Abstract
Purpose
Intestinal neuronal dysplasia (IND) is a congenital anomaly affecting gastrointestinal neural innervation, but the pathogenesis remains unclear. The homozygous Ncx/Hox11L.1 knockout (Ncx−/−) mice exhibit megacolon and enteric ganglia anomalies, resembling IND phenotypes. Sox10-Venus transgenic mouse were used to visualize enteric neural crest cells in real time. This study aims to establish a novel mouse model of Sox10-Venus+/Ncx−/− mouse to study the pathogenesis of IND.
Methods
Sox10-Venus+/Ncx−/− (Ncx−/−) (n = 8) mice and Sox10-Venus+/Ncx+/+ controls (control) (n = 8) were euthanized at 4–5 weeks old, and excised intestines were examined with fluorescence microscopy. Immunohistochemistry was performed on tissue sections with neural marker Tuj1.
Results
Ncx−/− mice exhibited dilated cecum and small intestine. Body weight of Ncx−/− mice was lower with higher ratio of small intestine length relative to body weight. The neural network (Sox10-Venus) was observed along the intestine wall in Ncx−/− and control mice without staining. Ectopic and increased expression of Tuj1 was observed in both small intestine and proximal colon of Ncx−/− mice.
Conclusion
This study has established a reliable animal model that exhibits characteristics similar to patients with IND. This novel mouse model can allow the easy visualization of ENS in a time- and cost-effective way to study the pathogenesis of IND.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Intestinal neuronal dysplasia (IND) is a disorder of the enteric nervous system (ENS) associated with intestinal dysmotility. IND patients present with a clinical condition similar to Hirschsprung's disease (HD) including severe constipation or intestinal obstruction [1]. However, the pathological features of IND are clearly different from HD, mainly hyperplasia of the submucosal and myenteric nerve plexus [2]. Since the first description of IND in 1971 [3], the definition of IND is still a subject of controversy. While the histopathological diagnostic criteria for IND undergo continuous revisions, the underlying causes and pathogenic mechanisms have not been elucidated.
Animal model is a critical tool for understanding the anatomy and pathophysiology of human disease and for exploring new treatments. Recently, genetic studies have provided strong evidence in animal models that IND is a real entity [4,5,6,7].
The Ncx (Enx, Hox11L.1) gene, one of the unclustered Hox genes belonging to the Hox11 gene family [8, 9], is specifically expressed in the dorsal root ganglia, cranial nerve ganglia (V, IX, and X), enteric ganglia, adrenal glands, and sympathetic ganglia [10]. Ncx is proven to be crucial to the development of ENS [10]. Homozygous mutant mice of Ncx−/− display megacolon with impaired colonic motility at the age of 3–5 weeks and have been verified to strongly resemble IND without any further major morphologic disorders [6, 7]. Histopathological evaluation revealed hyperplasia of myenteric ganglia similar to the phenotype observed in human IND type B [6, 7].
The transcription factor, Sox10, is expressed in glial cell lineage but not in enteric neurons such as ganglion glial cell differentiation [11,12,13]. Previously, we developed the genetically engineered transgenic Sox10-Venus mouse to visualize the behavior of migrating enteric neural crest cells (ENCCs) expressing green fluorescent protein (GFP), known as the Venus mouse [14]. The genetic regulation behind Sox10 gene expression and its mechanism in controlling ENS development are highly important to understand the pathogenesis of Sox10-related diseases including HD. Recently, we successfully created an endothelin receptor B (Ednrb) knockout mouse with Sox10 transgenic expression, which has been widely used to investigate the pathogenesis of HD through real-time visualization of ENCCs with Venus/GFP [15, 16].
With both the diagnostic criteria and pathogenesis of IND still controversial, an animal model is needed that can faithfully mimic the phenotypes of IND patients while providing us with a tool to study the cause of the disease. The aim of this study was to establish a novel mouse model of Sox10-Venus transgenic/Ncx−/− mouse that could allow the real-time visualization of ENS development in IND and to develop novel disease therapeutics.
Materials and methods
Animal model
We generated and raised Sox10-Venus transgenic mice (Sox10-Venus+) following a previously described protocol to visualize neural crest lineage cells [14]. We obtained heterozygote littermates (referred to as Ncx+/−) and homozygotes (referred to as Ncx−/−) from Dr. Masahiko Hatano [6]. Sox10-Venus+/Ncx−/− mice were raised at the Juntendo University School of Medicine in Tokyo, Japan, by initially crossing Ncx+/− mice with Sox10-Venus+ mice and repeat crossing with Sox10-Venus+/Ncx+/− mice. The genotypes of mice and embryos were determined by polymerase chain reaction. Mouse genotypes were determined using the 5′-TCACCTTCCCCTGGATGGAC-3′ upstream primer and the 5′-GATTGCACGCAGGTTCTCC-3′ Ncx downstream primer for the knockout allele. A total of 17 mice were euthanized via cervical dislocation at four to five weeks of age, comprising 8 Ncx−/−, 1 Ncx+/−, and 8 Ncx+/+ mice. All animal procedures underwent thorough review and received approval from the Juntendo University School of Medicine Animal Care and Use Committee (Institutional Review Board no. 2023032).
Morphological analysis using fluorescence
Body weights of each animal were measured before euthanization. The entire intestine was excised from each mouse, measured for length, fixed with 4% paraformaldehyde, and examined using fluorescence microscopy. Images were obtained using a BZX810 microscope (Keyence, Osaka, Japan).
Quantitative analysis of the area of Sox10-Venus in the intestine
To compare the area of Sox10-positive cell expression between Ncx−/− and control, we calculated the area with GFP expression divided by the total area of the entire intestinal tract in the region of interest using ‘Image J’ image analysis software (NIH, USA).
Immunostaining
After washing with phosphate buffered saline (PBS), specimens were immersed in 20% sucrose, embedded in OCT Mounting Compound (VWR International, Leuven, Belgium), frozen at − 80 °C, sectioned transversely at a thickness of 10 μm and mounted on Superfrost Plus slides (VWR International, Leuven, Belgium).
For direct Sox10-Venus fluorescence visualization, slides were washed with distilled water and mounted with coverslips using Vectashield mounting media containing DAPI (Vector Laboratories, USA).
For immunostaining, slices were rinsed with distilled water and blocked with goat serum, followed by incubation with the anti-Tuj1 primary antibodies (1:200) (mouse; BioLegend, California) overnight at 4 ℃, then incubated with anti-mouse HRP-conjugated secondary antibody (1:200) (DAKO, Sant Clara, CA) for 1 h at 37 ℃. All antibody incubation and washing steps were performed in PBS at pH7.4 diaminobenzidine (DAB, Dojindo, Kumamoto, Japan) solution was added and slides were monitored under microscope until color change. Slides were then counterstained with Hematoxylin and mounted with cover slip. Images were acquired on a confocal laser-scanning microscope (BZX810 microscope, Keyence, Japan).
Statistical analysis
Comparisons between the Ncx−/− and control groups were tested using an unpaired t test. Statistical significance was defined as p < 0.05. The GraphPad Prism 8.0 (GraphPad, SanDiego, California) software was used for data analysis.
Results
Lower body weight and megacolon in Ncx −/− mice
The gross anatomy of Ncx−/− mice indicated megacolon (Fig. 1a). Dilatation of the distal ileum, cecum and proximal colon in Ncx−/− mice was observed (Fig. 1b). The body weight of Ncx−/− mice was significantly lower than the control mice (16.9 ± 2.36 vs 20.7 ± 2.40, p < 0.05) without any further major morphologic disorders.
Gross morphological observation of intestinal tissue in Ncx−/− mouse and control mouse at 5 weeks. a Macroscopic comparison of intestine phenotype in control (left) and Ncx−/− (right) mice. b The entire dissected intestine from control and Ncx−/− mice. c Body weight (BW) and length of small intestine relative to body weight of both control and Ncx−/− mice (n = 8 for each group) (*p < 0.05)
Furthermore, the ratio of small intestine length to body weight was significantly higher in the Ncx−/− group than in the control group (1.82 ± 0.41 vs 1.22 ± 0.36, p < 0.05) (Fig. 1c).
Prescence of Sox10-Venus expression in the ENS of Ncx −/− mice
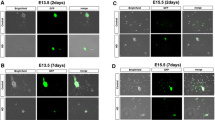
We confirmed that a complex network of nerve fibers/glia cells labeled using green fluorescence was visualized along the intestine wall without the need for antibody staining in both Ncx−/− and control mice (Fig. 2a). Looking at tissue sections, Sox10-Venus was mainly expressed in the neural plexus in intestine sandwiched between the muscle layers in the control proximal colon (Fig. 2b). However, in Ncx−/− mice, Sox10-Venus expression seemed to be uneven between the neural plexuses, specifically Ncx−/− mice has a higher expression of Sox10-Venus in the myenteric plexus compared to submucosal plexus. An analysis of the surface area covered by green fluorescence demonstrated higher region of Sox10-Venus expression in Ncx−/− proximal colon than in control proximal colon (5.83 ± 1.84 vs 2.60 ± 1.27, p < 0.05) (Fig. 2c).
Expression of Sox10-Venus in control and Ncx−/− intestine without immunostaining. a Whole tissue fluorescence image of unstained Sox10-Venus control and Ncx−/− proximal colon. b Tissue cross sections of unstained Sox10-Venus control and Ncx−/− proximal colon. c Percentage area of Sox10-Venus expression relative to total tissue section area
Ectopic neural expression in Ncx −/− mice
To study whether the Ncx−/− mice mimics phenotype of IND patients, immunohistochemical staining was carried out looking at the neural marker Tuj1. Increased expression of Tuj1 in the myenteric plexus layer was observed in Ncx−/− intestine, in addition to ectopic expression in the mucosal and muscular layers in both small intestine and proximal colon (Fig. 3). This shows that the Ncx−/− mice can be used as a faithful animal model to study IND.
Discussion
In this study, we have established a novel mouse model of IND, enabling the real-time visualization of ENS without the need for staining techniques. This model faithfully recapitulated the etiological factors and clinical features observed in human IND, including hyperganglionosis, ectopic ganglia, stunted weight growth, and the presence of megacolon. The creation of this novel mouse model holds significant promise for forthcoming investigations, as it facilitates in vivo imaging of individual cells, providing a valuable tool for detailed cellular-level research.
Granero Cendon et al. [17] reported that IND has an incidence of 1 in 7500 newborns, and studies examining rectal suction biopsies in various centers have reported isolated IND occurrences ranging from 0.3% to 40% [18, 19]. Isolated IND, which constitutes more than 95% of IND cases [18], is a malformation of the ENS characterized by abnormalities in the submucosal plexus. It was initially described by Melier-Ruge in 1971 as a condition where children exhibited clinical symptoms resembling HD due to ENS malformation [3]. In 1983, Fadda et al. [20] further classified IND into two distinct subtypes, both clinically and histologically. Currently, IND can be classified into two clinical and histological distinct subtypes. Type A IND (IND-A), accounting for less than 5% of the cases observed, can be histologically characterized by congenital aplasia or hypoplasia of the sympathetic innervation, with symptoms of intestinal obstruction, diarrhea, and bloody stools in the neonatal period due to delayed maturation of neuronal cells. In contrast, Type B IND (IND-B) is a permanent illness with hyperganglionosis, giant ganglia of the submucosal and myenteric plexus and ectopic ganglion cells, accounting for over 95% of all IND cases [18, 19, 21]. Thus, IND is now often used as synonymous with IND type B. In this study, we demonstrated that Ncx−/− mice has a higher expression of Sox10-Venus in the myenteric plexus compared to submucosal plexus. The fact that expression is not only elevated in the submucosal but also in the myenteric plexus is a feature very similar to that in humans and more supportive of this animal model [7].
The pathogenesis of IND is not completely understood and its etiology remains unknown. Nevertheless, numerous indications point toward a genetic basis, coupled with substantial involvement from environmental factors. However, findings from previous studies have not unveiled any specific genetic variants as the direct causes in IND-B patients [22, 23]. In fact, a low number of patients is a recurring problem in the analysis of the genetic basis of IND-B [22, 24,25,26], due to the low prevalence of the disease and the difficulties involved in the clinical diagnosis and management of patients. Moreover, some studies have suggested that the pathologic changes seen in IND might be a secondary phenomenon induced by congenital obstruction and inflammatory disease [1, 27]. Certainly, genetic research utilizing animal models can offer more compelling proof to validate IND as a genuine phenomenon.
Several animal models with mutations of various genes involved in ENS development, such as Ncx/Hox1L11, Spry2, Ednrb and Edn3, share histological features human IND-B phenotypes [2, 28,29,30], such as hyperganglionosis [23]. Recently, two different Ncx/Hox11L1 knockout mouse models and a heterozygous endothelin B receptor-deficient rat demonstrated abnormalities of the submucous plexus similar to that observed in human IND. In both Ncx/Hox11L1 knockout mouse model, homozygous mutant mice were viable, and approximately 50% of Ncx−/− mice developed megacolon at the age of 3–5 weeks. Histological and immunohistochemical analyses showed hyperplasia of the myenteric ganglia, a phenotype similar to that observed in IND [6, 7]. More recently, Von Boyen et al. [28] reported abnormalities of the ENS in heterozygous endothelin B receptor (Ednrb)-deficient rats resembling IND in humans. In general, Ncx−/− mouse is proposed as a model for IND. Further characterization of these mice may help to better define this controversial disease mechanism.
Sox10 plays a crucial role in cell fate specification and glial cell differentiation [11, 12]. It is reported that Sox10 is mainly expressed in glial cells of the peripheral nervous system but not in neurons in IND human colon [13, 31, 32]. We have previously created a mouse model of HD in which the ENS can be visualized without staining, a tool that has been very useful for investigation from the embryonic stage and at the cellular level leading to pathogenesis and therapeutic innovation [15]. In the present study, we confirmed the presence of Sox10 positive cells without staining, as observed in the small intestine and colon of both Sox10-Venus+/+/Ncx+/+ and Sox10-Venus+/+/Ncx−/− mice (Fig. 2.). Besides, immunohistochemical staining revealed increased expression of Tuj1, a neuron marker, in the myenteric plexus layer, in addition to ectopic expression in the mucosal and muscular layers in both small intestine and colon of Ncx−/− mice. Therefore, we confirmed that we have successfully established the animal model of IND using Sox10-Venus+/+/Ncx−/− mice which can be visualized without immunological staining, providing a more economical and unbiased approach with less physical and chemical manipulation. Similar to the advancements made in cell therapy and innovative treatments for HD, it is anticipated that this mouse model for IND will pave the way for a significant breakthrough in understanding its pathophysiology and the creation of novel therapeutics. Furthermore, through the transgenic labeling of the Sox10-expressing cells, ENCCs can be isolated and put to use in drug discovery studies targeting IND, an area that has not yet been explored. The application of this novel Sox10-Venus+/+/Ncx−/− mouse can open up new avenues for groundbreaking drug discovery investigations in the realm of IND.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Kapur RP, Reyes-Mugica M (2019) Intestinal neuronal dysplasia type B: an updated review of a problematic diagnosis. Arch Pathol Lab Med 143:235–243. https://doi.org/10.5858/arpa.2017-0524-RA
Yamataka A, Hatano M, Kobayashi H, Wang K, Miyahara K, Sueyoshi N et al (2001) Intestinal neuronal dysplasia-like pathology in Ncx/Hox11L.1 gene-deficient mice. J Pediatr Surg 36:1293–1296. https://doi.org/10.1053/jpsu.2001.25797
Meier-Ruge W (1971) Casuistic of colon disorder with symptoms of Hirschsprung’s disease (author’s transl). Verh Dtsch Ges Pathol 55:506–510
Liu W, Zhou T, Tian J, Yu X, Ren C, Cao Z et al (2022) Role of GDNF, GFRα1 and GFAP in a bifidobacterium-intervention induced mouse model of intestinal neuronal dysplasia. Front Pediatr. https://doi.org/10.3389/fped.2021.795678
Wang D, Gao N, Zhou T, Zhang Q, Wang J, Li A (2020) Effect of Neuroligin1 and Neurexin1 on the colonic motility in a mouse model of neuronal intestinal dysplasia. Gastroenterol Res Pract 2020:1–9. https://doi.org/10.1155/2020/9818652
Hatano M, Aoki T, Dezawa M, Yusa S, Iitsuka Y, Koseki H et al (1997) A novel pathogenesis of megacolon in Ncx/Hox11L.1 deficient mice. J Clin Investig 100:795–801. https://doi.org/10.1172/JCI119593
Shirasawa S, Yunker AMR, Roth KA, Brown GA, Horning S, Korsmeyer SJ (1997) Enx (Hox11L1)-deficient mice develop myenteric neuronal hyperplasia and megacolon. Nat Med 3:646–650. https://doi.org/10.1038/nm0697-646
Wen X-Y, Tang S, Breitman ML (1994) Genetic mapping of two mouse homeobox genes Tlx-1 and Tlx-2 to murine chromosomes 19 and 6. Genomics 24:388–390. https://doi.org/10.1006/geno.1994.1634
Dear TN, Sanchez-Garcia I, Rabbitts TH (1993) The HOX11 gene encodes a DNA-binding nuclear transcription factor belonging to a distinct family of homeobox genes. Proc Natl Acad Sci 90:4431–4435. https://doi.org/10.1073/pnas.90.10.4431
Hatano M, Iitsuka Y, Yamamoto H, Dezawa M, Yusa S, Kohno Y et al (1997) Ncx, a Hox11 related gene, is expressed in a variety of tissues derived from neural crest cells. Anat Embryol (Berl) 195:419–425. https://doi.org/10.1007/s004290050061
Kelsh RN (2006) Sorting outSox10 functions in neural crest development. BioEssays 28:788–798. https://doi.org/10.1002/bies.20445
Bondurand N, Natarajan D, Barlow A, Thapar N, Pachnis V (2006) Maintenance of mammalian enteric nervous system progenitors by SOX10 and endothelin 3 signalling. Development 133:2075–2086. https://doi.org/10.1242/dev.02375
Bondurand N, Natarajan D, Thapar N, Atkins C, Pachnis V (2003) Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development 130:6387–6400. https://doi.org/10.1242/dev.00857
Shibata S, Yasuda A, Renault-Mihara F, Suyama S, Katoh H, Inoue T et al (2010) Sox10- Venus mice: a new tool for real-time labeling of neural crest lineage cells and oligodendrocytes. Mol Brain 3:31. https://doi.org/10.1186/1756-6606-3-31
Miyahara K, Kato Y, Koga H, Lane GJ, Inoue T, Akazawa C et al (2010) Abnormal enteric innervation identified without histopathologic staining in aganglionic colorectum from a mouse model of Hirschsprung’s disease. J Pediatr Surg 45:2403–2407. https://doi.org/10.1016/j.jpedsurg.2010.08.039
Fujiwara N, Miyahara K, Nakazawa-Tanaka N, Akazawa C, Yamataka A (2022) In vitro investigation of the differentiation of enteric neural crest-derived cells following transplantation of aganglionic gut in a mouse model. Pediatr Surg Int 38:755–759. https://doi.org/10.1007/s00383-022-05105-2
Granero Cendón R, Millán López A, Moya Jiménez MJ, López Alonso M (2007) De Agustín Asensio JC [Intestinal neuronal dysplasia: association with digestive malformations]. Cir Pediatr 20:166–168
Puri P, Rolle U (2004) Variant Hirschsprung’s disease. Semin Pediatr Surg 13:293–299. https://doi.org/10.1053/j.sempedsurg.2004.10.017
Puri P, Gosemann J-H (2012) Variants of Hirschsprung disease. Semin Pediatr Surg 21:310–318. https://doi.org/10.1053/j.sempedsurg.2012.07.005
Fadda B, Maier W, Meier-Ruge W, Schärli A, Daum R (1983) Neuronale intestinale DysplasieEine kritische 10-Jahres-Analyse klinischer und bioptischer Diagnostik. Eur J Pediatr Surg 38:305–311. https://doi.org/10.1055/s-2008-1059994
Puri P (1997) Variant Hirschsprung’s disease. J Pediatr Surg 32:149–157. https://doi.org/10.1016/S0022-3468(97)90170-6
Gath R, Goessling A, Keller K-M, Koletzko S, Coerdt W, Müntefering H et al (2001) Analysis of the RET, GDNF, EDN3, and EDNRB genes in patients with intestinal neuronal dysplasia and Hirschsprung disease. Gut 48:671–675. https://doi.org/10.1136/gut.48.5.671
Sánchez-Mejías A, Fernández RM, Antiñolo G, Borrego S (2010) A new experimental approach is required in the molecular analysis of intestinal neuronal dysplasia type B patients. Exp Ther Med 1:999–1003. https://doi.org/10.3892/etm.2010.140
Borghini S, Di DM, Prato AP, Lerone M, Martucciello G, Jasonni V et al (2009) Search for pathogenetic variants of the SPRY2 gene in intestinal innervation defects. Intern Med J 39:335–337. https://doi.org/10.1111/j.1445-5994.2009.01907.x
Fava M, Borghini S, Cinti R, Cusano R, Seri M, Lerone M et al (2002) HOX11L1: a promoter study to evaluate possible expression defects in intestinal motility disorders. Int J Mol Med 10:101–106
Costa M (2000) Evaluation of the HOX11L1 gene as a candidate for congenital disorders of intestinal innervation. J Med Genet 37:9e–99. https://doi.org/10.1136/jmg.37.7.e9
Sacher P, Briner J, Hanimann B (1993) Is neuronal intestinal dysplasia (NID) a primary disease or a secondary phenomenon? Eur J Pediatr Surg 3:228–230. https://doi.org/10.1055/s-2008-1063549
von Boyen GBT (2002) Abnormalities of the enteric nervous system in heterozygous endothelin B receptor deficient (spotting lethal) rats resembling intestinal neuronal dysplasia. Gut 51:414–419. https://doi.org/10.1136/gut.51.3.414
Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, Kato R et al (2005) Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci 8:855–857. https://doi.org/10.1038/nn1485
Yanai T, Kobayashi H, Yamataka A, Lane GJ, Miyano T, Hayakawa T et al (2004) Acetylcholine-related bowel dysmotility in homozygous mutant NCX/HOX11L.1-deficient (NCX−/−) mice—evidence that acetylcholine is implicated in causing intestinal neuronal dysplasia. J Pediatr Surg 39:927–930. https://doi.org/10.1016/j.jpedsurg.2004.02.004
Young HM, Bergner AJ, Müller T (2003) Acquisition of neuronal and glial markers by neural crest-derived cells in the mouse intestine. J Comp Neurol 456:1–11. https://doi.org/10.1002/cne.10448
Liu Y-R, Ba F, Cheng L-J, Li X, Zhang S-W, Zhang S-C (2019) Efficacy of Sox10 promoter methylation in the diagnosis of intestinal neuronal dysplasia from the peripheral blood. Clin Transl Gastroenterol 10:e00093. https://doi.org/10.14309/ctg.0000000000000093
Acknowledgements
We thank Mr. Fumio Kanai for support for the animals in the Laboratory of Genome Research, Research Institute for Diseases of Old Age, Juntendo University Graduate School of Medicine and Ms. Mirei Takahashi for assistance in the experiments.
Funding
This work was supported by JSPS KAKENHI Grant Number (22K08721).
Author information
Authors and Affiliations
Contributions
NF performed animal experiments and the analysis of the experimental results, also wrote the whole manuscript. KM performed some of the animal experiments. NN-T and DL supervised some of the animal experiments. AP, AY, CA and MH provided advice and supervision. All the authors reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fujiwara, N., Miyahara, K., Lee, D. et al. A novel mouse model of intestinal neuronal dysplasia: visualization of the enteric nervous system. Pediatr Surg Int 39, 298 (2023). https://doi.org/10.1007/s00383-023-05585-w
Accepted:
Published:
DOI: https://doi.org/10.1007/s00383-023-05585-w