurwaerdere F, Louis E, Cabooter M, De Vos M, Fontaine F, Naegels S, Schurmans P, Stals H, Geboes K, Rutgeerts P (2002) Budesonide in collagenous colitis-a double-blind placebo-controlled trial with histological follow-up. Gastroenterology 122:20–25
Miehlke S, Heymer P, Bethke B, Bästlein E, Meier E, Bartram HP, Wilhelms G, Lehn N, Dorta G, DeLarive J, Tromm A, Bayerdörffer E, Stolte M (2002) Budesonide treatment for collagenous colitis–a randomized, double-blind, placebo-controlled, multicenter trial. Gastroenterology 123:978–984
Bonderup OK, Hansen JB, Birket-Smith L, Vestergaard V, Teglbjaerg PS, Fallingborg J (2003) Budesonide treatment of collagenous colitis: a randomised, double blind, placebo controlled trial with morphometric analysis. Gut 52:248–251
Madisch A, Heymer P, Voss C, Wigginghaus B, Bastlein E, Bayerdorffer E, Meier E, Schimming W, Bethke B, Stolte M, Miehlke S (2005) Oral budesonide therapy improves quality of life in patients with collagenous colitis. Int J Colorectal Dis 20:312–316
Miehlke S, Madisch A, Bethke B, Stolte M (2005) Time to remission with budesonide in collagenous colitis. Aliment Pharmacol Ther 21:1507–1508
Miehlke S, Madisch A, Voss C, Morgner A, Heymer P, Kuhlisch E, Bethke B, Stolte M (2005) Long-term follow-up of collagenous colitis after induction of clinical remission with budesonide. Aliment Pharmacol Ther 22:1115–1119
Gupta I, Gupta V, Parihar A, Gupta S, Ludtke R, Safayhi H, Ammon HP (1998) Effects of Boswellia serrata gum resin in patients with bronchial asthma: results of a double-blind, placebo-controlled, 6-week clinical study. Eur J Med Res 3:511–514
Sander O, Herborn G, Rau R (1998) Is H15 (resin extract of Boswellia serrata, “incense”) a useful supplement to established drug therapy of chronic polyarthritis? Results of a double-blind pilot study. Z Rheumatol 57(1):11–16
Kimmatkar N, Thawani V, Hingorani L, Khiyani R (2003) Efficacy and tolerability of Boswellia serrata extract in treatment of osteoarthritis of knee–a randomized double blind placebo controlled trial. Phytomedicine 10:3–7
Gerhardt H, Seifert F, Buvari P, Vogelsang H, Repges R (2001) Therapy of active Crohn disease with Boswellia serrata extract H 15. Z Gastroenterol 39:11–17
Gupta I, Parihar A, Malhotra P, Singh GB, Ludtke R, Safayhi H, Ammon HP (1997) Effects of Boswellia serrata gum resin in patients with ulcerative colitis. Eur J Med Res 2:37–43
Gupta I, Parihar A, Malhotra P, Gupta S, Ludtke R, Safayhi H, Ammon HP (2001) Effects of gum resin of Boswellia serrata in patients with chronic colitis. Planta Med 67:391–395
Chande N, McDonald JW, Macdonald JK (2005) Interventions for treating collagenous colitis. Cochrane Database Syst Rev 19:CD003575
Madisch A, Miehlke S, Eichele E, Bethke B, Mrwa J, Wilhelms G, Debus M, Bästlein E, Kuhlisch E, Stolte M (2005) Boswellia Serrata Extract for the Treatment of collagenous colitis: a randomized, double-Blind, placebo-controlled, multicenter Trial. Gastroenterology 128:A581
Sailer ER, Schweizer S, Boden SE, Ammon HP, Safayhi H (1998) Characterization of an acetyl-11-keto-beta-boswellic acid and arachidonate-binding regulatory site of 5-lipoxygenase using photoaffinity labeling. Eur J Biochem 256:364–368
Safayhi H, Mack T, Sabieray J Anazodo MI, Subramanian LR, Ammon HP (1992) Boswellic acids: novel, specific, nonredox inhibitors of 5-lipoxygenase. J Pharmacol Exp Ther 261/3:1143–1146
Safayhi H, Sailer ER, Ammon HPT (1995) Mechanism of 5-lipoxygenase inhibition by acetyl-11-keto-ß-boswellic acid. Mol Pharmacol 47:1212–1216
Safayhi H, Sailer ER, Ammon HPT (1996) 5-lipoxygenase inhibition by acetyl-11-keto-ß-boswellic acid (AKBA) by a novel mechanism. Phytomedicine 3/1:71
Krieglstein CF, Anthoni C, Rijcken EJ, Laukotter M, Spiegel HU, Boden SE, Schweizer S, Safayhi H, Senninger N, Schurmann G (2001) Acetyl-11-keto-beta-boswellic acid, a constituent of a herbal medicine from Boswellia serrata resin, attenuates experimental ileitis. Int J Colorectal Dis 16:88–95
Syrovets T, Buchele B, Gedig E, Slupsky JR, Simmet T (2000) Acetyl-boswellic acids are novel catalytic inhibitors of human topoisomerases I and II alpha. Mol Pharmacol 58:71–81
Acknowledgement
We thank R. Beckmann for assistance in data collection. We also thank M. Stewart for critical reviewing the manuscript. We are grateful to the following colleagues for recruiting patients to the study: R. Abuagela, H.-P. Bartram, P. Dietz, M. Dornberg, D. Fuchs, E. Grüger, C Haferland, J. Keymling, E. Meier, J. Morgenthaler, P. Rohde, G. Stegemann-Özdemir, and H. Wollmann.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madisch, A., Miehlke, S., Eichele, O. et al. Boswellia serrata extract for the treatment of collagenous colitis. A double-blind, randomized, placebo-controlled, multicenter trial. Int J Colorectal Dis 22, 1445–1451 (2007). https://doi.org/10.1007/s00384-007-0364-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00384-007-0364-1